Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
The Role of the Clinical Pharmacist on the Health Outcomes of Acute Exacerbations of Chronic Obstructive Pulmonary Disease (AECOPD)
Authors Gong Y, Chen Q, Zhang Y
Received 12 April 2022
Accepted for publication 2 August 2022
Published 15 August 2022 Volume 2022:17 Pages 1863—1870
DOI https://doi.org/10.2147/COPD.S370532
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Yanqing Gong,1,2,* Qiying Chen,1,* Yin Zhang1
1Department of Pharmacy, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, People’s Republic of China; 2Department of Pharmacy, Gaoxin Branch of the First Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yin Zhang, Department of Pharmacy, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, People’s Republic of China, Email [email protected]
Background: Clinical pharmacists play a significant role in clinical practice, but their work in the clinical pathway (CP) of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) remains undefined.
Methods: This prospective study included patients who met the discharge criteria during hospitalization at the department of respiratory medicine of the Second Affiliated Hospital of Fujian Medical University from March to December 2017 (no pharmacists involved) and from March 2018 to January 2019 (pharmacists involved). The adverse drug reaction (ADR) reporting rate, the average DDD number of antibacterial drugs, the per capita cost of pharmaceutical services, and the benefit-cost ratio (B/C) were analyzed.
Results and Discussion: Eighty participants were enrolled during the traditional period and eighty-five participants during the clinical pharmacist period. The average hospital stays (9.2± 0.4 vs 10.7± 0.6 days, P=0.032), the total cost of hospitalization expenses (¥ 14,058± 826 vs ¥ 18,765± 1434, P=0.004), the total cost of drugs (¥ 5717± 449 vs ¥ 8002± 755, P=0.004), and cost of antimicrobial drugs (¥ 3639± 379 vs ¥ 5636± 641, P=0.007) were all lower in the clinical pharmacist group than in the traditional group. The B/C was 10.38 and 5.05 in the total cost of hospitalization expenses and the total cost of drugs, respectively. The clinical pharmacists’ participation was independently associated with the total cost of hospitalization expenses (β=− 0.201, 95% confidence interval: − 0.390, − 0.055, P=0.010).
What is New and Conclusion: The participation of the clinical pharmacist in implementing an AECOPD CP significantly reduces patients’ hospitalization days, the total cost of hospitalization expenses, and antibiotic use and improves the B/C of AECOPD management. The clinical pharmacists’ participation was independently associated with the total hospitalization expenses.
Keywords: clinical pharmacist, acute exacerbation of chronic obstructive pulmonary disease, clinical pathway, pharmaceutical services, cost-benefit analysis
What is Known and Objective
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease characterized by persistent respiratory symptoms and airflow restriction.1 COPD prevalence among Chinese adults >20 years of age is 8.6%, and 13.7% among adults >40 years.2 Acute exacerbation of COPD (AECOPD) is characterized by respiratory symptoms worsening sharply beyond normal daily variations to the extent of requiring a change in therapy.1 It is often accompanied by a continuous decline of lung function, an important event during COPD and an important determining factor of mortality in such patients.3 In-hospital mortality for patients with COPD exacerbations is around 2.5% in general and 10% with hypercarbia;4 all-cause mortality within 3 years of hospitalization may be as high as 49%.1 COPD is the fourth leading cause of death in the world. In 2015, about 3.2 million people died of COPD, which is expected to become the third leading cause of death by 2030.5 COPD is the third leading cause of death in China, with more than 900,000 deaths in 2013.6
COPD has a huge economic burden worldwide.7,8 For example, in the Asia-Pacific region, the annual economic burden per capita is as high as $ 4500 and about $ 3072 in China.9,10 In 2019, a study on the economic burden of COPD in America, Europe, and Asia showed that the per capita cost of hospitalization in the United States, Italy, and Singapore was $ 6852, $ 8203, and $ 2335, respectively.11
A clinical pathway (CP) is a continuous improvement process based on the PDCA cycle (Deming Cycle). In the 1980s, to curb rising medical costs and increase the reasonable utilization of medical resources, and limit hospital compensation, the United States of America took the lead in implementing CP, which successfully curbed the rising clinical costs. As a standardized model for treating various diseases, CP has been widely used in many countries.12–15 The pilot work of CP in China started in 2009. Pilot CPs at the end of 2011 involved 23 hospitals in the country, a total of 110 hospitals, including 22 specialties and 331 diseases, and the national clinical path completion rate reached 89.4%.16 CP management has achieved good results in hospital management systems, including medical, nursing, and pharmaceutical management,16–19 including AECOPD.20–22 Still, there are problems such as a lack of correct understanding of CPs, small scope of popularization, inaccurate and imperfect evaluation standards of the CP, and lack of guidance in the knowledge management system.23,24 In addition, the functions and roles of pharmacists in the CPs of AECOPD are not widely recognized. In a CP study, pharmacists were responsible for teaching drug administration,20 but pharmacists could play other roles in managing COPD. Hudd reported in 202025 on the increasing role of pharmacists in managing COPD.
