Back to Journals » International Journal of General Medicine » Volume 15
The Role of Serum 1,25-Dihydroxy Vitamin D3 and PCT in Idiopathic Pulmonary Fibrosis
Authors Yang L , Zhai Z, Zhang J
Received 20 August 2022
Accepted for publication 27 October 2022
Published 8 November 2022 Volume 2022:15 Pages 8081—8092
DOI https://doi.org/10.2147/IJGM.S386984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Li Yang,1 Zhinan Zhai,2 Jinxiang Zhang3
1Department of Respiratory and Critical Care Medicine, Tianjin Chest Hospital, Tianjin, People’s Republic of China; 2Department of Medical Laboratory Science, Tianjin Chest Hospital, Tianjin, People’s Republic of China; 3Department of Nutrition, Tianjin Chest Hospital, Tianjin, People’s Republic of China
Correspondence: Li Yang, Department of Respiratory and Critical Care Medicine, Tianjin Chest Hospital, No. 261 Taierzhuang South Road, Jinnan District, Tianjin, 300222, People’s Republic of China, Tel +86 22-88185009, Email [email protected]
Objective: Biomarkers for the acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) are urgently needed to provide better patient management. We aimed to investigate whether serum 1,25(OH)2D3 (1,25-dihydroxy vitamin D3) levels predict AE-IPF and whether they could be a potential prognostic biomarker for IPF.
Participants and Methods: This prospective study included 72 patients with IPF (31 with stable IPF and 41 with AE-IPF). All participants were recruited during hospitalisation at Tianjin Chest Hospital and were followed up for at least 12 months. Demographics, comorbidities, arterial blood gas, and serum biochemical profile, radiological features, and anti-fibrotic therapy were evaluated. Serum concentrations of 1,25(OH)2D3 and transforming growth factor beta1 (TGFβ 1) were detected using enzyme-linked immunosorbent assay (ELISA). Risk factors for AE-IPF were identified using multivariate analysis. Prognostic factors were assessed using Kaplan-Meier and Cox regression analyses.
Results: Baseline values of alveolar-arterial oxygen difference (A-aDO2) (40.85 mmHg vs 29.2 mmHg, p =0.035), white blood cell counts (10.09 ± 4.2× 109/L vs 7.46 ± 7.84× 109/L, p < 0.001), percentage of monocytes (7.36 ± 1.36% vs 6.6 ± 1.2%, p =0.017), C-reactive protein (CRP) (2.1 mg/dL vs 1.12 mg/dL, p =0.015) and procalcitonin (PCT) (36.59% vs 3.23%, p < 0.001) were significantly higher in AE-IPF patients than in stable IPF patients. Instead, the mean concentration of serum calcium and 1,25(OH)2D3 at baseline were higher in IPF patients with stable disease than in those with acute exacerbation (2.17 ± 0.13 nmol/L vs 2.09 ± 0.13 nmol/L, p =0.023 and 16.62 pg/mL vs 11.58 pg/mL, p < 0.001, respectively). In multivariate analysis, a higher proportion of patients with lower serum 1,25(OH)2D3 levels experienced AE-IPF (OR 0.884, 95% CI 0.791– 0.987, p =0.029), and rising serum PCT level (PCT > 0.05 ng/mL) was associated with an increased risk of mortality (HR 3.664, 95% CI 1.010– 12.900, p =0.043).
Conclusion: Decreased serum 1,25(OH)2D3 is associated with an increased risk of acute exacerbation for patients with IPF. A high serum PCT level is predictive of worse prognosis in IPF patients. 1,25(OH)2D3 may be a potential biomarker for AE-IPF, while PCT could be a prognostic biomarker for IPF.
