Back to Journals » Drug Design, Development and Therapy » Volume 14
The Safety and Efficacy of Ultrasound-Guided Bilateral Dual Transversus Abdominis Plane (BD-TAP) Block in ERAS Program of Laparoscopic Hepatectomy: A Prospective, Randomized, Controlled, Blinded, Clinical Study
Authors Zhang J, Liu T, Zhou H, Fei Y, Yu X
Received 2 April 2020
Accepted for publication 23 June 2020
Published 21 July 2020 Volume 2020:14 Pages 2889—2898
DOI https://doi.org/10.2147/DDDT.S255385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Jun Zhang, Tieshuai Liu, Haiyan Zhou, Yue Fei, Xin Yu
Department of Anesthesiology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Xin Yu
Department of Anesthesiology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, 3 Qingchun Road East, Jianggan District, Hangzhou 310016, Zhejiang, People’s Republic of China
Tel +86 13588708514
Fax +86 0571-86006662
Email [email protected]
Purpose: Postoperative pain management for patients undergoing hepatic resection is still a challenge due to the risk of perioperative liver dysfunction. The transversus abdominis plane (TAP) block is a promising regional analgesic technique. However, the correct guidelines regarding the dose and regimen of local anesthetics in patients undergoing hepatic resection have yet to be established completely. This study aimed to evaluate the safety and efficacy of ultrasound-guided BD-TAP block with a large dose of ropivacaine in laparoscopic hepatectomy.
Patients and Methods: This prospective, blinded, randomized, controlled study was conducted with 50 patients who were scheduled for selective laparoscopic hepatectomy. Patients who received a BD-TAP block (3 mg/kg of ropivacaine diluted to 60 mL) with general anesthesia were categorized into the BD-TAP block group (n = 25), and those who received general anesthesia were categorized into the control group (n = 25). The primary outcomes were consumption of sufentanil within 48 hours post-operation and plasma ropivacaine concentration. The secondary outcomes were the severity of pain (at rest and upon coughing), nausea and/or vomiting, and quality of recovery.
Results: Compared with the control group, the patients in BD-TAP block group had a significant reduction of postoperative sufentanil consumption at 2 hours (P = 0.019), 24 hours (P = 0.001), and 48 hours (P = 0.001), and the visual analog scale (VAS) scores on coughing were significantly lower at postoperative 2 hours (P = 0.004). There were no statistically significant differences in postoperative nausea and/or vomiting, flatus, catheter removal, off-bed activity, liver function, or postoperative hospital stay. The mean peak total ropivacaine concentration was 1,067.85 ng/mL, which occurred 1 hour after administering the block, and mean free ropivacaine concentration was 52.32 ng/mL. The highest individual peak plasma concentration was 2,360.90 ng/mL at 45 min postinjection, and the free ropivacaine concentration was 139.29 ng/mL.
Conclusion: Ultrasound-guided BD-TAP block provides effective postoperative analgesia after laparoscopic hepatectomy. This study also confirms that ultrasound-guided BD-TAP blocks with 3 mg/kg ropivacaine during laparoscopic hepatectomy almost never results in the plasma ropivacaine concentrations associated with neurotoxicity.
Keywords: ropivacaine, laparoscopic hepatectomy, BD-TAP block, neurotoxicity
Introduction
Hepatic resection is a common treatment method for primary and secondary diseases (hepatolithiasis, hemangioma and cancer) of the liver. At present, the laparoscopic hepatectomy is widely used in clinical practice, and the number performed worldwide has increased exponentially.1,2 Compared with the traditional open hepatectomy, minimally invasive surgery combined with an enhanced recovery after surgery (ERAS) program can reduce perioperative stress response and postoperative complications and promote their recovery from surgery.3 Therefore, research on laparoscopic hepatectomy and ERAS is of great significance.
