Back to Journals » Infection and Drug Resistance » Volume 11
The rs1625579 T>G polymorphism in the miRNA-137 gene confers a risk of early-onset Kawasaki disease in a southern Chinese population
Authors Che D, Li J, Fu L, Pi L, Rong X, Wang Y , Xu Y , Huang P, Chu M, Gu X
Received 14 May 2018
Accepted for publication 13 June 2018
Published 3 August 2018 Volume 2018:11 Pages 1055—1060
DOI https://doi.org/10.2147/IDR.S174140
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Di Che,1,* Jiawen Li,2,* Lanyan Fu,1,* Lei Pi,1 Xing Rong,2 Yanfei Wang,3 Yufen Xu,1 Ping Huang,3 Maoping Chu,2 Xiaoqiong Gu1,4
1Department of Clinical Biological Resource Bank, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China; 2Children’s Heart Center, the Second Affiliated Hospital and Yuying Children’s Hospital, Institute of Cardiovascular Development and Translational Medicine, Wenzhou Medical University, Wenzhou, China; 3Department of Cardiology, Guangzhou Women and Children’s Hospital, Guangzhou Medical University, Guangzhou, China; 4Department of Clinical Lab, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
*These authors contributed equally to this work
Background: Kawasaki disease (KD) mainly manifests as excessive inflammation and vascular endothelial cell injury. This disease generally occurs in children younger than 5 years of age and is more severe in children younger than 12 months. KD affects males and females at a ratio of 1.5:1. Polymorphisms of the rs1625579 locus in the miR-137 gene are associated with schizophrenia susceptibility, and high glucose-induced upregulation of miR-137 in vascular endothelial cells promotes monocyte chemotaxis and inflammatory cytokine secretion in gestational diabetes mellitus. However, researchers have not reported whether rs1625579 is associated with KD susceptibility or onset. Therefore, we investigated the relationship between the miRNA-137 rs1625579 T>G polymorphism and KD susceptibility.
Methods: TaqMan real-time polymerase chain reaction was applied to determine the genotypes of 532 patients with KD (365 males and 167 females) and 623 control subjects (402 males and 221 females).
Results: Comparison of all cases with all controls revealed that the rs1625579 T>G polymorphism was not associated with KD susceptibility. However, a subgroup analysis revealed that subjects with the rs1625579 TG/GG genotypes exhibited a significantly higher onset risk for KD before 12 months of age than carriers of the TT genotype (adjusted age and gender odds ratio=1.99, 95% CI=1.04–3.83; P=0.039).
Conclusion: Our results indicate that the rs1625579 T>G polymorphism confers a risk of early-onset KD in southern Chinese children.
Keywords: Kawasaki disease, miRNA-137, rs1625579, susceptibility, early onset
Introduction
Kawasaki disease (KD) was first reported by Dr Kawasaki in 1967.1 The peak incidence of the onset of KD is from 9 to 11 months of age, and excessive inflammation and vascular endothelial cell (VEC) injury are its main pathological features.2,3Over a 10-year period, we observed a significant increase in the proportion of coronary artery stenosis in patients who were <12 months of age at KD onset compared with patients who were older than 1 year.4 Coronary artery embolism is a severe complication of KD caused by systemic vasculitis and is particularly common in infants and children aged <60 months.5,6 Teenagers and adults also suffer from KD,7 which affects males and females at a ratio of 1.5:1.5,6 Moreover, Leonardi et al reported a case of severe KD in a 3-month-old patient.8
The etiopathogenesis of KD is unknown, but the immune response, microbial infections, and genetic factors are thought to contribute to its development. A recent genome-wide association study identified some remarkable candidate genes associated with KD.9,10 KD is a type of autoimmune disease that causes immune-related multisystem vasculitis. Some polymorphisms in inflammation-related genes, such as BLK, CD40, HLA, COPB2 and FCGR2A, have been confirmed to be associated with KD susceptibility in the Han Chinese population.11–15 Polymorphisms in the ITPKC and SLC11A1 genes caused age-dependent elevations in the white blood cell count, platelet count, LDH level, and erythrocyte sedimentation rate in Korean patients with KD, particularly those aged <24 months.16 These studies provide insights into the etiology of KD, and thus, many interesting immune-related genes should be studied to assess their relationships with susceptibility to KD.