Keywords: idiopathic pulmonary fibrosis, acute exacerbation, 1,25(OH)2D3, procalcitonin
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrosing interstitial lung disease of unknown aetiology, with a median survival time of 2–4 years from diagnosis.1–6 The abnormal crosstalk between epithelial, endothelial, mesenchymal, and immune cells may play a key role in IPF.7 The clinical course of IPF is highly variable, and all patients experience disease progression.8 With the most severe and rapid disease progression, acute exacerbation (AE) leads to the highest number of deaths.9,10 AE in IPF patients is typically associated with poor prognosis.11 Several potential prognostic biomarkers for IPF have been reported, including inflammatory molecules, gene expression signatures, telomere length, circulating blood cells and circulating epithelial biomarkers.12–20 However, the predictors of AE in IPF patients remain unclear, especially when considering the modified diagnostic criteria for AE-IPF in recent years.21–23
Vitamin D is an important secosteroid hormone with a wide spectrum of biological activities
in metabolic syndrome, immunological diseases, cardiovascular diseases, renal diseases, and cancers.24,25 1,25(OH)2D3 (1,25-dihydroxy vitamin D3), the active metabolite of vitamin D, exerts protective effects on other multiple respiratory diseases.26,27 Recently, it has also been reported that supplementation with exogenous 1,25(OH)2D3 can partially prevent pulmonary fibrosis in vivo.28,29 These results highlight the need to study the clinical impact and underlying protective mechanisms of 1,25(OH)2D3 in IPF patients.
In this study, we aimed to correlate serum 1,25(OH)2D3 levels with AE in patients with IPF. A further aim was to identify independent prognostic factors of IPF patients.
Materials and Methods
Subject Design
We performed a prospective study of 72 patients with definite IPF (31 with stable IPF, 41 with AE-IPF). Patients were recruited during hospitalisation at Tianjin Chest Hospital between March 2018 and May 2020 (Figure 1). IPF diagnoses were made in accordance with the ATS/ERS/JRS/ALAT criteria.2 Patients with potential connective tissue disease, domestic and occupational environmental exposures, and a non-usual interstitial pneumonia (UIP) pattern of fibrosis on chest high-resolution computed tomography (HRCT) were excluded. 68 subjects (94.44%) with IPF were newly diagnosed. Patients who had lung cancer and those who refused follow-up or who were lost to follow-up were also excluded. AE-IPF diagnosis was established according to international criteria: (1) definitive diagnosis of IPF; (2) worsening of dyspnoea within 1 month; (3) new bilateral ground-glass opacity or consolidation on chest HRCT; and (4) absence of heart failure or other explanation.2,30 We followed the enrolled participants by telephone until April 2021, the median follow-up times were 20.7 months. All included participants had a clinical follow-up of at least 12 months.
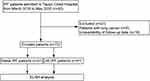 |
Figure 1 Patient enrolment flow diagram. Abbreviations: IPF, idiopathic pulmonary fibrosis; AE, acute exacerbation; ELISA, enzyme-linked immunosorbent assay. |
All patients in our study were treated according to clinical practice guidelines.1,4 The treatment regime of patients was as follows. Both group of patients received antibiotic, proton pump inhibitor, and oxygen therapies. Patients with stable IPF were treated with oral antibiotics, whereas patients with AE-IPF were treated with venous antibiotics (mainly empiric broad-spectrum antibiotics). Five patients (5/41,12.2%) in the AE-IPF group received antifungal therapy. We intravenously administered methylprednisolone on a routine basis in the AE-IPF group at a dose of 0.6–2mg/kg/day.31 In addition, a small number of patients in both groups received antioxidant therapy (N-acetylcysteine, NAC) and/or anti-fibrotic (pirfenidone) therapy. The study protocol was conducted in accordance with the principles of Declaration of Helsinki. All the participants provided informed consent. Ethical approval was granted by the ethics committee of Tianjin Chest Hospital (2018KY-020-01).
The basic and clinical information of all patients with IPF was collected at the time of enrolment. Arterial blood samples from all IPF patients were collected on the day of admission and venous blood specimens were collected on day 2 after admission. We quantified IPF severity using the Helbich HRCT scoring system, and the HRCT score was calculated as previously described.32
Serum Measurements
Venous blood samples were centrifuged at 600×g for 15 min, and serum was collected and stored at −80°C until further analysis. Serum transforming growth factor beta1 (TGFβ1) and 1,25(OH)2D3 concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Inc., Minneapolis, MN; MyBiosource, Inc., San Diego, CA).