Challenges in Postoperative Pain Control in Laparoscopic Hepatectomy
The liver is the primary organ of key proteins synthesis of plasma (albumin, clotting factors), and patients undergoing hepatic resection often have liver dysfunction, such as hypoproteinemia or coagulation dysfunction. Although epidural analgesia (EA) is identified as the gold standard of postoperative pain control for both open and laparoscopic abdominal surgery, and previous studies confirmed that EA improved pain control, reduced opioid requirements and faster returned to bowel function in patients undergoing colorectal surgery.4 However, EA may result in rare but serious complications such as epidural hematoma and neurological sequelae. These potential benefits of EA must be weighed against the theoretical risks of epidural hematoma and neurological sequelae in laparoscopic hepatectomy patients. Moreover, excessive use of opioid can lead to extended recovery from anesthesia, nausea and vomiting, ileus, and lengthened hospital stay.5 Thus, it is still challenging for surgeons and anesthesiologists to provide effective pain control during the perioperative period. Multimodal analgesia in an ERAS program involves using two or more non-opioid medications to both minimize pain and minimize opioid use after surgery.6 One component of this is the utilization of regional analgesic for postoperative pain control.
Laparoscopic hepatectomy requires anesthetization of both the upper intercostal TAP plexus (Th6–Th9) and lower classical TAP (CL-TAP) plexus (Th10–L1), and the upper intercostal and lower CL-TAP compartments do not communicate.7 The BD-TAP block is a promising regional analgesic technique, and previous studies have confirmed that an ultrasound-guided BD-TAP block with injections of the upper intercostal and lateral CL-TAP compartments to anesthetize the upper (Th6–Th9) and the lower (Th10–L1) abdominal wall could improve the severity of pain following major abdominal surgery and lead to low opioid consumption on postoperative day 1 and early mobilization.8 Thus, we recently projected the use of the ultrasound-guided BD-TAP block for treating postoperative analgesia in patients undergoing laparoscopic hepatectomy. The primary aim of the current study was to observe the effects of the postoperative ultrasound-guided BD-TAP block on pain and recovery after laparoscopic hepatectomy.
Safety of BD-TAP Block with Ropivacaine in Laparoscopic Hepatectomy
Ropivacaine is a long-acting local anesthetic that is frequently used in the regional block technique. One of the most important pharmacological characteristics of ropivacaine is that high systemic exposure to it often results in central nervous system disorders and cardiovascular disorders.9 In a study including individuals receiving titrated intravenous infusions of ropivacaine, it was indicated that the onset of neurological symptoms occurred at a mean total plasma ropivacaine concentration of 2.20 μg/mL and free ropivacaine concentration of 0.15 (0.08) μg/mL.10
In previous studies, potentially toxic plasma concentrations of ropivacaine have been assessed after ultrasound-guided CL-TAP blocks for cesarean sections and open major gynecological surgery.11,12 These studies revealed that 40% of patients undergoing a cesarean section had a total plasma ropivacaine concentration that exceeded the universally known toxic threshold of 2.20 µg/mL at some points after the block, whereas 36% of patients undergoing gynecological surgery exceeded the potentially toxic threshold-free concentration of 0.15 µg/mL. However, the above data are only suitable for patients with normal liver function using ropivacaine for a CL-TAP block. The BD-TAP block technique involves the injection of a relatively single high-dose of local anesthetic into transversus abdominis plane. The risk for ropivacaine systemic toxicity in hepatectomy patients undergoing a BD-TAP block should be well recognized because the metabolism and disposition of local anesthetics may be changed in such patients owing to postoperative liver dysfunction. At present, there is lack of clinical experience and data regarding patients undergoing hepatic resection using ropivacaine and receiving an ultrasound-guided BD-TAP block. Other primary aims of this study were to determine the peak and mean venous plasma ropivacaine concentrations after ultrasound-guided BD-TAP block using 3 mg/kg ropivacaine diluted with 0.9% saline to a total volume of 60 mL (15 mL each point) in patients undergoing laparoscopic hepatectomy under general anesthesia and to measure the time course of plasma ropivacaine concentration within 24 hours postinjection.
Patients and Methods
Ethics Approval and Informed Consent
Approval was obtained from the Ethics Committee of Sir Run Run Shaw Hospital affiliated with the Zhejiang University School of Medicine (approval number: 20170615–1), and written informed consent was obtained from all participants. The trial has been registered at http://www.chictr.org.cn/index.aspx (registration number: ChiCTR-INR-17012243 and ChiCTR-ONC-17012245). This trial was conducted in accordance with the Declaration of Helsinki.