Non-coding miRNAs, which are ~20 nucleotides in length, are involved in the regulation of gene expression and affect protein-coding genes that participate in various biological processes, including the immune response.17 miRNAs are associated with many diseases, including diabetes mellitus, congenital heart disease, coronary artery disease, Parkinson’s disease, and inflammatory bowel diseases.18–22 KD is one of the most common causes of acute febrile systemic vasculitis,3 and recent studies have reported that overexpression of miRNA-137 inhibits upregulation of the inflammatory cytokines IL-6, VCAM-1 and ICAM-1, and human umbilical vein endothelial cell angiogenesis in vitro.23 Next-generation sequencing has identified >20 miRNAs that distinguish patients with fever from patients with KD; 10 of these miRNAs were selected for further analysis by quantitative polymerase chain reaction,24 but the authors did not mention a relationship between miRNA-137 and KD. Using frozen serum samples, Jia et al identified a set of four serum exosomal miRNAs that distinguished patients with KD from other patients with fever and healthy individuals,25 but this set of miRNAs did not include miRNA-137. The miRNA-137 variant rs1625579 was identified as the strongest predictor of the risk of schizophrenia in a genome-wide association study.26 Askari et al found that a 31-year-old man with schizophrenia also had microscopic polyangiitis, which is a type of small vessel vasculitis.27 As shown in some case reports, cerebral hemorrhage, blood vessel expansion, and other phenomena occur in the brains of patients with KD, which suggests that these patients suffer from cerebral vascular inflammation.28,29 Upregulated miR-137 expression may play a crucial role in high glucose-induced VEC dysfunction by promoting monocyte chemotaxis and adhesion to VECs in gestational diabetes mellitus.23 Because miRNA-137 is involved in VEC injury and inflammation and the rs1625579 T>G polymorphism is related to schizophrenia, which may be accompanied by vasculitis, KD may have some relationship with miRNA-137 polymorphisms. However, no study has investigated the association of the miRNA-137 rs1625579 T>G polymorphism with KD susceptibility. In the present case–control study, we investigated the association between this polymorphism and KD susceptibility in a southern Chinese population comprising 532 children with KD and 623 healthy controls.
Materials and methods
Ethics statement
The study was approved by the Medical Ethics Committee of Guangzhou Women and Children’s Medical Center (2014073009) and was conducted according to the International Ethical Guidelines for Research Involving Human Subjects stated in the Declaration of Helsinki. Informed written consent was obtained from the guardians of the patients and controls.
Study population
Most of the participants reside in southern China. A total of 532 patients who had been recently diagnosed with KD, and 623 healthy controls were recruited from January 2012 to January 2017. KD was diagnosed according to the American Heart Association guidelines.3 Each participant provided 2 mL of fresh blood. Total genomic DNA extracted from 200 µL of each specimen yielded a sufficient amount for the genomic DNA analysis. The remaining specimens were stored in the clinical biological sample bank at our hospital for further study.
DNA extraction and genotyping
Genomic DNA was extracted from 200 µL of blood collected from each participant using a TIANamp Blood DNA Kit (centrifugal column; Tiangen, Beijing city, China) according to the manufacturer’s specifications. Each 384-well plate contained positive and negative samples, which were used for comparisons. TaqMan real-time polymerase chain reaction was performed with an ABI Q6 instrument (Thermo Fisher Scientific) to genotype miRNA-137 rs1625579 polymorphisms.
Statistical analysis
The genotype distributions of the control group, which were expected to be in Hardy–Weinberg equilibrium, were confirmed using a goodness-of-fit chi-squared test, and the differences in variables and genotype frequency distributions between the patients and controls were tested using a two-sided chi-squared test. The relationship between the miRNA-137 rs1625579 T>G polymorphism and KD susceptibility was described by calculating odds ratios and 95% CIs through a univariate logistic regression analysis. Multivariate analyses were performed after adjusting for gender and age. The relationships between KD susceptibility and genotypes were analyzed in different subgroups based on age, gender, and the presence of coronary artery lesions (CALs) or coronary artery aneurysms (CAAs). According to the Japanese Kawasaki Disease Research Committee and the coronary z score, CALs were defined as lesions with a luminal diameter ≥3.0 mm in a child <5 years of age or ≥4.0 mm in a child ≥5 years of age, a segment with an internal diameter ≥1.5 times larger than an adjacent segment or a clearly irregular luminal contour. According to the internal diameters of the coronary vessels, patients with CALs were divided into groups with dilatations or a small CAA (<5.0 mm), middle CAA (5.0–8.0 mm) and large CAA (>8.0 mm).3 The SAS software (version 9.4; SAS Institute, Cary, NC, USA) was used to rapidly perform all statistical analyses.
Ethical approval and consent to participate
This study was performed with the approval of the Institutional Committee of Guangzhou Women and Children’s Medical Center (2014073009). All participants provided written informed consent.