Statistical Analyses
Data analysis was performed with SPSS statistical software (version 22.0, SPSS, Inc., Chicago, IL). Continuous variables are represented as median (interquartile range) or mean ± SD, while categorical variables are described as frequencies and percentages. Continuous variables were compared using Student’s t-test, or Mann–Whitney U-test; categorical variables were compared using χ2 test, or Fisher exact test. The survival probability of IPF patients was summarised using the Kaplan-Meier method, and group comparisons were tested using Log rank test. Multivariate logistic regression analysis was performed to detect independent predictors of AE-IPF. Univariate and multivariate Cox proportional hazards regression model were used to identify the effects of the variables on survival in patients with IPF. Differences were considered statistically significant when the P-value (p) was < 0.05 (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
Patient Characteristics
A total of 72 patients were included in this present analysis. The baseline patient characteristics are summarised in Table 1. The mean age for the stable IPF group was 69.84 ± 8.24 years; the mean age for the AE-IPF group was 70.38 ± 11.19 years and 71% were male in both groups. There were no significant differences in body mass index (BMI), or smoking history between the two groups. In terms of arterial blood gas analysis, the median alveolar-arterial oxygen difference (A-aDO2) in the AE-IPF group was significantly higher than that in the stable IPF group (40.85mmHg vs 29.2mmHg, p =0.035). The AE-IPF group had significantly higher white blood cell (WBC) counts (10.09 ± 4.2×109/L vs 7.46 ± 7.84×109/L, p <0.001) and a higher percentage of monocytes (7.36 ± 1.36% vs 6.6 ± 1.2%, p =0.017) than the stable IPF group. The C-reactive protein (CRP) level for AE-IPF patients was 2.1 mg/dL, which was significantly higher than that for stable patients (1.12 mg/dL, p =0.015). Mean procalcitonin (PCT) was also found to be significantly elevated. Of note, in the AE-IPF group, 15 patients (15/41, 36.59%) had elevated PCT levels, whereas in the stable IPF group, only one patient (1/31, 3.23%) had elevated PCT levels. Moreover, compared to patients with stable IPF, those in the AE-IPF group had lower serum calcium levels (2.09 ± 0.13 nmol/L vs 2.17 ± 0.13 nmol/L, p =0.023). Patient clinical comorbidities and family history appear in Table 2. Finally, we found a similar rate of comorbidities and family history in the two groups.
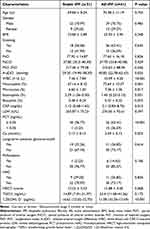 |
Table 1 Summary of Patient baseline characteristics |
 |
Table 2 Comorbidities and Family History for IPF |
Serum Concentration of TGFβ1 and 1,25(OH)2D3
10 patients in stable IPF group and 11 patients in AE-IPF group were administered oral daily 600mg calcium (as calcium carbonate) and vitamin D3 (125 international units). No significant difference was observed in the serum levels of TGFβ1 between patients with stable IPF and those with AE-IPF. However, serum levels of 1,25(OH)2D3 was significant lower in patients with AE-IPF than in those with stable IPF (p = 0.001) (Figure 2A and B).
Overall Survival Analysis
According to the Kaplan–Meier analysis, AE-IPF patients showed significantly higher overall mortality (8/31, 25.81%) than stable IPF patients (25/41, 60.98%; Log rank test, p = 0.0014) (Figure 3A). Of 41 AE-IPF patients, 13 (13/41, 31.71%) died within the first year after the onset of an AE episode; stable IPF patients had a significantly lower 1-year mortality rate of 9.68% (3/31) (p = 0.023).
Risk Factors of IPF Exacerbation
Variables with statistically significant differences (p < 0.05) between the two groups were further tested using multivariate logistic regression. Our test revealed that the 1,25(OH)2D3 serum level was an independent risk factor of AEs in IPF patients (odd ratio [OR] = 0.884; 95% CI = 0.791–0.987; p = 0.029) (Table 3). Receiver operating characteristic (ROC) curve analysis showed that the optimal cut-off value of serum 1,25(OH)2D3 level to predict AE-IPF was 15.08ng/mL (area under the ROC curve = 0.755, 95% CI = 0.631–0.879) (Figure 3B). A lower serum 1,25(OH)2D3 level (< 15.08 pg/mL) was associated with an increased risk of IPF exacerbation.