Case Selection, Random and Blind Method
We recruited 50 ASA physical status I–III patients scheduled for laparoscopic hepatectomy, in a randomized, blinded controlled clinical trial. Participants were randomized to undergo BD-TAP block (n = 25) or to receive standard care (n = 25). The allocation sequence was generated by a random number table, and group allocation was concealed in sealed opaque envelopes that were opened by an anesthesiologist who performed the BD-TAP block after closure of the skin at the end of the surgery. As the intervention procedure was different between the two groups, it was impossible to blind the anesthesiologist who performed BD-TAP block; however, all patients, the anesthesiologist who administered general anesthesia, data managers, and the staff providing postoperative care were blinded to the group assignment. The exclusion criteria were history of allergy to ropivacaine, tolerance toward opioid analgesics, inability to use patient-controlled intravenous analgesia (PCIA), operation conversion to laparotomy, mental disorder, and body mass index (BMI) >40.
ERAS Protocol
The ERAS protocol for all patients was used to standardize the perioperative treatment in both groups. It comprises the following: (1) preoperative education, (2) no bowel preparation, (3) shortening fasting time, (3) three-dimensional reconstruction of liver, (4) no premedication, (5) no gastric tube, (6) thrombus prophylaxis, (7) minimally invasive surgery, (8) avoidance of hypothermia and fluid overload, (9) multimodal analgesia, (10) early catheter removal, (11) early oral nutrition, (12) early off-bed activity, (13) respiratory rehabilitative exercise, (14) nutritional rehabilitation support, and (15) follow-up.
Methods of Anesthesia
All participants received a standardized general anesthetic. The internal jugular vein access and invasive arterial blood pressure were obtained in the preoperative preparing room. Electrocardiogram, invasive arterial blood pressure, pulse oxygen saturation, end-tidal carbon dioxide, temperature, and entropy were monitored. After preoxygenation, anesthesia was induced with intravenous sufentanil (0.5 µg/kg to a maximum of 50 µg), cisatracurium (0.2 mg/kg), and propofol (2.0 mg/kg). Tracheal intubation was performed after the administration of muscle relaxants and general anesthesia was maintained through inhalation of sevoflurane (0.6–1.0 MAC), propofol (1.0–2.0 mg/kg/min), and remifentanil (0.1–0.4 µg/kg/min). The entropy index was maintained at the range of 40–60, and 0.2 µg/kg sufentanil was added before the end of the operation. After surgery, a standardized postoperative analgesic regimen was initiated for both groups. This included intravenous flurbiprofen axetil at 50 mg every 8 h and PCIA. The PCIA pump was programmed in an only-bolus mode without a basal rate, allowing for a 2 µg sufentanil bolus injection every 5 min with a maximum of 40 µg every 4 h. Sufentanil was administered on demand if the VAS scores were >3 on assessment; PONV was treated with 3-mg intravenous granisetron (in the postanesthesia care unit) or 10-mg intravenous metoclopramide (on the ward).
Ultrasound-Guided BD-TAP Block
In the experimental group, a skilled anesthesiologist who performed BD-TAP block exited the room after closure of the skin. The skin was disinfected with a 5% povidone-iodine solution. Ultrasound images were procured using a SonoSite S Series ultrasound machine (FUJIFILM SonoSite, Inc. Bothell, Washington, USA) with a high-frequency linear US transducer (HFL38x/13-6 MHz). The blocks were administered using a 120-mm regional anesthesia needle (B-Braun Medical, Melsungen AG, Germany) via an in-plane approach in a medial-to-lateral direction. The participants received total 3 mg/kg of ropivacaine (Naropin, AstraZeneca, Sweden), which was diluted to 60 mL (15 mL each point). While bilaterally administering the upper abdomen intercostal TAP plexus (Th6–Th9), the insertion point was as high and medially as possible based on each individual anatomy features. The local anesthetic were injected either between the posterior rectus sheath and transversus abdominis muscles (medial intercostal TAP block) or between the internal oblique and transversus abdominis muscles lateral to the aponeurosis (lateral intercostal TAP block).3
Follow-Up Visits
As the primary outcome parameter, we evaluated the cumulative sufentanil consumption within 48 hours post-operation. The secondary endpoints were assessed within 48 hours after surgery. The severity of pain at rest and on coughing was assessed using a 10-cm VAS (0–10). The occurrence and level of nausea and/or vomiting were recorded and evaluated via a categorical scale (0, none; 1, mild; 2, moderate; 3, severe). Moreover, we evaluated the time from surgery until catheter removal, flatus, off-bed activity, postoperative hospital stay, and perioperative changes in liver function.