Results
Population characteristics
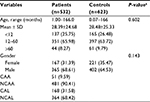
A total of 532 KD cases and 623 healthy controls comprised the population analyzed in our study. The demographics of all participants are shown in Table 1. The average age of KD onset was 28.39 months. The KD group comprised 365 (68.61%) male patients and 167 (31.39%) female patients. No differences in age (P=0.602) or gender (P=0.143) were observed between the patients with KD and healthy controls. An analysis of the degree of coronary artery damage in the KD cases revealed 51 (9.59%) patients with CAAs and 481 (90.41%) patients without CAAs (NCAAs), as well as 168 (31.58%) patients with CALs and 364 (68.42%) patients without CALs (NCALs).
Relationship between the miRNA-137 rs1625579 T>G polymorphism and KD susceptibility
To explore the association between miRNA-137 rs1625579 T>G polymorphism and KD susceptibility, we detected the genotype frequency distributions of KD cases and controls. As shown in Table 2, the controls satisfied the conditions for Hardy–Weinberg equilibrium (P=0.791). The genotype frequency distributions of the miRNA-137 rs1625579 polymorphisms were 85.58% (TT), 13.47% (TG), and 0.95% (GG) in the KD group and 87.94% (TT), 11.74% (TG), and 0.32% (GG) in the controls. No significant relationship was observed between the rs1625579 T>G polymorphism and KD susceptibility.
Stratification analysis
KD is a disease that related to age and gender, and CAL is the most common complication of KD. After stratifying the subjects by age, gender, and the degree of damage of the coronary artery, we explored the relationship between the rs1625579 T>G polymorphism and KD susceptibility. As illustrated in Table 3, when the patients were stratified by age after adjusting for gender, the TG/GG genotypes of the rs1625579 T>G polymorphism contributed to a higher occurrence of KD compared with carriers of the TT genotype in patients aged <12 months (adjusted odds ratio=1.99, 95% CI=1.04–3.83; P=0.039). We also studied other subgroups stratified by gender (adjusted for age), the degree of damage of the coronary artery by CALs and NCALs or CAAs and NCAAs (adjusted for gender and age), but did not detect other notable relationships.
Discussion
The association between KD susceptibility and rs1625579 T>G polymorphism was analyzed in our case–control investigation. No significant association between the rs1625579 T>G polymorphism and KD susceptibility was noted in the patients (Table 2). The subgroup analysis indicated that the miRNA-137 rs1625579 TG/GG genotypes increased the risk of KD in patients aged l<12 months (Table 3). KD affects children aged between 6 months and 5 years. However, in our subgroup analysis, we did not conclude that the occurrence of CALs or CAAs in patients with KD aged <1 year was related to the miRNA-137 rs1625579 T>G polymorphism. We intend to collect more samples from patients with KD aged <1 year and presenting with coronary complications to confirm this discovery.
This study is the first to examine miRNA-137 rs1625579 polymorphisms in patients with KD. Recent research showed that overexpression of miRNA-137 significantly inhibited tumor necrosis factor alpha-induced protein 1 (TNFAIP1) production both in vivo and in vitro,30 and miRNA-137 suppressed vascular smooth muscle cell proliferation and migration.31 Overexpression of miR-137 inhibited upregulation of the inflammatory cytokines IL-6, VCAM-1 and ICAM-1, and human umbilical vein endothelial cell angiogenesis in vitro.23 Central nervous system vasculitis has been reported to cause psychosis.27 Chen et al noted remarkable relationships between autoimmune diseases, such as psoriasis, Graves’ disease and pernicious anemia, and schizophrenia.32 KD is a type of autoimmune disease that causes immune-related multisystem vasculitis. Based on our results, doctors should be more aware of vasculitis in the brains of patients with KD, particularly in patients aged <1 year. The majority of brain growth occurs from birth to 2 years of age, and the brain is easily injured during this period.33 The hypothalamus is the area most likely to be affected. A meta-analysis by Zhang et al revealed that the rs1625579 single-nucleotide polymorphism in the miR-137 gene might be involved in schizophrenia susceptibility in a Chinese Han population,34 but another meta-analysis by Pu and Xiao did not identify a significant association between rs1625579 and schizophrenia in an Asian population.35 The finding that rs1625579 in miRNA-137 is associated with schizophrenia in southern Chinese patients has been verified.36 Schizophrenia is associated with decreases in the volumes of many brain structures, including the hypothalamus.37 Thus, rs1625579 polymorphisms are associated with the development of the hypothalamus, particularly in infants, which may explain why children in that age group are prone to vasculitis, especially if they are diagnosed with KD.