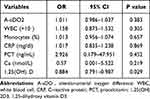 |
Table 3 Multivariate Logistic Regression Analysis for an Acute Exacerbation of Idiopathic Pulmonary Fibrosis Related to Risk Factors |
Prognostic Factors for the Survival of IPF Patients
In univariate Cox proportional-hazards analyses, higher A-aDO2, higher WBC counts, a higher percentage of monocytes, higher serum CRP and PCT levels and lower serum 1,25(OH)2D3 levels were significantly associated with poor survival (Table 4). In multivariate analysis, only PCT (hazard ratio [HR] = 3.664; 95% CI = 1.010–12.900; p = 0.043) was associated with mortality in IPF patients (Figure 4A). Kaplan–Meier survival curves for IPF patients sorted by PCT level were then generated (Figure 4B). Mortality was significantly higher in IPF patients with PCT > 0.05 ng/mL (Log rank test, p < 0.0001). The overall mortality was 87.5% (14/16) and 1-year mortality was 68.75% (11/16) in IPF patients with PCT > 0.05 ng/mL. This observation was in sharp contrast to the IPF patients with PCT ≤ 0.05 ng/mL who had a lower overall (33.93%, 19/56) and 1-year (8.93%, 5/56) mortality.
 |
Table 4 Prognostic Factors in Univariate Cox Proportional Hazards Regression Analyses with IPF for OS |
Discussion
In this study, we showed that 1,25(OH)2D3 was associated with AE in patients with IPF. IPF patients with lower serum 1,25(OH)2D3 levels (< 15.08pg/mL) had a higher risk of AE. However, Kaplan–Meier and multivariate Cox regression analysis demonstrated that serum PCT level, rather than 1,25(OH)2D3, was the only significant predictor of OS in patients with IPF. IPF patients with a PCT > 0.05 ng/mL had a higher overall mortality than those with a PCT ≤ 0.05 ng/mL.
The causal role of infection and microbial dysbiosis in AE-IPF has been highlighted by several studies.33,34 A prospective study involving 20 AE-IPF patients and 15 stable IPF patients showed that AE-IPF is associated with an increase bacterial burden.35 Weng et al found that AE-IPF patients had higher bacterial IgM levels in the serum than stable IPF patients. In the same study, higher rates of viral infection were detected in nasopharyngeal swabs of patients AE-IPF.36 Wootton et al identified that Torque Teno virus infection was predominant in patients with IPF with AE.37 A recent study by McElroy et al suggested that viral and bacterial infections induce AE-related death in IPF.38 In response to infection and dysbiosis of the lung microbiome, inflammatory markers, such as WBC counts, CRP levels, and PCT levels, were elevated,39–41 which is consistent with our results. We also observed that the AE-IPF group had a substantially higher A-aDO2 than the stable IPF group, which reflected marked pulmonary diffusion impairment in patients with AE-IPF.42
While an increased percentage of blood monocytes in the AE-IPF group was identified in our study which aligns with a previous report,21 we found that it was not an independent risk factor for AE or OS. A higher blood monocyte count was recently reported to be associated with shortened OS in IPF patients.18 Kreuter et al stratified 2067 IPF patients into three groups for blood monocyte count (<0.6 K/uL, 0.6-<0.95 K/uL, and ≥0.95 K/uL). They confirmed a significant relationship between an elevated blood monocyte count and increased risks of progression, hospitalisation, and OS.15 Together, these findings suggest that blood monocyte count, not the percentage of blood monocytes, deserves special attention in IPF patients.
Vitamin D deficiency or insufficiency was highly prevalent in IPF patients; 34–56.3% of patients had low serum vitamin D levels according to the Endocrine Society Clinical Practice Guideline.43–45 However, whether the serum level of 1,25(OH)2D3 is similarly reduced in IPF patients has not been reported. We showed for the first time that the serum 1,25(OH)2D3 concentration in IPF patients is far lower than that in healthy individuals.46 Meanwhile, AE-IPF patients have a significantly lower serum 1,25(OH)2D3 concentration than stable IPF patients. Here, we further demonstrated that the 1,25(OH)2D3 serum level was an independent risk factor of AE in IPF patients, suggesting that 1,25(OH)2D3 may provide a predictive value for AE of IPF. Our data suggest that a serum 1,25(OH)2D3 cut-off of < 15.08pg/mL is correlated with AE of IPF.