Therefore, this study aimed to define the participation of pharmacists in the CP of AECOPD. There are currently many clinical pharmacists in respiratory specialties in China, but there are few reports on how to intervene in the treatment of AECOPD. The results might show that the participation of clinical pharmacists in implementing the AECOPD clinical pathway can significantly improve patient management.
Methods
Study Design and Participants
This prospective study was approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University and in accordance with the principles of the Declaration of Helsinki, written informed consent was obtained from the participants. The study included patients who met the discharge criteria during hospitalization at the department of respiratory medicine of the Second Affiliated Hospital of Fujian Medical University from March to December 2017 and from March 2018 to January 2019. The inclusion criteria were 1) met the diagnostic criteria of COPD from the “GOLD Global Initiative for Chronic Obstructive Pulmonary Disease: Global Strategy for Diagnosis, Treatment, and Prevention of COPD (2019 Version)1”, 2) met the diagnostic criteria for AECOPD,1 with at least two main symptoms (dyspnea aggravating, purulent sputum increasing, and sputum volume increasing), or one main symptom combined with one secondary symptom (runny nose, stuffy nose, wheezing, sore throat, and cough) lasting for at least two days, which can be accompanied by an obvious aggravation of inflammation and fever, and 3) clear awareness and basic language communication skills. The exclusion criteria were 1) needed tracheal intubation or mechanical ventilation; 2) tumor or other serious life-threatening disease complications, 3) unstable coronary heart disease, uncontrolled drug-induced congestive heart failure, severe uncontrolled hypertension, recent myocardial infarction, or severe pulmonary hypertension, or 4) history of mental illness, cognitive dysfunction, or communication impairment.
The participants from 2017 were included in the traditional group. The patients from 2018–2019 were included in the clinical pharmacist group since clinical pharmacists were involved in their management.
Treatments
All participants received standardized treatments.3 No clinical pharmacists were involved in the traditional group. Relevant information from the participants was recorded directly from the electronic medical records. Patients in the traditional group were treated with the standard clinical pathway of the AECOPD in our hospital, which mainly included the first diagnosis of chronic obstructive pulmonary disease with acute exacerbation (ICD-10: J44.100), chronic obstructive emphysema bronchitis with acute exacerbation (ICD-10: J44.100 X001), the treatment plan is mainly implemented according to《 Clinical Diagnosis and Treatment Guide-Respiratory Volume》 (Compiled by Chinese Medical Association, People’s Medical Publishing House) and 《 COPD Diagnosis and Treatment Guide (2018 Revised Edition)》 (Chronic obstructive pulmonary Disease Group, Respiratory Branch of Chinese Medical Association).Clinical pharmacists participated in managing participants in the clinical pharmacist group, carried out various clinical pharmacy work, and adopted the PDCA cycle method. Patients in the clinical pharmacist group, we added clinical Pharmacists’ Paths (Appendix 1) on the basis of the standard clinical paths.
