Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 15
The Role of Single Nucleotide Polymorphisms in Transporter Proteins and the Folate Metabolism Pathway in Delayed Methotrexate Excretion: A Case Report and Literature Review
Authors Wang J, Zhao YT, Sun MJ, Chen F, Guo HL
Received 31 May 2022
Accepted for publication 29 September 2022
Published 2 November 2022 Volume 2022:15 Pages 919—926
DOI https://doi.org/10.2147/PGPM.S376797
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Jun Wang,1 Yue-Tao Zhao,2,3 Meng-Jiao Sun,1 Feng Chen,3 Hong-Li Guo3
1Department of Hematology and Oncology, Children’s Hospital of Nanjing Medical University, Nanjing, 210008, People’s Republic of China; 2School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, 210009, People’s Republic of China; 3Pharmaceutical Sciences Research Center, Department of Pharmacy, Children’s Hospital of Nanjing Medical University, Nanjing, 210008, People’s Republic of China
Correspondence: Hong-Li Guo, Children’s Hospital of Nanjing Medical University, 72 Guangzhou Road, Nanjing, 210008, People’s Republic of China, Email [email protected]
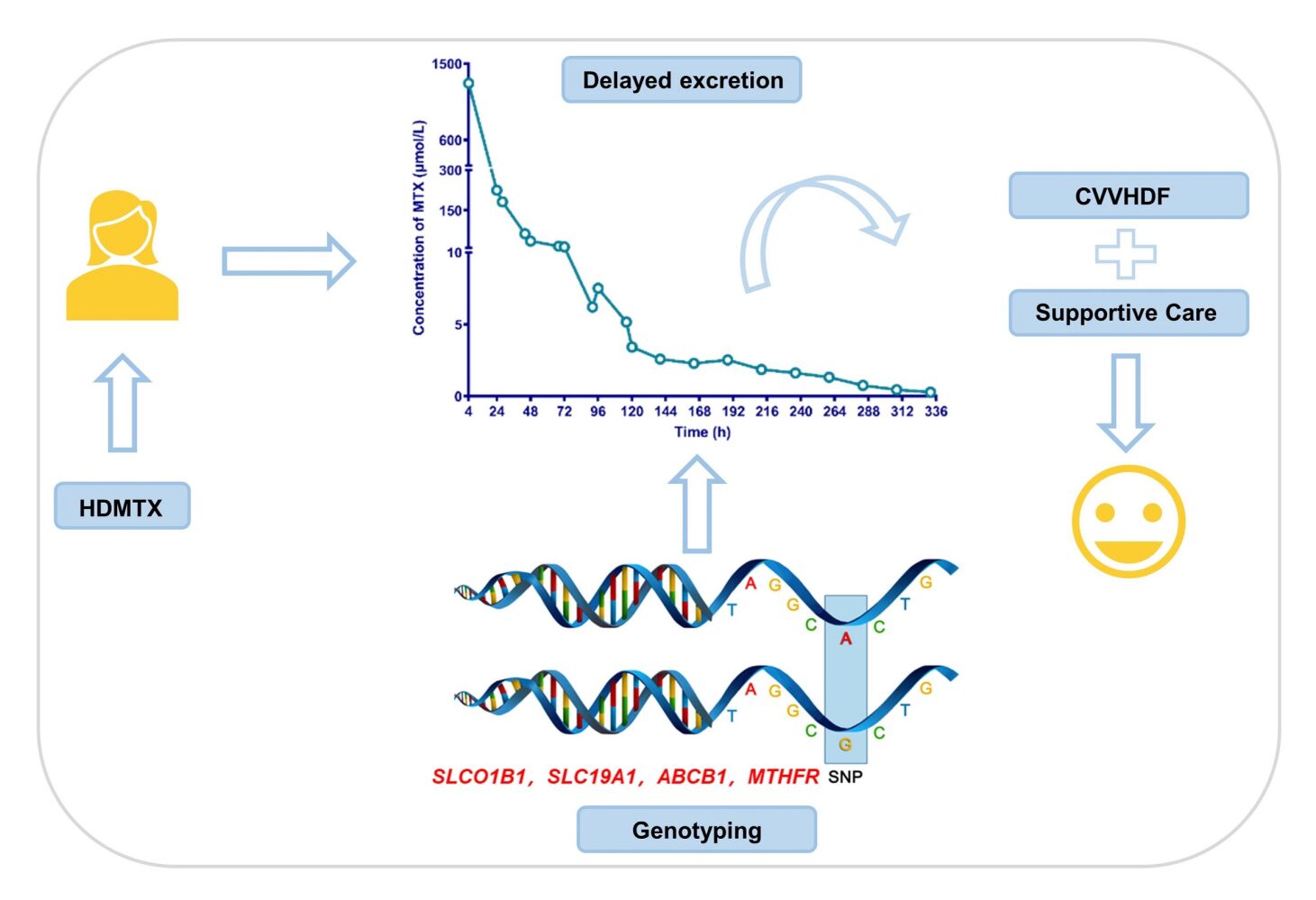
Abstract: High-dose methotrexate (HDMTX) is a pivotal component of the chemotherapeutic regimens of osteosarcoma. However, the use of HDMTX is limited by an increased risk of dose-dependent toxicity. It is thought that the plasma levels and therapy-related toxicity of MTX could be associated with single nucleotide polymorphisms (SNPs) within MTX metabolism pathway genes. Here, we report a case of a paediatric osteosarcoma girl with delayed MTX excretion who was successfully managed using supportive measures and continuous veno-venous haemodiafiltration. We further identified the cause that could account for delayed elimination by genotyping analysis. The results showed that variations have been found in SLCO1B1, SLC19A1, ABCB1 and MTHFR, all those were reported to have a strong association with delayed elimination of MTX in clinical studies. After comprehensive consideration of genotype and clinical phenotype, the second course of HDMTX was administered to this patient at a half reduced dose. We