Low serum vitamin D levels have been reported as a negative prognostic factor for interstitial lung disease. A previous study by Tzilas et al showed that low serum vitamin D contribute to high all-cause mortality in patients with IPF.47 A study by Gao et al assessed the correlation between serum vitamin D levels and prognosis of connective tissue disease-interstitial lung disease (CTD-ILD). They found an association between low serum vitamin D levels and poor prognosis.48 Furthermore, low serum 1,25(OH)2D3 levels were predictive of increased mortality in patents with sepsis.49 These results suggest a possible link between low serum 1,25(OH)2D3 levels and poor prognosis in IPF patients. Hence, we aimed to investigate whether serum 1,25(OH)2D3 levels affect the OS. A multivariate Cox proportional hazards model was used; however, serum 1,25(OH)2D3 levels did not influence OS. This is not consistent with the finding in the pulmonary fibrosis model; 1,25(OH)2D3 supplements have been shown to reduce the degree of fibrosis and increase lifespan in a BLM-induced pulmonary fibrosis mouse model.29 Further studies are needed to address this issue.
Unlike 1,25(OH)2D3, the serum PCT level was first identified to predict death in IPF based on multivariate Cox hazard proportional regression analysis in our study. We subsequently employed Kaplan-Meier analysis. Remarkably, Kaplan-Meier analysis also revealed it to be a significant prognostic factor for OS. We chose to stratify IPF patients by a value of PCT above 0.05 ng/mL, due to serum PCT having a detection limit of 0.05ng/mL within the trial. The TRIAGE study, a large prospective multicentre study, demonstrated that PCT was a strong and independent prognostic predictor of 30-day mortality in emergency department patients.50 In the TRIAGE study, when using a cut-off of 0.05ng/mL, the mortality rate increased to 1%. The discrepancy in mortality rates between our study and the TRAIGE study may have resulted from differences in the studied populations and follow-up periods. Because patients with concurrent IPF and lung cancer were reported to have higher serum PCT levels than those with IPF alone,51 to rule out the interfering effect of lung cancer, we excluded IPF patients who had concurrent lung cancer before enrolment or developed lung cancer during the follow-up period. Our results suggest that serum PCT in IPF patients should be evaluated at a concentration above 0.05 ng/mL.
On comparing the AE-IPF and stable IPF groups, lower serum calcium levels were detected in the AE-IPF group. This suggests that patients with AE-IPF might be more prone to developing osteoporosis and fractures.52 1,25(OH)2D3 attenuates the epithelial-mesenchymal transition (EMT) in lung epithelial cells by inhibiting TGF-β signaling.53–56 An inverse correlation between vitamin D and serum TGF-β1 levels has also been observed.57 Therefore, we examined serum TGF-β1 in IPF patients and found that its level was not linked to AE or OS, suggesting that it cannot be used as a potential biomarker for predicting IPF.
Our study has some limitations. Firstly, most patients were clinically diagnosed with IPF; only one patient was pathologically diagnosed with IPF by lung biopsy (surgery). Reasons for the lack of biopsy included patient declined biopsy, unsafe biopsy, and poor tolerance of biopsy. Secondly, we did not have complete pulmonary function information for some IPF patients because these patients were in critical condition at diagnosis, and pulmonary function tests were not feasible. Thirdly, the sample size of the study was small. Finally, this was a single-centre study, restricted to a limited number of IPF enrolees and local practice.
Conclusion
The present study had two important findings: 1) In IPF patients, decreased serum 1,25(OH)2D3 levels are associated with an increased risk of AE; 2) a higher PCT level is predictive of worse prognosis in patients with IPF. The novelty of the present study lies in the identification of serum 1,25(OH)2D3 and PCT that predict AE and OS for IPF, respectively. We recommend that serum 1,25(OH)2D3 and PCT should be routinely measured to stratify patients at diagnosis who are at risk of more AE and worse prognosis.