The clinical pharmacists in the clinical pharmacist group participated as follows. Clinical pharmacists (three anti-infective pharmacists, bachelor’s degree or above, pharmacist-in-charge) participated in drug treatment and pharmacy rounds. They conducted medical order reviews (such as drug selection, indications, route of administration, frequency and dose of medication, medication course, combination medication, compatibility, etc.) and made recommendations for individual administration. In addition, medication education was provided, including basic knowledge about COPD, correct use of inhalators, adverse reactions and precautions of drugs, countermeasures for adverse reactions, and health guidance after discharge.
Indicators
The outcomes were total cost of hospitalization expenses, cost of drugs, adverse drug reaction (ADR), the average DDD number of antibacterial drugs, combined use of antimicrobial drugs, and benefit-cost ratio (B/C).
The ADR reporting rate was defined as the total number of reports (including the number of adverse reactions reported by doctors, the number of adverse reactions reported by clinical pharmacists to remind physicians, and the number of adverse reactions reported by clinical pharmacists) divided by the total number of each group.
The DDD value of each medicine was the limited daily dose of each medicine, referring to the “Dictionary of Medicines and DDD Value of Antimicrobial Clinical Application Monitoring Network” of the Chinese Ministry of Health.
The average DDD number of antibacterial drugs was the sum of the DDD numbers of all antibacterial drugs used during the hospitalization of each group of participants divided by the total number of participants in each group. The number of DDD was the cumulative value of the total amount of antibacterial drugs (g) used by each participant divided by the DDD value of the corresponding antibacterial drugs.
The combined use of antimicrobial drugs was defined as the simultaneous use of two or more antibiotics by a patient on any day during hospitalization. The corresponding combined use rate of antibiotics in each group was obtained by dividing the number of cases in this situation by the total number of cases and multiplying by 100%.
Cost Calculation
The traditional group cost calculation includes the total hospitalization expenses = treatment + nursing expenses + examination fee + bed fee + the total cost of drugs. And the clinical pharmacist group cost calculation includes the total hospitalization expenses = treatment + nursing expenses + examination fee + bed fee + the total cost of drugs + the cost of pharmaceutical services.
The cost in this study mainly referred to the cost of implementing pharmaceutical services. Per capita cost of pharmaceutical services in the intervention group = total time of clinical pharmacist intervention during the intervention × hourly salary of clinical pharmacist ÷ number of participants receiving the intervention. Since the traditional group did not receive pharmaceutical services, the cost was zero. The lowered per capita the total hospitalization expenses and the total cost of drugs were used as the efficiency index. The B/C referred to the ratio of benefit to cost during the intervention period. If B/C was >1, this CP was economical. In this study, cost mainly refers to the cost of implementing pharmaceutical services. The benefit indicators were the reduced the total of hospitalization expenses and the total cost of drugs.
Sample Size
The sample size required for the comparison of two samples was calculated by the following formula: 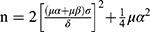 ,26 where n is the sample size, σ is the population standard deviation, δ=μ1- μ2 is the difference between the two population means, and α=0.05. Because the intervention was expected to be effective, one-sided u0.05=1.64, β=0.10, and u0.10=1.28 were used. Among them, σ and δ are obtained through pre-experiment. The pre-experiment used the drug cost as the primary data. The calculated value of σ was 3754, and the value of δ was 2093. Therefore, the required sample size was 56 cases for each group.
,26 where n is the sample size, σ is the population standard deviation, δ=μ1- μ2 is the difference between the two population means, and α=0.05. Because the intervention was expected to be effective, one-sided u0.05=1.64, β=0.10, and u0.10=1.28 were used. Among them, σ and δ are obtained through pre-experiment. The pre-experiment used the drug cost as the primary data. The calculated value of σ was 3754, and the value of δ was 2093. Therefore, the required sample size was 56 cases for each group.
Statistical Analysis
All data were analyzed using SPSS 23.0 (IBM, Armonk, NY, USA). The continuous data were presented as the maximum and minimum values and means ± standard deviation and were analyzed using Student’s t-test. The categorical data were presented as n (%) and analyzed using the chi-square or Fisher’s exact test. Univariable analysis was used to analyze the risk factors for the total cost of hospitalization expenses. P-values <0.05 were defined as statistically significant.