also performed a literature review to summarize the pharmacogenetic factors that influence HDMTX pharmacokinetics or MTX-related adverse effects in osteosarcoma patients. It is suggested that the potential risk of delayed MTX elimination is worthy of clinical attention, and the implementation of genotyping should be considered to ensure therapeutic safety.
Keywords: high-dose methotrexate, osteosarcoma, delayed excretion, single nucleotide polymorphisms
Graphical Abstract:
Introduction
Osteosarcoma (OS) is the most common malignant bone tumour and mostly affects teenagers.1 High-dose methotrexate (HDMTX) combined with doxorubicin and cisplatin is the “gold standard” chemotherapy for osteosarcoma.2 Unfortunately, because the dose of HDMTX (8–12 g/m2) is dozens of times more than the common dose, patients receiving this strategy often suffer from significant toxicities of the kidneys, the liver and gastrointestinal tract as well.3 Therefore, supportive measures, including folate supplementation, fluid hyperhydration, and urine alkalization before and during MTX treatment, are used to facilitate renal elimination to reduce HDMTX-related toxicities.4 Nevertheless, there is still considerable interpatient variability in the clearance of HDMTX, with severe delayed MTX excretion seen from 0.86 to 1.8% in youths with OS.5 Factors including age, hydration status and the concurrent use of nephrotoxic agents are shown to influence MTX clearance.6 Furthermore, recent pharmacogenetic studies have investigated the effects of several genes and polymorphisms on MTX clearance,7,8 which may help us to better understand the pharmacokinetic variability and improve patient outcomes.
Here, we report a case of paediatric osteosarcoma in whom delayed MTX excretion was successfully managed using continuous veno-venous haemodiafiltration (CVVHDF) and supportive measures. Most importantly, polymorphisms in genes encoding transporter proteins or folate pathway genes partially account for delayed MTX excretion. According to the pharmacogenetic results, a reduced MTX dose (6 g/m2) was optimized for the second course of chemotherapy and completed uneventfully.