Sample Collection
After the block, venous blood samples were obtained by aspiration from the central vein access. Blood samples (5 mL) were collected at 15, 30, 45, 60, and 90 min and 2, 4, 6, and 24 h following the block. If the tracheal tube had been removed, patients were asked whether they experienced perioral tingling, a metallic taste, tinnitus, visual disturbances, or slurred speech each time blood sample was taken. These samples were temporarily stored in a refrigerator, and the blood samples were centrifuged for 15 min at 1100 g within 2 h of sampling. Following centrifugation of these samples, the plasma was stored at −80°C until being assayed. The total plasma concentration of ropivacaine was measured via liquid chromatography–tandem mass spectrometry (LC–MS/MS). The binding rate of ropivacaine to human plasma protein in samples obtained from participants undergoing laparoscopic hepatectomy was determined via equilibrium dialysis.
Ropivacaine Quantification
For the total plasma ropivacaine concentration, 100 µL of plasma was added to a tube with 400 µL acetonitrile mixed with 100 ng/mL internal standard, vortex oscillated, and centrifuged at 13,000 rpm 4°C for 10 min. The upper 200 µL was taken and placed in the inner lining pipe for LC–MS/MS (10°C) sample analysis. Standard solutions of ropivacaine at concentrations of 0.40, 1.00, 2.00, 4.00, 10.0, 20.0, 50.0, and 100 μg/mL were prepared by precisely absorbing the reserve solution of ropivacaine and diluting it with methanol. Standard curve samples with ropivacaine concentrations of 20.0, 50.0, 100, 200, 500, 1000, 2000, and 5000 ng/mL were prepared by adding 5.0 µL of each standard solution to 95 µL blank human plasma and operated according to the item of “human plasma sample processing.” The standard curve of ropivacaine in human plasma was obtained with the peak area ratio of the sample and internal standard as the ordinate, the concentration (ng/mL) as the abscissa, and 1/×2 as the weight coefficient. The results showed that the linearity of ropivacaine was good in the range of 20–5000 ng/mL. For the incubated sample, 10.0 µL of plasma sample was taken and placed into a 1.5-mL Eppendorf tube. Then, 90.0 µL phosphate buffer was added, and the solution was mixed evenly; 200 µL acetonitrile was mixed with 100 ng/mL internal standard loratadine, vortex oscillated, and centrifuged at 14,000 rpm and 10°C for 10 min. The supernatant underwent LC–MS/MS for sample analysis. At 24 h after the block, if the concentration of ropivacaine in the plasma was lower than the lower limit of quantification, the default concentration of ropivacaine was zero.
Sample Size Estimation
In our preliminary study, 20 patients who were prepared for laparoscopic hepatectomy were evenly divided into two groups. Based on the preliminary study, we projected a mean standard deviation (SD) 24 h sufentanil consumption of 53.80 (35.57) μg and 19.40 (17.56) μg in the control group and BD-TAP group, respectively. The sample of patients was calculated as 22 in each group through statistics software STATA/SE 12.0 (α = 0.05 two sides, β = 0.1 one side). We decided to recruit 25 patients per group into the study to minimize any effect of data loss.
Statistical Analysis
The IBM SPSS Statistics version 22 software was used for the statistical analyses. Continuous variables were compared using Student’s t-test or Mann–Whitney U-test as appropriate. Categorical data were determined using the Chi-square test or Fisher’s exact test. Continuous variables were presented as means (SD) or medians (interquartile range). The categorical data were presented as frequencies. A P-value < 0.05 was considered statistically significant.
Results
In total, 50 patients were enrolled in the study. Three patients were eliminated from the study due to surgery conversion to laparotomy. The other 47 patients, 24 were categorized to undergo an ultrasound-guided BD-TAP block with ropivacaine, and 23 were categorized to receive standard therapy. There were no statistic differences between the two groups in terms of patient characteristics and surgical procedures (Table 1).