Results
Characteristics of the Participants
A total of 203 cases were screened, but 38 were excluded. Therefore, 80 participants were enrolled during the traditional period and 85 during the clinical pharmacist period (Figure 1). There were no significant differences in patient characteristics between the clinical pharmacist and traditional groups (all P>0.05) (Table 1).
 |
Table 1 Characteristics of the Participants |
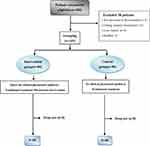 |
Figure 1 Participant flowchart. |
Economic Effect
The average hospital stay was 9.2±0.4 days in the clinical pharmacist group and 10.7±0.6 days in the traditional group (P=0.032). The total cost of hospitalization expenses was ¥ 14,058±826 in the clinical pharmacist group and ¥ 18,765±1434 in the traditional group (P=0.004). The total cost of drugs was ¥ 5717±449 in the clinical pharmacist group and ¥ 8002±755 in the traditional group (P=0.004). Similar results were observed for the antimicrobial drugs (¥ 3639±379 vs 5636±641, P=0.007) (Table 2).
 |
Table 2 Patients’ Hospitalization Days and Medical Expenses Table |
With reference to the clinical pharmacy service standards of the top three hospitals in China,27 the average hourly salary of clinical pharmacists was set at ¥ 43.72. The daily clinical working hours of the clinical pharmacists in the clinical pharmacist group were 4 h, and the intervention lasted 20 days each month. For a total of 880 h, the total time cost was ¥ 38,473.6 (43.72 ¥/h × 880 h), and the average time cost for each participant to receive pharmaceutical services was ¥ 452.6 /participant (¥38,473.6 ÷ 85 participants). The cost of clinical pharmacist services in the traditional group was zero. Compared with the traditional group, the average total hospitalization expenses and average drug cost of the clinical pharmacist group were reduced by ¥ 4698 and ¥ 2284, respectively, and the benefit-cost ratio (B/C) was 10.38 and 5.05, respectively.
The use of antibacterial drugs is shown in Table 3. In the traditional group, 29 participants received antimicrobial drugs, accounting for 36.3% of the total number of patients; in the clinical pharmacist group, 21 participants received antimicrobial drugs, accounting for 24.7% of the total number of patients (P=0.128). The average DDD number of antibacterial drugs was 18.1±1.5 in the traditional group, those values in the clinical pharmacist group were 13.6±1.1.
 |
Table 3 Usage of Antibiotics |
Analysis of Factors Influencing the Total of Hospitalization Expenses
Age, sex, smoking history, comorbidities, and pharmacist participation were used for univariable analysis (the total of hospitalization expenses were log-transformed). The results showed that age, sex, smoking history, and comorbidities were not associated with the total of hospitalization expenses (P>0.05). The influence of pharmacists’ participation on the total of treatment cost was statistically different (P=0.010) (Table 4).
 |
Table 4 Multiple Linear Regression Analysis of Influencing Factors of the Total of Hospitalization Expenses |
Discussion
Clinical pharmacists play a significant role in clinical practice, but their work in the CP of AECOPD remains undefined. Therefore, this study aimed to study the role of clinical pharmacists in the CP of AECOPD. The results suggest that the participation of the clinical pharmacist in implementing an AECOPD CP reduced patients’ hospitalization days, the total of healthcare expenses, and antibiotic use and improved the B/C of AECOPD management.