Abbreviations
IPF, idiopathic pulmonary fibrosis; AE, acute exacerbation; OS, overall survival; 1,25(OH)2D3, 1,25-dihydroxy vitamin D3; TGFβ1, transforming growth factor beta1; HRCT, high-resolution computed tomography; ELISA, enzyme-linked immunosorbent assay; BMI, body mass index; GERD, gastroesophageal reflux disease; CAD, coronary artery disease; CVD, cerebrovascular disease; VTE, venous thrombus embolism; PaO2, partial pressure of arterial oxygen; PaCO2, partial pressure of arterial carbon dioxide; FiO2, fraction of inspired oxygen; PaO2/FiO2, oxygenation index; A-aDO2, alveolar-arterial oxygen difference; WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase; PCT, procalcitonin; NAC, N-acetylcysteine; ROC, receiver operating characteristic; HR, hazard ratio; OR, odd ratio.
Data Sharing Statement
All data and materials are available from the corresponding author upon reasonable request.
Ethical Approval
The study was approved by the ethics committee of Tianjin Chest Hospital (2018KY-020-01).
Consent
Informed consent was obtained from all individual participants included in the study.
Funding
This study was supported by Tianjin Medical and Health Science and Technique Project, China (KJ20224), Tianjin Key Medical Discipline (Specialty) Construction Project, China (TJYXZDXK-049A), and Tianjin Chest Hospital Fund, China (2018XKZ32).
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi:10.1164/rccm.2009-040GL
2. Raghu G, Remy-Jardin M, Myers JL, et al.; American Thoracic Society ERSJRS, Latin American Thoracic S. Diagnosis of idiopathic pulmonary fibrosis. An Official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. doi:10.1164/rccm.201807-1255ST
3. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205:e18–e47. doi:10.1164/rccm.202202-0399ST
4. Raghu G, Rochwerg B, Zhang Y, et al.; European Respiratory s, Japanese Respiratory S, Latin American Thoracic A. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–e19. doi:10.1164/rccm.201506-1063ST
5. King TE, Albera C, Bradford WZ, et al. All-cause mortality rate in patients with idiopathic pulmonary fibrosis. Implications for the design and execution of clinical trials. Am J Respir Crit Care Med. 2014;189:825–831. doi:10.1164/rccm.201311-1951OC
6. Ruaro B, Pozzan R, Confalonieri P, et al. Gastroesophageal reflux disease in idiopathic pulmonary fibrosis: viewer or actor? To treat or not to treat? Pharmaceuticals. 2022;15:1033. doi:10.3390/ph15081033
7. Ma H, Wu X, Li Y, Xia Y. Research progress in the molecular mechanisms, therapeutic targets, and drug development of idiopathic pulmonary fibrosis. Front Pharmacol. 2022;13:963054. doi:10.3389/fphar.2022.963054
8. Khanna D, Tashkin DP, Denton CP, Renzoni EA, Desai SR, Varga J. Etiology, risk factors, and biomarkers in systemic sclerosis with interstitial lung disease. Am J Respir Crit Care Med. 2020;201:650–660. doi:10.1164/rccm.201903-0563CI
9. Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi:10.1164/rccm.201006-0894CI
10. Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med. 2014;190:773–779. doi:10.1164/rccm.201403-0566OC
11. Ryerson CJ, Cottin V, Brown KK, Collard HR. Acute exacerbation of idiopathic pulmonary fibrosis: shifting the paradigm. Eur Respir J. 2015;46:512–520. doi:10.1183/13993003.00419-2015
12. Herazo-Maya JD, Sun J, Molyneaux PL, et al. Validation of a 52-gene risk profile for outcome prediction in patients with idiopathic pulmonary fibrosis: an international, multicentre, cohort study. Lancet Respir med. 2017;5:857–868. doi:10.1016/S2213-2600(17)30349-1
13. Jenkins RG, Simpson JK, Saini G, et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir med. 2015;3:462–472. doi:10.1016/S2213-2600(15)00048-X
14. Korthagen NM, van Moorsel CH, Barlo NP, et al. Serum and BALF YKL-40 levels are predictors of survival in idiopathic pulmonary fibrosis. Respir Med. 2011;105:106–113. doi:10.1016/j.rmed.2010.09.012
15. Kreuter M, Lee JS, Tzouvelekis A, et al. Monocyte count as a prognostic biomarker in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2021;204:74–81. doi:10.1164/rccm.202003-0669OC
16. Maher TM, Oballa E, Simpson JK, et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir med. 2017;5:946–955. doi:10.1016/S2213-2600(17)30430-7
17. Richards TJ, Kaminski N, Baribaud F, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi:10.1164/rccm.201101-0058OC