Case Summary
A 12-year-old girl was diagnosed with osteosarcoma of the right proximal femur and received chemotherapy in our department of haematology and oncology. Her laboratory test results, including the indicators of liver and kidney function, were all in the normal range. Her first course of HDMTX was administered at 12 g/m2. Six hours before MTX infusion, she was intravenously hydrated with 500–1000 mL/m2 of 5% glucose together with 5 mL/kg of 5% NaHCO3 solution. MTX was dissolved in 500 mL of D5W and infused over 4 hours. After this MTX infusion, she was hydrated with the same fluid to a total of 3000–4000 mL/m2 per day during the first 48 hours. Her 4-hour MTX level was 1270.08 μmol/L. Her 24-hour and 28-hour MTX levels were 224.64 and 181.4 μmol/L, respectively (Figure 1A). Thus, leucovorin was immediately escalated to 200 mg/m2 and administered every 6 hours. The girl developed acute kidney injury (AKI) with an increasing serum creatinine level of 197 μmol/L (Figure 1B). She also suffered from acute liver failure with prothrombin activity less than 40%, an increased ALT and AST levels (1247 U/L and 823 U/L, respectively, Figure 1B). She was recommended to be transferred to pediatric intensive care unit (PICU) for CVVHDF management. Furthermore, repeated blood routine tests showed that the number of white blood cells, the proportion of neutrophils and the PCT levels were significantly higher than normal. The patient developed a fever, so bacteremia was considered, and meropenem was used to protect against infection. During CVVHDF, omeprazole was given to protect the stomach, atomolan and compound glycyrrhizin were administered to protect the liver, and leucovorin rescue was continued at a dose that was administered according to the MTX concentration. After a total of 5 days for CVVHDF, serum ALT and AST levels were decreased to 285 U/L and 30 U/L, respectively, and the serum creatinine level was 79.9 μmol/L (Figure 1B). The MTX concentration was decreased to 2.28 μmol/L. Then the patient was transferred back to the general ward for further treatment. Over the following hospital days, her MTX level decreased very slowly and finally reached to 0.28 μmol/L after 14 days of high-dose leucovor in rescue (Figure 1A). Another two days later, indicators of liver and kidney function returned to normal.
 |
Figure 1 Time course of plasma methotrexate concentrations (A) and medications and liver/renal function (B). |
To identify the cause that could account for delayed elimination and to explore a suitable dosage strategy for the next chemotherapy of MTX, we selected the following candidate genetic variants, rs1051296 in SLC19A1; rs4149056 and rs11045879 in SLCO1B1; rs1045642, rs2032582 and rs1128503 in ABCB1; rs717620 in ABCC2; rs1801131 and rs1801133 in MTHFR, for genotyping by real-time PCR. As shown in Table 1, variations have been found in SLCO1B1, SLC19A1, ABCB1 and MTHFR, all of which were reported to have a strong association with delayed elimination of MTX in clinical studies.
 |
Table 1 Genotype of the Targeted SNPs |
After comprehensive consideration of genotype and clinical phenotype, the second course of HDMTX was administered to this patient at a half reduced dose (6 g/m2). The 4-hour and 24-hour MTX levels were 699.84 and 6.84 μmol/L, respectively. Continuous leucovorin rescue was performed according to the MTX concentration. After 3 days, the MTX level was decreased to 0.22 μmol/L (Figure 1A). Following recovery, she proceeded to chemotherapy comprising of cisplatin (60 mg/m2·d) and epirubicin (37.5 mg/m2·d) and completed the course uneventfully.
Discussion
Although HDMTX (8–12 g/m2) has been shown to be the most effective single agent in the neoadjuvant therapy of OS, delayed excretion of MTX can result in life-threatening toxicity that may lead to treatment cessation, irreversible organ damage, and even death. As it excreted primarily by renal route, the precipitation of MTX or its relatively insoluble metabolites in the renal tubules might be one of the most likely mechanisms for acute nephrotoxicity and then result in delayed excretion.9 More important, delayed MTX excretion may occur in the absence of any predisposing factor.6 In this case, the girl suffered from “severe” delayed excretion (C24h >50 μmol/L) and acute liver and kidney injury. She was successfully managed using supportive cares and CVVHDF.