In this single-center prospective study, the differences in hospitalization days, drug costs, and the average DDD number of antibacterial drugs among the traditional and clinical pharmacist groups were analyzed. The difference in the male-female ratio between the two groups was in line with the survey results of Wang,28 and there were no significant differences in the general characteristics between the two groups (all P>0.05), indicating that the two patient populations were similar despite being from two different periods. Still, the pharmacist intervention decreased the average hospitalization days, total hospitalization costs, total drug costs, and antibacterial drug costs. Based on an estimated hourly salary of ¥ 43.72,29 the benefit-cost ratio of the total hospitalization cost and total drug cost of the clinical pharmacist group was greater than 1, indicating that the participation of clinical pharmacists in the implementation of the clinical pathway has a positive role in reducing medical costs and saving medical resources, and has a good economic potential. These results are supported by previous studies.30–32
Nishimura et al20 reported a valid CP for AECOPD that involves pharmacists for teaching, but no comparison was made with a CP without pharmacists. Gillespie et al33 showed that the involvement of pharmacists in COPD management led to a 16% reduction in hospital visits, 47% reduction in emergency department visits, and 80% reduction in drug-related readmissions. Marin Armero et al34 showed that involving pharmacists in smoking cessation of COPD patients led to 43.5% of patients completely ceasing smoking. Medication reviews by pharmacists led to an 8% reduction in a mean hospital stay or an 11% reduction in patients >80 years of age.35 A pharmacist-led bundle improved outpatient management of COPD and decreased phone calls.36 A study showed that pharmacist-led training improved inhaler techniques and shortened the training time.37 Hence, pharmacists can play multiple important roles in the CP of AECOPD.
In this study, clinical pharmacists developed and followed the clinical pharmacist CP table and implemented pharmaceutical services through full participation. The PDCA cycle management model was used to manage the path of clinical pharmacists, and the role of clinical pharmacists in the treatment of AECOPD patients was studied with a single-center prospective research method. However, due to the limited research time on this subject and the limited number of cases included, the AECOPD clinical pharmacist path form still needs to be further improved. Therefore, future research is expected to expand the content of clinical pharmacist services on more levels by expanding the sample size, promoting clinical pharmacists to serve patients better, and increasing the recognition of medical staff and patients to clinical pharmacists.
This study has limitations. It was a single-center study, limiting the representativeness. In the future, multiple centers should participate. Secondly, due to the limited time of the project, the number of participants that could be enrolled was limited. Thirdly, the traditional group was from a different period. Although the characteristics of the two groups were similar, changes in management between the two periods cannot be excluded, introducing bias. In the future, the traditional and experimental groups should be set to the same period.
What is New and Conclusion
By formulating, optimizing, and following the “Clinical Pharmacist Path Table”, clinical pharmacists can be important members of the AECOPD CP. The participation of the clinical pharmacist in implementing an AECOPD CP significantly reduced patients’ hospitalization days, healthcare expenses, and antibiotic use and improved the B/C of AECOPD management.
Acknowledgments
Thanks to the Second Affiliated Hospital of Fujian Medical University for providing us with a good experimental platform, thanks to all the teachers of clinical pharmacy for their support in my thesis and care in my life, and thanks to all the doctors of the respiratory department for their support in my work and thesis.
Funding
This study was supported by the Fujian Provincial Department of Science and Technology (2020, No. 2020J01213).
Disclosure
Yanqing Gong and Qiying Chen share first authorship. All authors declare that they have no conflict of interest.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2020 Report). Global Initiative for Chronic Obstructive Lung Disease, Inc; 2020.
2. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. PubMed PMID: 29650248. doi:10.1016/S0140-6736(18)30841-9
3. Bai CX, Cai BQ, Feng YL. Chinese Expert Consensus on the diagnosis and treatment of acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Int J Respir. 2017;37:1042–1057.
4. Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163(10):1180–1186. PubMed PMID: 12767954. doi:10.1001/archinte.163.10.1180
5. Abdulsalim S, Unnikrishnan MK, Manu MK, Alrasheedy AA, Godman B, Morisky DE. Structured pharmacist-led intervention programme to improve medication adherence in COPD patients: a randomized traditionalled study. Res Social Adm Pharm. 2018;14(10):909–914. PubMed PMID: 29104008. doi:10.1016/j.sapharm.2017.10.008
6. Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–272. PubMed PMID: 26510778. doi:10.1016/S0140-6736(15)00551-6
7. Dang-Tan T, Ismaila A, Zhang S, Zarotsky V, Bernauer M. Clinical, humanistic, and economic burden of chronic obstructive pulmonary disease (COPD) in Canada: a systematic review. BMC Res Notes. 2015;8:464. PubMed PMID: 26391471; PubMed Central PMCID: PMCPMC4578756. doi:10.1186/s13104-015-1427-y
8. Chen X, Wang N, Chen Y, Xiao T, Fu C, Xu B. Costs of chronic obstructive pulmonary disease in urban areas of China: a cross-sectional study in four cities. Int J Chron Obstruct Pulmon Dis. 2016;11:2625–2632. PubMed PMID: 27799761; PubMed Central PMCID: PMCPMC5079691. doi:10.2147/COPD.S118523
9. Wang Y, Ghoshal AG, Bin Abdul Muttalif AR, et al. Quality of life and economic burden of respiratory disease in Asia-pacific-Asia-pacific burden of respiratory diseases study. Value Health Reg Issues. 2016;9:72–77. PubMed PMID: 27881264. doi:10.1016/j.vhri.2015.11.004
10. Li J, Feng R, Cui Y. Analysis of the economic burden of medical treatment of COPD patients in tertiary hospitals in China. China Health Econ. 2015;34(9):66–68.