18. Scott MKD, Quinn K, Li Q, et al. Increased monocyte count as a cellular biomarker for poor outcomes in fibrotic diseases: a retrospective, multicentre cohort study. Lancet Respir med. 2019;7:497–508. doi:10.1016/S2213-2600(18)30508-3
19. Stuart BD, Lee JS, Kozlitina J, et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir med. 2014;2:557–565. doi:10.1016/S2213-2600(14)70124-9
20. Tzouvelekis A, Herazo-Maya JD, Slade M, et al. Validation of the prognostic value of MMP-7 in idiopathic pulmonary fibrosis. Respirology. 2017;22:486–493. doi:10.1111/resp.12920
21. Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi:10.1164/rccm.200810-1534OC
22. Neighbors M, Cabanski CR, Ramalingam TR, et al. Prognostic and predictive biomarkers for patients with idiopathic pulmonary fibrosis treated with pirfenidone: post-hoc assessment of the CAPACITY and ASCEND trials. Lancet Respir med. 2018;6:615–626. doi:10.1016/S2213-2600(18)30185-1
23. Raghu G, Richeldi L, Jagerschmidt A, et al. Idiopathic pulmonary fibrosis: prospective, case-controlled study of natural history and circulating biomarkers. Chest. 2018;154:1359–1370. doi:10.1016/j.chest.2018.08.1083
24. Trombetta AC, Smith V, Gotelli E, et al. Vitamin D deficiency and clinical correlations in systemic sclerosis patients: a retrospective analysis for possible future developments. PLoS One. 2022;12:e0179062. doi:10.1371/journal.pone.0179062
25. Trehan N, Afonso L, Levine DL, Levy PD. Vitamin D deficiency, supplementation, and cardiovascular health. Crit Pathw Cardiol. 2017;13:109–118. doi:10.1097/HPC.0000000000000122
26. Herr C, Greulich T, Koczulla RA, et al. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12:31. doi:10.1186/1465-9921-12-31
27. Pfeffer PE, Hawrylowicz CM. Vitamin D and lung disease. Thorax. 2012;67:1018–1020. doi:10.1136/thoraxjnl-2012-202139
28. Tsujino I, Ushikoshi-Nakayama R, Yamazaki T, Matsumoto N, Saito I. Pulmonary activation of vitamin D3 and preventive effect against interstitial pneumonia. J Clin Biochem Nutr. 2019;65:245–251. doi:10.3164/jcbn.19-48
29. Zhang Z, Yu X, Fang X, et al. Preventive effects of vitamin D treatment on bleomycin-induced pulmonary fibrosis. Sci Rep. 2015;5:17638. doi:10.1038/srep17638
30. Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med. 2016;194(3):265–275. doi:10.1164/rccm.201604-0801CI
31. Arai T, Tachibana K, Sugimoto C, et al. High-dose prednisolone after intravenous methylprednisolone improves prognosis of acute exacerbation in idiopathic interstitial pneumonias. Respirology. 2017;22(7):1363–1370. doi:10.1111/resp.13065
32. Helbich TH, Heinz-Peer G, Eichler I, et al. Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology. 1999;213(2):537–544. doi:10.1148/radiology.213.2.r99nv04537
33. Han MK, Zhou Y, Murray S, et al.; Investigators C. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir med. 2014;2(7):548–556. doi:10.1016/S2213-2600(14)70069-4
34. Takahashi Y, Saito A, Chiba H, et al. Impaired diversity of the lung microbiome predicts progression of idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):34. doi:10.1186/s12931-018-0736-9
35. Molyneaux PL, Cox MJ, Wells AU, et al. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res. 2017;18:29. doi:10.1186/s12931-017-0511-3
36. Weng D, Chen XQ, Qiu H, et al. The role of infection in acute exacerbation of idiopathic pulmonary fibrosis. Mediators Inflamm. 2019;2019:5160694. doi:10.1155/2019/5160694
37. Wootton SC, Kim DS, Kondoh Y, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:1698–1702. doi:10.1164/rccm.201010-1752OC
38. McElroy AN, Invernizzi R, Laskowska JW, et al. Candidate role for toll-like receptor 3 L412F polymorphism and infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2022;205:550–562. doi:10.1164/rccm.202010-3880OC
39. Ding J, Chen Z, Feng K. Procalcitonin-guided antibiotic use in acute exacerbations of idiopathic pulmonary fibrosis. Int J Med Sci. 2013;10:903–907. doi:10.7150/ijms.4972
40. Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132:214–220. doi:10.1378/chest.07-0323
41. Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37:356–363. doi:10.1183/09031936.00159709
42. Hantzidiamantis PJ, Amaro E. Physiology, alveolar to arterial oxygen gradient. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022.