To the best of our knowledge, KD is an age- and gender-related disease that generally occurs in children aged <5 years and is more severe in children aged <12 months.3,38 However, why KD occurs in children younger than 5 years old is unclear. Wang et al reported that miRNA-137 appeared to influence age at onset in patients with schizophrenia, but might not be related to susceptibility.39 Similarly, based on the results from the present study, the rs1625579 T>G polymorphism in miRNA-137 is not associated with KD susceptibility in patients with KD. However, further subgroup analyses revealed a significantly earlier onset of KD in subjects with the rs1625579 TG/GG genotypes in whom disease onset occurred before 12 months of age than in patients with the TT genotype. Thus, our study suggests that the rs1625579 T>G polymorphism confers a risk of early-onset KD in southern Chinese children. This factor may be one reason why onset of KD is early in children aged <12 months. The limitations of this study are related to the insufficient number of patients with coronary aneurysms, and further studies with a larger sample size are needed to confirm the results.
Acknowledgments
We thank Jin-xin Wang and Lan-yan Fu for assisting with the DNA extraction and genotyping and An-qi Zhang for providing technical guidance. We thank the Clinical Biological Resource Bank of Guangzhou Women and Children’s Medical Center for providing all of the clinical samples. This study was supported by grants from the Guangdong Natural Science Fund, China (grant number 2016A030313836), the Guangdong Science and Technology Project, China (grant numbers 2014A020212012, 2014A020212613, and 2014A020212023), the Guangzhou Science and Technology Program Project, China (grant numbers 201510010159, 201607010011, 201707010270, and 201804010035), the Guangzhou Medical and Health Technology Projects, China (grant numbers 20161A010030 and 20171A011260), and the National Key Basic Research and Development Program (973 Program), China (grant number 2015CB755402).
Disclosure
The authors report no conflicts of interest in this work.
References
Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16(3):178–222. | ||
Yanagawa H, Nakamura Y, Yashiro M, Uehara R, Oki I, Kayaba K. Incidence of Kawasaki disease in Japan: the nationwide surveys of 1999–2002. Pediatr Int. 2006;48(4):356–361. | ||
Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747–2771. | ||
Zhang L, Yu M, Xie X, et al. Follow up and review of patients with Kawasaki disease complicated with giant coronary artery aneurysms for a decade: a single-institution experience. Zhonghua Er Ke Za Zhi. 2015;53(1):40–44. | ||
Tacke CE, Breunis WB, Pereira RR, Breur JM, Kuipers IM, Kuijpers TW. Five years of Kawasaki disease in the Netherlands: a national surveillance study. Pediatr Infect Dis J. 2014;33(8):793–797. | ||
Cimaz R, Fanti E, Mauro A, Voller F, Rusconi F. Epidemiology of Kawasaki disease in Italy: surveillance from national hospitalization records. Eur J Pediatr. 2017;176(8):1061–1065. | ||
Fraison JB, Sève P, Dauphin C, et al. Kawasaki disease in adults: observations in France and literature review. Autoimmun Rev. 2016;15(3):242–249. | ||
Leonardi S, Barone P, Gravina G, et al. Severe Kawasaki disease in a 3-month-old patient: a case report. BMC Res Notes. 2013;6:500. | ||
Kim KY, Kim DS. Recent advances in Kawasaki disease. Yonsei Med J. 2016;57(1):15–21. | ||
Chang LS, Hsu YW, Lu CC, et al. CYP2E1 gene polymorphisms related to the formation of coronary artery lesions in Kawasaki disease. Pediatr Infect Dis J. 2017;36(11):1039–1043. | ||
Tsai FJ, Lee YC, Chang JS, et al. Identification of novel susceptibility loci for Kawasaki disease in a Han Chinese population by a genome-wide association study. PLoS One. 2011;6(2):e16853. | ||
Yan Y, Ma Y, Liu Y, et al. Combined analysis of genome-wide-linked susceptibility loci to Kawasaki disease in Han Chinese. Hum Genet. 2013;132(6):669–680. | ||
Duan J, Lou J, Zhang Q, et al. A genetic variant rs1801274 in FCGR2A as a potential risk marker for Kawasaki disease: a case–control study and meta-analysis. PLoS One. 2014;9(8):e103329. | ||