The pharmacokinetics of HDMTX showed large individual variability. A reduced effective blood volume or the concurrent use of nephrotoxic agents were known to be related to the development of delayed MTX excretion.10 However, the patient had no signs of decreased blood volume and did not receive any nephrotoxic agent. Before administration, her renal function was normal, and her hydration status was standard. The reason for delayed MTX excretion is worth exploring. Recently, growing evidence suggested that genetic variants of metabolic enzymes11,12 or transporters13,14 involved in MTX elimination could provide a mechanistic explanation for the variability of its pharmacokinetics. Furthermore, these variants might be potential predictors for personalized pharmacotherapy. Therefore, we focused on the pharmacogenomics of MTX to illustrate the causes of delayed MTX elimination.
Generally, pharmacogenetic studies have identified various genes that contribute to the vast interindividual variation in MTX pharmacokinetics and are mainly involved in transporter genes (SLCO1B1, SLC19A1, ABCB1, and ABCC2) or folate pathway genes (MTHFR, MTR, MTRR, and DHFR). However, those studies that focused on OS patients were very limited. We first searched PubMed with a combination of the keywords “pharmacogenetic” AND “methotrexate” AND “osteosarcoma”; the information of these studies is summarized and presented in Table 2. Of those genes, the SNPs on ABCG2,7 ABCB1, ABCC3,15 and SLCO1B116 were associated with MTX-PK parameters. Other genetic variants, such as MTHFR (rs1801133), RFC1 (rs1051266), GSTP1 (rs1695), and MTR (rs1805087) were shown to be associated with an increased risk of HDMTX-related adverse effects.17–23 In addition, rs4148416 in ABCC3 and three SNPs in ABCB1, rs4148737, rs1128503 and rs10276036 were associated with overall survival.24
 |
Table 2 Pharmacogenetic Literatures Information of HD-MTX in OS |
Interestingly, SLCO1B1 was considered to be the only gene that reliably demonstrates an effect on MTX pharmacokinetics in a recent systematic review.25 Notably, rs4149081 and rs11045879 in SLCO1B1 have been strongly associated for the first time with MTX clearance.26,27 The rs11045879 variant was found in this patient, which may partially account for the delayed excretion. However, one study showed variants in SLCO1B1 explained up to 10% of the interpatient variability in the clearance of HDMTX.26 Hence, other MTX transporters could be of interest. Among them, the most studied was SLC19A1. Overall, SLC19A1 is an important transporter responsible for folate homeostasis and the uptake of endogenous reduced folates and anti-folate xenobiotics including MTX.28 In Chinese children with acute lymphoblastic leukaemia, delayed elimination of MTX was more frequent in rs1051296 mutant carriers than in wild-type patients.29 This girl carried a heterozygous variant of SLC19A1, increasing the risk of delayed elimination. On the other hand, pumping out MTX from the cell was mediated by highly polymorphic ABC (specifically the ABCB1, ABCC2, ABCC4 and ABCG2) transporters.30 Polymorphisms in these genes have been studied in early studies, however, the results are inconsistent.7,21 This girl has two heterozygous mutations in rs1045642 and rs2032582 in ABCB1, which might lead to a decrease in MTX clearance.8
In addition, the girl carried a T variant of rs1801133 in MTHFR. MTHFR is responsible for the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate. It has been confirmed that reduced MTHFR expression is related to MTX-induced toxicity in patients with leukaemia.31 Furthermore, both SNPs of MTHFR C677T (rs1801133) and MTHFR A1298C (rs1801131) have been suggested to be accompanied by decreased enzyme activity.32 Some studies found that the T allele in rs1801133 was associated with decreased elimination of MTX, while few studies found a clinically significant association between MTX elimination and rs1801131.33,34 Therefore, we hypothesized that carrying with the T allele may aggravate the plasma MTX level and increase the risk of adverse effects in this case.