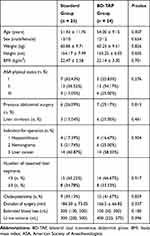 |
Table 1 Patient Characteristics and Surgical Procedures |
Follow-Up Visits Outcome
Compared with the control group, only the VAS scores on coughing were significantly lower in the BD-TAP block group at 2 hours after surgery (P = 0.004); the patients had a dramatic reduction of sufentanil consumption at postoperative 2 hours (P = 0.019), 24 hours (P = 0.001) and 48 hours (P = 0.001) (Figure 1, Table 2). There was no statistic difference in the incidence and severity of postoperative nausea and vomiting (PONV) in the two groups within 24 h post-operation (Table 3).
 |
Table 2 Postoperative VAS Scores and Cumulative Sufentanil Consumption of the Study Population |
 |
Table 3 PONV Scores of the Study Population |
There was no significant difference between the two groups in time to removal of urinary catheter, flatus, or off-bed activity. In this study, three patients in the control group were hospitalized for 21, 20, and 22 days because of postoperative fever, hyperbilirubinemia, and hypertension, respectively. Two patients in the BD-TAP group were hospitalized for 27 days and 25 days because of gastroparesis and endoscopic retrograde cholangiopancreatography (ERCP), respectively. No differences were found in the postoperative hospital stay between the two groups either (Table 4).
 |
Table 4 Postoperative Quality of Recovery of the Study Population |
In the control group, one patient was excluded due to incomplete data of liver function. There was no statistic difference in the recovery of liver function between the two groups (Table 5).
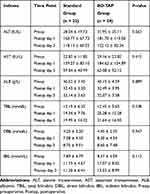 |
Table 5 Quantitative Assessment of Liver Function of the Study Population |
Plasma Ropivacaine Concentrations
A total of 24 patients received an ultrasound-guided BD-TAP block with ropivacaine. For the last 18 patients, the sample collection at 6 h post-block was deleted due to the concern that it would have resulted in disturbing sleep of the patients; the significance of this time was not very great.
At the end of surgery, the ultrasound-guided BD-TAP blocks were performed using a mean (SD) dose of ropivacaine of 181.04 (29.26) mg; the mean concentration was 0.35–0.25%.
The mean peak total ropivacaine concentration was 1,067.85 ng/mL, which occurred 1 h after the administering block. The maximum measured total plasma concentration was 2,360.90 ng/mL at 45 min postinjection. The median concentrations were 954.45 ng/mL for up to 45 min postinjection. The time course of the total serum ropivacaine concentration is presented in Figure 2.
 |
Figure 2 Total plasma ropivacaine concentrations: (A) Mean (SD); (B) Median (minimum to maximum); (C) Scatter plot of total plasma ropivacaine. |
The binding rate of ropivacaine to human plasma protein was determined via equilibrium dialysis. When the concentrations of ropivacaine were 500, 1000, and 2000 ng/mL, the binding rates of human plasma protein were 95.6 ± 1.080%, 95.1 ± 0.654%, and 94.1 ± 0.487%, respectively. The mean-free peak concentration measured at 1 h was 52.32 ng/mL. The maximum-free plasma concentration was 139.29 ng/mL. The median-free concentration was 41.99 ng/mL at 45 min postinjection.
We did not observe any symptoms of neurological toxicity, and no critical cardiovascular instability was found in any patient. There were no other complications attributable to the ultrasound-guided BD-TAP block.
Discussion
The benefits of optimal postoperative pain control include a decrease in the postoperative stress response, postoperative complication, and length of stay. Moreover, effective pain control improves rehabilitation and enhances recovery after surgery.13 Our study was conducted based on an ERAS program and intended to assess the safety and efficacy of ultrasound-guided BD-TAP block in the management of postoperative pain, PONV, and postoperative hospital stay after laparoscopic hepatectomy.
The studies reported thus far utilized ultrasound-guided CL-TAP blocks bilaterally for intestinal surgery and cesarean sections. CT-TAP blocks improve pain scores, reduce the incidence of PONV, and reduce postoperative opioid consumption.14–16 However, they can only be utilized for surgery involving the lower CL-TAP plexus (Th10–L1), and upper intercostal TAP plexus (Th6–Th9) and lower CL-TAP plexus (Th10–L1) compartments do not communicate.7 To our knowledge, no studies have assessed the safety and efficacy of ultrasound-guided BD-TAP blocks on postoperative pain control in laparoscopic hepatectomy.
