Back to Journals » OncoTargets and Therapy » Volume 9
The role of semaphorin 4D as a potential biomarker for antiangiogenic therapy in colorectal cancer
Authors Ding X, Qiu L, Zhang L, Xi J, Li D, Huang X, Zhao Y, Wang X, Sun Q
Received 22 October 2015
Accepted for publication 26 January 2016
Published 8 March 2016 Volume 2016:9 Pages 1189—1204
DOI https://doi.org/10.2147/OTT.S98906
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Faris Farassati
Xiaojie Ding,1,2,* Lijuan Qiu,1,2,* Lijuan Zhang,3 Juemin Xi,1,2 Duo Li,1,2 Xinwei Huang,1,2 Yujiao Zhao,1,2 Xiaodang Wang,1,2 Qiangming Sun1,2
1Institute of Medical Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, 2Molecular Epidemiology Joint Laboratory, Yunnan Key Laboratory of Vaccine Research & Development on Severe Infectious Diseases, 3Department of Pathology, The Third Affiliated Hospital of Kunming Medical University (Yunnan Provincial Tumor Hospital), Kunming, People’s Republic of China
*These authors contributed equally to this work
Background: Semaphorin 4D (Sema4D) belongs to the class IV semaphorins, and accumulating evidence has indicated that its elevated level may be one strategy by which tumors evade current antiangiogenic therapies. The biological roles of Sema4D in colorectal cancer (CRC), however, remain largely undefined. This study was designed to investigate the effects of Sema4D on tumor angiogenesis and growth in CRC, especially in different vascular endothelial growth factor (VEGF) backgrounds.
Methods: The expression of Sema4D in human CRC was evaluated by immunohistochemical analysis of tumors and their matching normal control tissues. The expression level of Sema4D and VEGF was investigated in different CRC cell lines. To evaluate the contributions of Sema4D to tumor-induced angiogenesis, two CRC cell lines with opposite VEGF backgrounds were infected with lentiviruses expressing Sema4D or Sema4D short hairpin RNA, followed by in vitro migration and in vivo tumor angiogenic assays.
Results: Immunohistochemical analysis of human CRC revealed high levels of Sema4D in a cell surface pattern. In all, 84.85% of CRC samples analyzed exhibited moderate to strong Sema4D expression. The positive ratios of Sema4D staining for well, moderately, and poorly differentiated cancers were 71.43%, 96.67%, and 77.27%, respectively. Sema4D is highly expressed in five different CRC cell lines, while VEGF expression level varies among these cell lines. HCT-116 showed the lowest VEGF level, while Caco-2 showed the maximum VEGF level. In vitro migration results show that regardless of cell type and VEGF background, Sema4D showed an enhanced in vitro proangiogenic effect to induce the migration of human umbilical vein endothelial cells. Finally, in vivo tumor angiogenic assays demonstrated that Sema4D alone can elicit a significant angiogenic response to promote tumor growth independently of VEGF.
Conclusion: Targeting Sema4D might serve as a parallel option for antiangiogenic therapy for CRC, particularly when traditional anti-VEGF therapies fail or tumors develop resistance to strategies targeting a single angiogenic signaling pathway.
Keywords: semaphorin 4D, VEGF, colorectal cancer, angiogenesis, migration, xenografts
Introduction
Antiangiogenic agents have become an attractive option for cancer therapy. These agents mostly target vascular endothelial growth factor (VEGF) and its receptor (VEGFR) in combination with chemotherapy for the treatment of cancer. However, in both preclinical and clinical settings, the benefits are at best transitory and are followed by a restoration of tumor growth and progression. The resistance of tumors to antiangiogenic therapies is becoming increasingly relevant. In fact, a distinction should be made between anti-VEGF/VEGFR and antiangiogenic therapy. The unsatisfactory results from targeting the former should not be interpreted as a negation of the latter. It has already been noticed that other angiogenic factors can be upregulated in tumors following or even during anti-VEGF therapies, allowing them to evade or overcome angiogenic inhibition that targets a single factor or only one signaling pathway.1,2 Emerging evidence has shown that semaphorin 4D (Sema4D) may be one of these proangiogenic factors elaborated by cells in response to VEGF loss.3
Semaphorins and their functional receptors, the plexins, were initially described as axon guidance factors participating in directing growth cones of axons to their right position during the development of the nervous system. In recent years, semaphorins have been found to function outside the nervous system serving as regulators of cell proliferation and migration and activators of lymphocytes.4,5 Semaphorins and plexins are expressed in a variety of tissues besides the nervous system.6 The neuronal and vascular systems share some common guidance signals.7 Interestingly, during embryonic development, there is a close space–time relationship between growing neurons and developing blood vessels.8 This suggests an intriguing link in the signal transduction circuits between those controlling axon guidance and those involved in angiogenesis. In addition to the already well-studied functions and signaling pathways, a variety of semaphorins and plexins are known to take part in the physiological and pathological development of blood vessels.9 Sema4D, a member of the class IV semaphorins, transduces a signal by directly binding to its high affinity receptor Plexin-B1. Emerging evidence has indicated that it also possesses a previously unrecognized function: a compensatory angiogenic factor which could promote tumor growth and angiogenesis.6,10,11 Sema4D is overexpressed by some malignancies and plays a role in tumor-induced angiogenesis similar to VEGF, while the Sema4D–RhoA signaling axis could recruit pericytes and regulate vascular permeability through endothelial production of PDGF-B and ANGPTL4, whereas VEGF lacks these effects.12 In this study, we investigated the influence of Sema4D on tumor growth and vascularity in colorectal carcinoma (CRC), especially in different VEGF backgrounds. We propose that targeting these proteins might provide complementary or parallel options for antiangiogenic therapy of CRC.