Based on the genotype results of this patient and previous literatures,35 we speculate that genetic polymorphisms in the transporters and folate pathways at least partially account for delayed MTX elimination. We consider that implementation of genotyping before HDMTX chemotherapy may help predict MTX treatment response. However, it is worth noting that the pharmacogenetics of MTX are still at the bench level. There is a lack of a definite quantified association between each genetic polymorphism and the efficacy or adverse effects of HDMTX. Therefore, only one pharmacogenetic variant alone may not have sufficient predictive power. A panel consisting of multiple genes should be considered in the clinic.
Here, we describe a valuable case of delayed MTX elimination, possibly attributed to genetic mutations in transporters (SLCO1B1, SLC19A1 and ABCB1) and the folate pathway (MTHFR). The potential risk of delayed MTX elimination is worthy of clinical attention, and the implementation of genotyping should be considered to ensure therapeutic safety.
Abbreviations
HDMTX, high-dose methotrexate; MTX, methotrexate; SNPs, single nucleotide polymorphisms; SLCO1B1, solute carrier organic anion transporter family member 1B1; SLC19A1, solute carrier family 19 member 1; ABCB1, ATP binding cassette subfamily B member 1; MTHFR, methylenetetrahydrofolate reductase; OS, osteosarcoma; CVVHDF, continuous veno-venous haemodiafiltration; D5W, dextrose 5% water solution; AKI, acute kidney injury; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PICU, pediatric intensive care unit; PCT, proealcitonin; PCR, polymerase chain reaction; ABCC2, ATP binding cassette subfamily C member 2; MTR, 5-methyltetrahydrofolate-homocysteine methyltransferase; MTRR, 5-methyltetrahydrofolate-homocysteine methyltransferase reductase; DHFR, dihydrofolate reductase; ABCG2, ATP binding cassette subfamily G member 2; ABCC3, ATP binding cassette subfamily C member 3; PK, pharmacokinetics; RFC1, reduced folate carrier protein 1; GSTP1, glutathione S-transferase pi 1; ABC, ATP binding cassette family; ABCC4, ATP binding cassette subfamily C member 4.
Patient Consent Statement
Written informed consent was obtained from the patient’s mother for the publication of this case report. The study was approved by the Ethics Committee of Children’s Hospital of Nanjing Medical University (202203007-1).
Acknowledgments
This research was supported by the Nanjing Medical Science and Technique Development Foundation (QRX 17074) and the Specially Appointed Medical Expert Project of the Jiangsu Commission of Health (2019). This study was also supported by the Scientific Research Support Foundation for Young Scholars at the Children’s Hospital of Nanjing Medical University (2020).
Disclosure
Yue-Tao Zhao is a visiting graduate student from China Pharmaceutical University. The authors declare no other conflicts of interest in this work.
References
1. Belayneh R, Fourman MS, Bhogal S, et al. Update on osteosarcoma. Curr Oncol Rep. 2021;23(6):71. doi:10.1007/s11912-021-01053-7
2. Isakoff MS, Bielack SS, Meltzer P, et al. Osteosarcoma, current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33(27):3029–3035. doi:10.1200/JCO.2014.59.4895
3. Abe K, MAEDA-MINAMI A, Ishizu T, et al. Risk factors for hepatic toxicity of high-dose methotrexate in patients with osteosarcoma. Anticancer Res. 2022;42(2):1043–1050. doi:10.21873/anticanres.15565
4. Brown P, Inaba H, Annesley C, et al. Pediatric acute lymphoblastic leukemia, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(1):81–112.