In this study, the patients who received an ultrasound-guided BD-TAP block had considerably reduced cumulative sufentanil consumption at postoperative 2, 24, and 48 hours. The data were consistent with the previous study.17 Moreover, this reduction at postoperative 24 hours is on a larger range (63.46% vs. 41.70%) compared with previous study on ultrasound-guided subcostal TAP blocks in patients undergoing an open right hepatectomy.18 The data demonstrate that the BD-TAP block was a more effective and perfect regional analgesic technique. Compared with the control group, only the VAS scores on coughing were significantly lower in the BD-TAP block group at 2 hours after surgery (P = 0.004).
The prevention of PONV is an important concern after laparoscopic hepatectomy in ERAS programs. In this study, the incidence of PONV in the BD-TAP block group was obviously lower: 47.83% of patients had nausea and/or vomiting in the control group, whereas only 29.17% of patients complained of those symptoms in the BD-TAP group. Yet for all that, the percentage and severity of patients suffering from nausea and/or vomiting were no statistically significant differences in both groups, and the incidence of PONV was higher in both groups. The most likely reason is increasing duration of surgery and use of volatile anesthetics in Laparoscopic Hepatectomy.19 In future study, we need to further optimize perioperative management and increase the sample size. It has been demonstrated that the combined effect of a TAP block and less opioids analgesics is advantageous in shortening the time to first flatus, oral intake, and catheter removal.20 Additionally, some previous studies have also reported the validity of a TAP block in reducing the length of stay.20,21 We found that the time to flatus was not significantly shorter in the BD-TAP block group than in the control group (34.77 ± 15.31 vs. 42.30 ± 19.26 h) in the present study. One possible reason for this is that the sample size evaluation index of our study was based on the 24 h sufentanil consumption; hence, it may be necessary to continue to increase the sample size in future experiments. Additionally, there was no significant difference between the two groups in the time to urinary catheter removal, off-bed activity, or postoperative hospital stay. It may be that these indicators were already included in the ERAS program, e.g., all patients being given fluid 6 h after surgery and having their catheters removed as early as possible to encourage them to complete off-bed activities. Meanwhile, the surgeon determined the length of stay based on previous clinical experience and discharge protocol in our hospital, which might not have been perfect. We believe that larger sample and prospective studies are necessary to analyze the effect of ultrasound-guided TAP block on length of stay.
Plasma Ropivacaine Concentration
Ropivacaine is a long-acting local anesthetic and it is frequently used in the regional block. The central nervous systems (CNS) toxicity of ropivacaine previously has been reported, showing the onset of symptoms of CNS toxicity at a mean total plasma concentration of 2.20 µg/mL and a free plasma concentration of 0.15 (0.08) µg/mL.10 Plasma ropivacaine concentration has been assessed in the CL-TAP block technique. When patients received total 3 mg/kg of ropivacaine diluted with 0.9% saline to form a total volume of 40 mL (mean concentration, 0.5%) for elective major open gynecological surgery, ten patients (36%) exceeded the potentially toxic threshold-free concentration of 0.15 µg/mL.11 In another study, patients received a total dose of 2.5 mg/kg of ropivacaine diluted with 0.9% saline to form a total volume of 40 mL (mean concentration, 0.5%) for postoperative analgesia after a cesarean section. The total plasma ropivacaine concentrations exceeded the potentially toxic threshold concentration of 2.20 µg/mL in 12 patients (40%).12 Although these studies found that clinically important toxicity is unlikely with this technique, the findings in these studies indicate the potential that total 2.5–3 mg/kg (mean concentration, 0.5%) may be excessive in some patients. In laparoscopic hepatectomy, the correct guidelines regarding the dose and regimen of ropivacaine for BD-TAP block have yet to be established completely. In this study, participants received an ultrasound-guided BD-TAP block for total 3 mg/kg (BMI 22.14 ± 3.30) of ropivacaine diluted 60 mL (mean concentration, 0.35–0.25%). The mean peak total ropivacaine concentration was 1,067.85 ng/mL, which appeared at 1 h after the block, and the corresponding mean free ropivacaine concentration was 52.32 ng/mL. Only one patient had total ropivacaine concentration that exceeded the toxic threshold of ropivacaine of 2.20 µg/m at 45 min after the block, with the highest individual value (2,360.90 ng/mL), and no complained of any symptoms of neurological toxicity and critical cardiovascular. These data demonstrate that the use of 3 mg/kg of ropivacaine diluted with 0.9% saline to a total volume of 60 mL in an ultrasound-guided BD-TAP block in laparoscopic hepatectomy almost never exceeded the potentially toxic threshold concentrations of ropivacaine, even in patients with cirrhosis or major hepatectomy.