Materials and methods
Cell culture
Normal human colon mucosal epithelial cell line NCM460, packaging cell 293T, human umbilical vein endothelial cells (HUVEC), and colorectal cancer (CRC) cell lines, including HCT-116, Caco-2, LoVo, HT-29, COLO-205, were kept by the Institute of Medical Biology, Chinese Academy of Medical Science and Peking Union Medical College. All cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), 2 mM glutamine, 100 U penicillin/0.1 mg/mL streptomycin, and cultured in 5% CO2, 37°C incubator.
Human tissues
CRC samples were obtained from the Third Affiliated Hospital of Kunming Medical University in Southwest China from 2011 to 2014. All samples were obtained from paraffin-embedded samples. Written consent was obtained from each participant patients. The study protocol was approved by the institutional ethics committee (Institute of Medical Biology, Chinese Academy of Medical Sciences), and complied with the 1974 Helsinki declaration and its later amendments or comparable ethical standards. In addition, we performed the analysis of human tissue histological slides according to the protocol of Basile et al.13
Lentiviral vector system for gene transfer
Gateway lentiviral vector system was a generous gift from Professor John R Basile (Department of Oncology and Diagnostic Sciences, University of Maryland Dental School, Baltimore, MD, USA). The following lentiviral vectors were included: lentivirus coding for Sema4D short hairpin RNA (shRNA) (oligonucleotides based on the following sequence worked best to knock down Sema4D 5′-GGCCTGAGGACCTTGCAGAAGA-3′), which was directed at knocking down the expression of Sema4D, and a Sema4D super-expressor coding lentivirus (Sema4D gene Accession: NM_006378.3, Forward primer: 5′-GCGGATCCATGAGGATGTGCACCCC-3′, reverse primer: 5′-TGCGCGGCCGCTCAGTCTCCATCTGCG-3′), which helped to increase expression of Sema4D. Control lentiviruses were manufactured with enhanced green fluorescent protein expression vectors. Lentiviral vectors were packed and infections performed as previously reported.14,15
Enzyme-linked immunosorbent assay
Culture medium of CRC cells was collected for detecting Sema4D and VEGF by an enzyme-linked immunosorbent assay (ELISA). Concentration of VEGF was detected by a VEGF-165 ELISA kit (YAD-R0168, Reanta, Beijing, China) according to the manufacturer’s instructions. Double antibody sandwich method was used for the detection of Sema4D. Briefly, rabbit anti-Sema4D (sc20928, Santa Cruz Biotechnology Inc., Dallas, TX, USA) antibody was diluted to 0.4 μg/mL in a coating buffer (0.05 M carbonate–bicarbonate buffer, pH 9.6), and used as the capture antibody to coat microtiter plates at 4°C overnight. The wells was washed three times with Phosphate Buffer Solution + 0.5% Tween-20 (PBST, pH 7.2), then coated with 5% skim milk in PBST. Then, the plate was ready for detecting Sema4D. The plate was incubated at 37°C for 1 hour with 100 μL cell culture medium. After washing, mouse anti-Sema4D (610670, BD Biosciences, San Jose, CA, USA,) antibody was added to each well. The wells were washed again, and incubated with horseradish peroxidase (HRP)-conjugated goat antimouse IgG, then detected by TMB substrate solution (Thermo Fisher Scientific), 100 μL per well. Color reaction was stopped by adding 100 μL stop solution to each well followed by reading absorbance at 450 nm.