11. Anees UR, Ahmad Hassali MA, Muhammad SA, et al. The economic burden of chronic obstructive pulmonary disease (COPD) in the USA, Europe, and Asia: results from a systematic review of the literature. Expert Rev Pharmacoecon Outcomes Res. 2020;20(6):661–672. PubMed PMID: 31596632. doi:10.1080/14737167.2020.1678385
12. Croce D, Lazzarin A, Rizzardini G, et al. HIV clinical pathway: a new approach to combine guidelines and sustainability of anti-retroviral treatment in Italy. PLoS One. 2016;11(12):e0168399. PubMed PMID: 28030621; PubMed Central PMCID: PMCPMC5193418. doi:10.1371/journal.pone.0168399
13. Soobrian J, Tyrrell A, Li C. Using a disease pathway management approach to improve the quality of breast cancer care in Ontario. J Clin Oncol. 2016;34(7_suppl):109. doi:10.1200/jco.2016.34.7_suppl.109
14. Panagiotidis I, Christoulas D, Terpos E. Inhibition of receptor activator of nuclear factor kappa-B ligand pathway for the management of aggressive osteosarcoma. Ann Transl Med. 2016;4(24):510. PubMed PMID: 28149872; PubMed Central PMCID: PMCPMC5233538 interest to declare. doi:10.21037/atm.2016.11.75
15. Killoran J, Balboni TA, Krishnan MS, Krishnan MS, Taylor A, Martin NE. Development and implementation of a clinical pathway for radiation of bone metastases on a palliative radiation oncology service. J Clin Oncol. 2016;34(26_suppl):170. doi:10.1200/jco.2016.34.26_suppl.170
16. Zhang Z. Clinical effect of pharmacists participating in the clinical path of chronic obstructive pulmonary disease. J Clin Rational Drug Use. 2018;11(24):16–18.
17. Madran B, Keske S, Uzun S, et al. Effectiveness of clinical pathway for upper respiratory tract infections in emergency department. Int J Infect Dis. 2019;83:154–159. PubMed PMID: 31051280. doi:10.1016/j.ijid.2019.04.022
18. Tarin T, Feifer A, Kimm S, et al. Impact of a common clinical pathway on length of hospital stay in patients undergoing open and minimally invasive kidney surgery. J Urol. 2014;191(5):1225–1230. PubMed PMID: 24270130. doi:10.1016/j.juro.2013.11.030
19. DeLaroche AM, Sivaswamy L, Farooqi A, Kannikeswaran N. Pediatric stroke clinical pathway improves the time to diagnosis in an emergency department. Pediatr Neurol. 2016;65:39–44. PubMed PMID: 27743748. doi:10.1016/j.pediatrneurol.2016.09.005
20. Nishimura K, Yasui M, Nishimura T, Oga T. Clinical pathway for acute exacerbations of chronic obstructive pulmonary disease: method development and five years of experience. Int J Chron Obstruct Pulmon Dis. 2011;6:365–372. PubMed PMID: 21760723; PubMed Central PMCID: PMCPMC3133508. doi:10.2147/COPD.S20423
21. Plishka CT, Rotter T, Penz ED, et al. Effects of clinical pathways for COPD on patient, professional, and systems outcomes: a systematic review. Chest. 2019;156(5):864–877. PubMed PMID: 31150639. doi:10.1016/j.chest.2019.04.131
22. Ban A, Ismail A, Harun R, Abdul Rahman A, Sulung S, Syed Mohamed A. Impact of clinical pathway on clinical outcomes in the management of COPD exacerbation. BMC Pulm Med. 2012;12:27. PubMed PMID: 22726610; PubMed Central PMCID: PMCPMC3479064. doi:10.1186/1471-2466-12-27
23. Fang Z. Analysis of the application status of clinical path management mode in hospital management. China Med Herald. 2017;14(10):166–169.