Along with the absolute serum levels, the rate of rise of serum local anesthetic concentration is closely related to toxicity. In this study, the plasma levels increased more slowly than in other CL-TAP block studies (mean concentration, 0.5%), where in peak levels occurred at 30 min11 and 35.5 min,12 respectively, which occurred at the 1-h measurement in this study. Upon combining the results of this study with those of previous studies, it is not difficult to infer that the mean peak total ropivacaine concentration and time of its emergence are not only determined by specific technical approaches and the total amount of ropivacaine22 but may also be related to the concentration of ropivacaine used.
Limitations
Two potential limitations of this study should be considered. Firstly, the study assessment of postoperative analgesia was limited to postoperative 2, 24, and 48 hours. The main reason is that all patients had preoperative education on the use of analgesia pump. Moreover, excessive follow-ups resulted in disturbing patients during the early hours of the night.23 Secondly, Alpha-1 acid glycoprotein (AAG) concentration was not measured in our protocol. AAG was the major plasma protein responsible for the binding of basic lipophilic drugs, and ropivacaine was reported to preferably bind to AAG.24 The AAG binding is saturable and more easily replaced by other drugs because the plasma AAG concentration fluctuates greatly among different individuals, especially among patients with inflammatory and certain comorbidities. Nonetheless, the binding rate of ropivacaine in our study is similar to that in the previous study (93.87%).11
Conclusion
In conclusion, the BD-TAP block technique provides effective postoperative analgesia after laparoscopic hepatectomy. This study also confirms that a BD-TAP block with 3 mg/kg ropivacaine upon laparoscopic hepatectomy almost never results in the plasma ropivacaine concentrations associated with neurotoxicity.
Abbreviations
BD-TAP, bilateral dual transversus abdominis plane; TAP, transversus abdominis plane; VAS, visual analog scale; ERAS, enhanced recovery after surgery; EA, epidural analgesia; CL-TAP, classical TAP; ASA, American Society of Anesthesiologists; PCIA, patient-controlled intravenous analgesia; BMI, body mass index; LC–MS/MS, liquid chromatography–tandem mass spectrometry; SD, standard deviation; PONV, post Operative Nausea And Vomiting; ERCP, endoscopic retrograde cholangiopancreatography; Preop, preoperative; Postop, postoperative; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; CNS, central nervous systems; AAG, Alpha-1 acid glycoprotein.
Data Sharing Statement
The datasets supporting the results of this study are available from the corresponding author.
Ethics and Consent Statement
This study was approved by the Ethics Committee of Sir Run Run Shaw Hospital affiliated with the Zhejiang University School of Medicine. Written informed consent was obtained from all subjects prior to the study. This trial was conducted in accordance with the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2804 patients. Ann Surg. 2009;250(5):831–841. doi:10.1097/SLA.0b013e3181b0c4df
2. Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250(1):103–111. doi:10.1097/SLA.0b013e3181ad6660
3. Dasari BV, Rahman R, Khan S, et al. Safety and feasibility of an enhanced recovery pathway after a liver resection: prospective cohort study. HPB. 2015;17(8):700–706. doi:10.1111/hpb.12447
4. Guay J, Nishimori M, Kopp SL. Epidural local anesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting, and pain after abdominal surgery: a cochrane review. Anesth Analg. 2016;123(6):1591–1602. doi:10.1213/ANE.0000000000001628
5. Simpson JC, Bao X, Agarwala A. Pain management in Enhanced Recovery after Surgery (ERAS) protocols. Clin Colon Rectal Surg. 2019;32(2):121–128. doi:10.1055/s-0038-1676477
6. Tan M, Law LS, Gan TJ. Optimizing pain management to facilitate enhanced recovery after surgery pathways. Can J Anaesth. 2015;62(2):203–218. doi:10.1007/s12630-014-0275-x