43. Faverio P, Fumagalli A, Conti S, et al. Nutritional assessment in idiopathic pulmonary fibrosis: a prospective multicentre study. ERJ Open Res. 2022;8(1):00443–2021. doi:10.1183/23120541.00443-2021
44. Rinaldi S, Balsillie C, Truchon C, Al-Mubarak A, Mura M, Madill J. Nutrition implications of intrinsic restrictive lung disease. Nutr clin pract. 2022;37:239–255. doi:10.1002/ncp.10849
45. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi:10.1210/jc.2011-0385
46. Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab. 2013;98:973–979. doi:10.1210/jc.2012-2114
47. Tzilas V, Bouros E, Barbayianni I, et al. Vitamin D prevents experimental lung fibrosis and predicts survival in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2019;55:17–24. doi:10.1016/j.pupt.2019.01.003
48. Gao Y, Zhao Q, Qiu X, et al. Vitamin D levels are prognostic factors for connective tissue disease associated interstitial lung disease (CTD-ILD). Aging. 2020;12:4371–4378. doi:10.18632/aging.102890
49. Nguyen HB, Eshete B, Lau KH, Sai A, Villarin M, Baylink D. Serum 1,25-dihydroxyvitamin D: an outcome prognosticator in human sepsis. PLoS One. 2013;8:e64348. doi:10.1371/journal.pone.0064348
50. Sager R, Wirz Y, Amin D, et al. Are admission procalcitonin levels universal mortality predictors across different medical emergency patient populations? Results from the multi-national, prospective, observational TRIAGE study. Clin Chem Lab Med. 2017;55:1873–1880. doi:10.1515/cclm-2017-0144
51. Mohamed S, Abdelhaffez A, Abd El-Aziz N. Serum procalcitonin in patients with combined Lung Cancer and Idiopathic Pulmonary Fibrosis (LC-IPF). Cureus. 2020;12:e9507. doi:10.7759/cureus.9507
52. Caffarelli C, Gonnelli S, Tomai Pitinca MD, et al. Idiopathic pulmonary fibrosis a rare disease with severe bone fragility. Intern Emerg Med. 2016;11:1087–1094. doi:10.1007/s11739-016-1501-z
53. Fischer KD, Agrawal DK. Vitamin D regulating TGF-β induced epithelial-mesenchymal transition. Respir Res. 2014;15:146. doi:10.1186/s12931-014-0146-6
54. Ramirez AM, Wongtrakool C, Welch T, Steinmeyer A, Zügel U, Roman J. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J Steroid Biochem Mol Biol. 2010;118:142–150. doi:10.1016/j.jsbmb.2009.11.004
55. Tan ZX, Chen YH, Xu S, et al. Calcitriol inhibits bleomycin-induced early pulmonary inflammatory response and epithelial-mesenchymal transition in mice. Toxicol Lett. 2016;240:161–171. doi:10.1016/j.toxlet.2015.10.022
56. Xue L, Li B, Zhang Z. 1α,25-dihydroxyvitamin D3 attenuates TGF-β-induced pro-fibrotic effects in human lung epithelial cells through inhibition of epithelial-mesenchymal transition. Nutrients. 2017;6:980.
57. Isik S, Ozuguz U, Tutuncu YA, et al. Serum transforming growth factor-beta levels in patients with vitamin D deficiency. Eur J Intern Med. 2012;23:93–97. doi:10.1016/j.ejim.2011.09.017
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