24. Huang X. Development and current status of clinical path management. Chin Med Records. 2014;2014(11):22–24.
25. Hudd TR. Emerging role of pharmacists in managing patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2020;77(19):1625–1630. PubMed PMID: 32699897; PubMed Central PMCID: PMCPMC7499078. doi:10.1093/ajhp/zxaa216
26. Sun Z. Medical Statistics.
27. Zhang HX, Li X, Huo HQ, Liang P, Zhang JP, Ge WH. Pharmacist interventions for prophylactic antibiotic use in urological inpatients undergoing clean or clean-contaminated operations in a Chinese hospital. PLoS One. 2014;9(2):e88971. PubMed PMID: 24586465; PubMed Central PMCID: PMCPMC3934870. doi:10.1371/journal.pone.0088971
28. Wang B, Miao Q. Research progress of gender differences in chronic obstructive pulmonary disease. Chin J Clin. 2013;7(12):5564–5567.
29. Han R, Zhao Z. Expert consensus on Chinese pharmaceutical service standards and charges. Drug Eval. 2016;13(14):8–15.
30. Zhong H, Ni XJ, Cui M, Liu XY. Evaluation of pharmacist care for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Clin Pharm. 2014;36(6):1230–1240. PubMed PMID: 25330865. doi:10.1007/s11096-014-0024-9
31. Tommelein E, Mehuys E, Van Hees T, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): a randomized traditionalled trial. Br J Clin Pharmacol. 2014;77(5):756–766. PubMed PMID: 24117908; PubMed Central PMCID: PMCPMC4004396. doi:10.1111/bcp.12242
32. van Boven JF, Stuurman-Bieze AG, Hiddink EG, Postma MJ, Vegter S. Medication monitoring and optimization: a targeted pharmacist program for effective and cost-effective improvement of chronic therapy adherence. J Manag Care Spec Pharm. 2014;20(8):786–792. PubMed PMID: 25062071. doi:10.18553/jmcp.2014.20.8.786
33. Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized traditionalled trial. Arch Intern Med. 2009;169(9):894–900. PubMed PMID: 19433702. doi:10.1001/archinternmed.2009.71
34. Marin Armero A, Calleja Hernandez MA, Perez-Vicente S, Martinez-Martinez F. Pharmaceutical care in smoking cessation. Patient Prefer Adherence. 2015;9:209–215. PubMed PMID: 25678779; PubMed Central PMCID: PMCPMC4319467. doi:10.2147/PPA.S67707
35. Hohl CM, Partovi N, Ghement I, et al. Impact of early in-hospital medication review by clinical pharmacists on health services utilization. PLoS One. 2017;12(2):e0170495. PubMed PMID: 28192477; PubMed Central PMCID: PMCPMC5305222. doi:10.1371/journal.pone.0170495
36. Smith AL, Palmer V, Farhat N. Hospital-based clinical pharmacy services to improve ambulatory management of chronic obstructive pulmonary disease. J Pharm Technol. 2017;33(1):8–14. doi:10.1177/8755122516675635
37. Nguyen TS, Nguyen TLH, Van Pham TT, Hua S, Ngo QC, Li SC. Pharmacists’ training to improve inhaler technique of patients with COPD in Vietnam. Int J Chron Obstruct Pulmon Dis. 2018;13:1863–1872. PubMed PMID: 29928117; PubMed Central PMCID: PMCPMC6001739. doi:10.2147/COPD.S163826
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