5. Widemann BC, Balis FM, Kempf-Bielack B, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100(10):2222–2232. doi:10.1002/cncr.20255
6. Lee KM, Lee HW, Kim SY, et al. Two pediatric osteosarcoma cases with delayed methotrexate excretion, its clinical course and management. Cancer Res Treat. 2011;43(1):67–70. doi:10.4143/crt.2011.43.1.67
7. Lui G, Treluyer J-M, Fresneau B, et al. A pharmacokinetic and pharmacogenetic analysis of osteosarcoma patients treated with high-dose methotrexate, data from the OS2006/Sarcoma-09 Trial. J Clin Pharmacol. 2018;58(12):1541–1549. doi:10.1002/jcph.1252
8. Esmaili MA, Kazemi A, Faranoush M, et al. Polymorphisms within methotrexate pathway genes, relationship between plasma methotrexate levels, toxicity experienced and outcome in pediatric acute lymphoblastic leukemia. Iran J Basic Med Sci. 2020;23(6):800–809. doi:10.22038/ijbms.2020.41754.9858
9. Buchen S, Ngampolo D, Melton RG, et al. Carboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failure. Br J Cancer. 2005;92(3):480–487. doi:10.1038/sj.bjc.6602337
10. Misaka KO, Suga Y, Staub Y, et al. Risk factors for delayed elimination of methotrexate in children, adolescents and young adults with osteosarcoma. In Vivo. 2020;34(6):3459–3465. doi:10.21873/invivo.12185
11. Frikha R, Elloumi M, Rebai T, et al. MTHFR 677T-1298C haplotype in acute lymphoblastic leukemia, impact on methotrexate therapy. J Oncol Pharm Pract;2021. 10781552211017193. doi:10.1177/10781552211017193
12. Yazicioglu B, Kaya Z, Güntekin Ergün S, et al. Influence of folate-related gene polymorphisms on high-dose methotrexate-related Toxicity and prognosis in Turkish children with acute lymphoblastic leukemia. Turk J Haematol. 2017;34(2):143–150. doi:10.4274/tjh.2016.0007
13. Yang L, Wu H, Gelder TV, et al. SLCO1B1 rs4149056 genetic polymorphism predicting methotrexate toxicity in Chinese patients with non-Hodgkin lymphoma. Pharmacogenomics. 2017;18(17):1557–1562. doi:10.2217/pgs-2017-0110
14. Han J, Liu L, Meng L, et al. Effect of Polymorphisms of ABCB1 and MTHFR on methotrexate-related toxicities in adults with hematological malignancies. Front Oncol. 2021;11:759805. doi:10.3389/fonc.2021.759805
15. Hegyi M, Arany A, Semsei AF, et al. Pharmacogenetic analysis of high-dose methotrexate treatment in children with osteosarcoma. Oncotarget. 2017;8(6):9388–9398. doi:10.18632/oncotarget.11543
16. Goricar K, Kovač V, Jazbec J, et al. Influence of the folate pathway and transporter polymorphisms on methotrexate treatment outcome in osteosarcoma. Pharmacogenet Genomics. 2014;24(10):514–521. doi:10.1097/FPC.0000000000000083
17. Lambrecht L, Sleurs C, Labarque V, et al. The role of the MTHFR C677T polymorphism in methotrexate-induced toxicity in pediatric osteosarcoma patients. Pharmacogenomics. 2017;18(8):787–795. doi:10.2217/pgs-2017-0013
18. Hattinger CM, Biason P, Iacoboni E, et al. Candidate germline polymorphisms of genes belonging to the pathways of four drugs used in osteosarcoma standard chemotherapy associated with risk, survival and toxicity in non-metastatic high-grade osteosarcoma. Oncotarget. 2016;7(38):61970–61987. doi:10.18632/oncotarget.11486
19. Jabeen S, Holmboe L, Alnæs GIG, et al. Impact of genetic variants of RFC1, DHFR and MTHFR in osteosarcoma patients treated with high-dose methotrexate. Pharmacogenomics J. 2015;15(5):385–390. doi:10.1038/tpj.2015.11
20. Hagleitner MM, Coenen MJH, Aplenc R, et al. The role of the MTHFR 677C>T polymorphism in methotrexate-induced liver toxicity: a meta-analysis in patients with cancer. Pharmacogenomics J. 2014;14(2):115–119. doi:10.1038/tpj.2013.19
21. Windsor RE, Strauss SJ, Kallis C, et al. Germline genetic polymorphisms may influence chemotherapy response and disease outcome in osteosarcoma, a pilot study. Cancer. 2012;118(7):1856–1867. doi:10.1002/cncr.26472
22. Patino-Garcia A, Zalacaín M, Marrodán L, et al. Methotrexate in pediatric osteosarcoma, response and toxicity in relation to genetic polymorphisms and dihydrofolate reductase and reduced folate carrier 1 expression. J Pediatr. 2009;154(5):688–693. doi:10.1016/j.jpeds.2008.11.030
23. Muller J, Kralovánszky J, Adleff V, et al. Toxic encephalopathy and delayed MTX clearance after high-dose methotrexate therapy in a child homozygous for the MTHFR C677T polymorphism. Anticancer Res. 2008;28(5B):3051–3054.