Lou J, Zhong R, Shen N, et al. Systematic confirmation study of GWAS-identified genetic variants for Kawasaki disease in a Chinese population. Sci Rep. 2015;5:8194. | ||
Wang W, Lou J, Lu XZ, et al. 8p22-23-rs2254546 as a susceptibility locus for Kawasaki disease: a case–control study and a meta-analysis. Sci Rep. 2014;4:4247. | ||
Kim KY, Bae YS, Ji W, Shin D, Kim HS, Kim DS. ITPKC and SLC11A1 gene polymorphisms and gene–gene interactions in Korean patients with Kawasaki disease. Yonsei Med J. 2018;59(1):119–127. | ||
Pritchard CC, Cheng HH, Tewari M. microRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13(5):358–369. | ||
Hashimoto N, Tanaka T. Role of miRNAs in the pathogenesis and susceptibility of diabetes mellitus. J Hum Genet. 2017;62(2):141–150. | ||
Leggio L, Vivarelli S, L’Episcopo F, et al. microRNAs in Parkinson’s Disease: from pathogenesis to novel diagnostic and therapeutic approaches. Int J Mol Sci. 2017;18(12):2698. | ||
Borghini A, Andreassi MG. Genetic polymorphisms offer insight into the causal role of microRNA in coronary artery disease. Atherosclerosis. 2018;269:63–70. | ||
Gao X, Yang L, Luo H, Tan F, Ma X, Lu C. A rare Rs139365823 polymorphism in pre-miR-138 is associated with risk of congenital heart disease in a Chinese population. DNA Cell Biol. 2018;37(2):109–116. | ||
Soroosh A, Koutsioumpa M, Pothoulakis C, Iliopoulos D. Functional role and therapeutic targeting of microRNAs in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2018;314(2): G256–G262. | ||
Peng HY, Li HP, Li MQ, Hp L, Mq L. High glucose induces dysfunction of human umbilical vein endothelial cells by upregulating miR-137 in gestational diabetes mellitus. Microvasc Res. 2018;118:90–100. | ||
Kuo HC, Hsieh KS, Ming-Huey Guo M, et al. Next-generation sequencing identifies micro-RNA-based biomarker panel for Kawasaki disease. J Allergy Clin Immunol. 2016;138(4):1227–1230. | ||
Jia HL, Liu CW, Zhang L, et al. Sets of serum exosomal microRNAs as candidate diagnostic biomarkers for Kawasaki disease. Sci Rep. 2017;7:44706. | ||
Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–976. | ||
Askari A, Saadeh A, Buheis NI. Microscopic polyangiitis presenting as schizophrenia. Rheumatol Int. 1999;18(5–6):215–217. | ||
Gitiaux C, Kossorotoff M, Bergounioux J, et al. Cerebral vasculitis in severe Kawasaki disease: early detection by magnetic resonance imaging and good outcome after intensive treatment. Dev Med Child Neurol. 2012;54(12):1160–1163. | ||
Shprakh VV, Surikova ZV, Bregel LV, Subbotin VM. A case of the cerebral vasculitis at the late stage of Kawasaki disease. Zh Nevrol Psikhiatr Im S S Korsakova. 2014;114(11):107–110. | ||
Zhao Y, Li S, Xia N, Shi Y, Zhao CM. Effects of XIST/miR-137 axis on neuropathic pain by targeting TNFAIP1 in a rat model. J Cell Physiol. 2018;233(5):4307–4316. | ||
Pan J, Li K, Huang W, Zhang X. miR-137 inhibited cell proliferation and migration of vascular smooth muscle cells via targeting IGFBP-5 and modulating the mTOR/STAT3 signaling. PLoS One. 2017;12(10):e0186245. | ||
Chen SJ, Chao YL, Chen CY, et al. Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. Br J Psychiatry. 2012;200(5):374–380. | ||
Knickmeyer RC, Gouttard S, Kang C, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28(47):12176–12182. | ||
Zhang P, Bian Y, Liu N, et al. The SNP rs1625579 in miR-137 gene and risk of schizophrenia in Chinese population: a meta-analysis. Compr Psychiatry. 2016;67:26–32. | ||
Pu X, Xiao X. No evidence of an association between MIR137 rs1625579 and schizophrenia in Asians: a meta-analysis in 30843 individuals. Psychiatr Genet. 2016;26(5):203–210. | ||
Ma G, Yin J, Fu J, et al. Association of a miRNA-137 polymorphism with schizophrenia in a Southern Chinese Han population. Biomed Res Int. 2014;2014:751267. | ||
Haijma SV, van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129–1138. | ||
Yanagawa H, Nakamura Y, Yashiro M, et al. Incidence survey of Kawasaki disease in 1997 and 1998 in Japan. Pediatrics. 2001;107(3):E33. | ||
Wang S, Li W, Zhang H, et al. Association of microRNA137 gene polymorphisms with age at onset and positive symptoms of schizophrenia in a Han Chinese population. Int J Psychiatry Med. 2014;47(2):153–168. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.