7. Borglum J, Jensen K, Christensen AF, et al. Distribution patterns, dermatomal anesthesia, and ropivacaine serum concentrations after bilateral dual transversus abdominis plane block. Reg Anesth Pain Med. 2012;37(3):294–301. doi:10.1097/AAP.0b013e31824c20a9
8. Borglum J, Maschmann C, Belhage B, Jensen K. Ultrasound-guided bilateral dual transversus abdominis plane block: a new four-point approach. Acta Anaesthesiol Scand. 2011;55(6):658–663. doi:10.1111/j.1399-6576.2011.02430.x
9. Ollier E, Heritier F, Bonnet C, et al. Population pharmacokinetic model of free and total ropivacaine after transversus abdominis plane nerve block in patients undergoing liver resection. Br J Clin Pharmacol. 2015;80(1):67–74. doi:10.1111/bcp.12582
10. Knudsen K, Beckman Suurkula M, Blomberg S, Sjovall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997;78(5):507–514. doi:10.1093/bja/78.5.507
11. Griffiths JD, Barron FA, Grant S, Bjorksten AR, Hebbard P, Royse CF. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;105(6):853–856. doi:10.1093/bja/aeq255
12. Griffiths JD, Le NV, Grant S, Bjorksten A, Hebbard P, Royse C. Symptomatic local anaesthetic toxicity and plasma ropivacaine concentrations after transversus abdominis plane block for caesarean section. Br J Anaesth. 2013;110(6):996–1000. doi:10.1093/bja/aet015
13. Kehlet H. Surgical stress: the role of pain and analgesia. Br J Anaesth. 1989;63(2):189–195. doi:10.1093/bja/63.2.189
14. El-Dawlatly AA, Turkistani A, Kettner SC, et al. Ultrasound-guided transversus abdominis plane block: description of a new technique and comparison with conventional systemic analgesia during laparoscopic cholecystectomy. Br J Anaesth. 2009;102(6):763–767. doi:10.1093/bja/aep067
15. Maeda A, Shibata SC, Wada H, et al. The efficacy of continuous subcostal transversus abdominis plane block for analgesia after living liver donation: a retrospective study. J Anesth. 2016;30(1):39–46. doi:10.1007/s00540-015-2085-x
16. Bharti N, Kumar P, Bala I, Gupta V. The efficacy of a novel approach to transversus abdominis plane block for postoperative analgesia after colorectal surgery. Anesth Analg. 2011;112(6):1504–1508. doi:10.1213/ANE.0b013e3182159bf8
17. McDonnell JG, Curley G, Carney J, et al. The analgesic efficacy of transversus abdominis plane block after cesarean delivery: a randomized controlled trial. Anesth Analg. 2008;106(1):186–191, table of contents. doi:10.1213/01.ane.0000290294.64090.f3
18. Erdogan MA, Ozgul U, Ucar M, et al. Effect of transversus abdominis plane block in combination with general anesthesia on perioperative opioid consumption, hemodynamics, and recovery in living liver donors: the prospective, double-blinded, randomized study. Clin Transplant. 2017;31(4). doi:10.1111/ctr.12931
19. Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102(6):1884–1898. doi:10.1213/01.ANE.0000219597.16143.4D
20. Conaghan P, Maxwell-Armstrong C, Bedforth N, et al. Efficacy of transversus abdominis plane blocks in laparoscopic colorectal resections. Surg Endosc. 2010;24(10):2480–2484. doi:10.1007/s00464-010-0989-y
21. Favuzza J, Brady K, Delaney CP. Transversus abdominis plane blocks and enhanced recovery pathways: making the 23-h hospital stay a realistic goal after laparoscopic colorectal surgery. Surg Endosc. 2013;27(7):2481–2486. doi:10.1007/s00464-012-2761-y
22. Rettig HC, Lerou JG, Gielen MJ, Boersma E, Burm AG. The pharmacokinetics of ropivacaine after four different techniques of brachial plexus blockade. Anaesthesia. 2007;62(10):1008–1014. doi:10.1111/j.1365-2044.2007.05197.x
23. Niraj G, Searle A, Mathews M, et al. Analgesic efficacy of ultrasound-guided transversus abdominis plane block in patients undergoing open appendicectomy. Br J Anaesth. 2009;103(4):601–605. doi:10.1093/bja/aep175
24. Huang Z, Ung T. Effect of alpha-1-acid glycoprotein binding on pharmacokinetics and pharmacodynamics. Curr Drug Metab. 2013;14(2):226–238.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