24. Caronia D, Patiño-Garcia A, Peréz-Martínez A, et al. Effect of ABCB1 and ABCC3 polymorphisms on osteosarcoma survival after chemotherapy, a pharmacogenetic study. PLoS One. 2011;6(10):e26091. doi:10.1371/journal.pone.0026091
25. Taylor ZL, Vang J, Lopez-Lopez E, et al. Systematic review of pharmacogenetic factors that influence high-dose methotrexate pharmacokinetics in pediatric malignancies. Cancers (Basel). 2021;13(11):2837. doi:10.3390/cancers13112837
26. Trevino LR, Shimasaki N, Yang W, et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol. 2009;27(35):5972–5978. doi:10.1200/JCO.2008.20.4156
27. Ramsey LB, Panetta JC, Smith C, et al. Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood. 2013;121(6):898–904. doi:10.1182/blood-2012-08-452839
28. Uhlen M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi:10.1126/science.1260419
29. Wang SM, Sun LL, Zeng WX, et al. Effects of a microRNA binding site polymorphism in SLC19A1 on methotrexate concentrations in Chinese children with acute lymphoblastic leukemia. Med Oncol. 2014;31(7):62. doi:10.1007/s12032-014-0062-0
30. Mikkelsen TS, Thorn CF, Yang JJ, et al. PharmGKB summary, methotrexate pathway. Pharmacogenet Genomics. 2011;21(10):679–686. doi:10.1097/FPC.0b013e328343dd93
31. Chae H, Kim M, Choi SH, et al. Influence of plasma methotrexate level and MTHFR genotype in Korean paediatric patients with acute lymphoblastic leukaemia. J Chemother. 2020;32(5):251–259. doi:10.1080/1120009X.2020.1764280
32. Chiusolo P, Giammarco S, Bellesi S, et al. The role of MTHFR and RFC1 polymorphisms on toxicity and outcome of adult patients with hematological malignancies treated with high-dose methotrexate followed by leucovorin rescue. Cancer Chemother Pharmacol. 2012;69(3):691–696. doi:10.1007/s00280-011-1751-4
33. Mahmoud LB, Mdhaffar M, Frikha R, et al. Use of MTHFR C677T polymorphism and plasma pharmacokinetics to predict methotrexate toxicity in patients with acute lymphoblastic leukemia. Adv Clin Exp Med. 2018;27(8):1061–1068. doi:10.17219/acem/69802
34. Choi YJ, Park H, Lee JS, et al. Methotrexate elimination and toxicity: MTHFR 677C>T polymorphism in patients with primary CNS lymphoma treated with high-dose methotrexate. Hematol Oncol. 2017;35(4):504–509. doi:10.1002/hon.2363
35. Hu YH, Zhou L, Wang SS, et al. Methotrexate disposition in pediatric patients with acute lymphoblastic leukemia, what have we learnt from the genetic variants of drug transporters. Curr Pharm Des. 2019;25(6):627–634. doi:10.2174/1381612825666190329141003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