Immunoblot analysis
Cells infected with the indicated lentiviral constructs were lysed in RIPA buffer (Solarbio, Beijing, China) with protease inhibitors (0.5 mM phenylmethylsulfonyl fluoride, Sigma-Aldrich Inc., Milwaukee, Wisconsin, USA) for 20 minutes at 4°C. After centrifugation, protein concentrations were measured using the BCA Protein Assay Kit (TIANGEN Biotech, Beijing, China). An amount of 50 μg protein from each sample was subjected to SDS-PAGE and transferred onto a polyvinylidene fluoride (PVDF) membrane (Immobilon P, Merck Millipore Corporation, Billerica, MA, USA). The membrane was cut into sections based on protein markers, and then probed for Sema4D, Plexin-B1, and VEGF, with α-tubulin selected as a loading control. The antibodies used were as follows: mouse anti-Sema4D (610670, BD Biosciences); mouse anti-Plexin-B1 (sc28372, Santa Cruz A8); mouse anti-VEGF (sc74584, Santa Cruz Biotechnology Inc., Dallas, TX, USA); and VEGFR2 (9698, Cell Signaling Technology, Danvers, MA, USA), rabbit anti-α-tubulin (Epitomics, Inc., Burlingame, CA, USA). The membranes were then incubated with the appropriate secondary antibodies (goat anti-mouse IgG [Heavy and Light Chain] peroxidase labeled [KPL], goat anti-rabbit IgG [Heavy and Light Chain] peroxidase labeled, KPL, Gaithersburg, MD, USA). Proteins were detected using Immobilon Western Chemiluminescent HRP Substrate (Millipore).
Tubulogenesis assays
Soluble Sema4D-expressing plasmid (pSeqTag2B S4D) was a generous gift from Dr John R Basile of the University of Maryland Dental School. This plasmid was transfected into 293T cells. The culture medium was collected after 48 hours and purified with BeaverBeads™ His-tag Protein Purification Kit (Beaver Nano-Technologies, Su Zhou, People’s Republic of China) according to the manufacturer’s instructions. In all, 2×104 HUVEC were subcultured on 96-well plates coated with 50 μL BD Matrigel Basement Membrane Matrix (BD Biosciences, Bedford, MA, USA) per well and cultured in 5% CO2, 37°C incubator with serum-free media containing the indicated factors (Sema4D) or not. Cells were then photographed at 2.5 hours.
In vitro migration assay
Serum-free DMEM was first conditioned by cells infected with the indicated lentivirus, and then placed in the bottom wells of a Boyden chamber to serve as chemoattractants. DMEM with 0.1% bovine serum albumin (BSA) was used as a negative control, and DMEM with 10% fetal bovine serum was used as a positive control. Serum-free DMEM containing 1×106/mL HUVEC cells were added to the top chamber. The two chambers were separated by polycarbonate membranes with a pore size of 8 microns (cat#: PRB8, Neuro Probe, Inc., Gaithersburg, MD, USA) coated with 10 mg/mL fibronectin (cat #1918-FN, R&D Systems, Inc, Minneapolis, MN, USA). The migration assay was performed as previously described.16 After 7 hours, the chamber was disassembled and the membrane was fixed, hematoxylin and eosin stained, and then scanned and analyzed with NIH image software, with cell migration expressed as pixel intensity. Each experiment was performed six times and the average and standard deviation were calculated. Cell migration was expressed as membrane-staining intensity relative to the negative control.
Xenografts of CRC cell lines
The two CRC cell lines HCT-116 and Caco-2, uninfected or infected with indicated lentivirus ex vivo, were injected subcutaneously into nude mice. Each injection contained 2×106 cells resuspended in 100 μL of serum-free DMEM with an equal volume of liquid Matrigel Basement Membrane Matrix (BD Biosciences, Bedford, MA, USA). Tumor size was measured using digital calipers every 4 days. The tumor volume was calculated as 0.512× width2 × length (mm3). The weight of the tumor was determined after dissection at the end of the experiment, then tumors were harvested for further examination.13 All animal studies were approved in accordance with the guidelines issued by the Ethical Committee of Institute of Medical Biology, Chinese Academy of Medical Sciences.
Immunohistochemistry
Parts of tumor tissues were fixed by formalin, deparaffinized, and rehydrated. Antigen retrieval was performed in sodium citrate (10 mM, pH 6.0) in a microwave oven for 20 minutes. The slides were then rinsed with PBS, blocked in 1% BSA, and incubated for 1 hour at 37°C with primary antibodies diluted in 1% BSA in PBS (mouse anti-Sema4D,1:100 dilution, BD Biosciences). The slides were then washed in PBS and incubated with secondary antibody (goat antimouse IgG [H + L] antibody, peroxidase labeled, KPL) for 30 minutes at 37°C. The slides were developed in enhanced HRP-DAB Kit (PA110, TIANGEN Biotech), counterstained with hematoxylin, dehydrated, and mounted. Images were taken with a Nikon Eclipse TE2000-U microscope system.
Judgment about the histological slides was determined by two independent observers, including an experienced doctor. Percentage of stained tumor cells and staining intensity were taken into consideration for estimating whether Sema4D was expressed. The staining intensity was rated as follows: 1 point (weak intensity), 2 points (moderate intensity), and 3 points (strong intensity). The percentages of stained tumor cells were marked as follows: 2 points (11%–50% positive tumor cells), 3 points (51%–80% positive cells), and 4 points (more than 81% positive cells). The two parts of the scores were added up for the final result for judging Sema4D expression. A score lower than 3 points was negative, and any scores 3 points or higher were judged to be positive.
Immunofluorescence
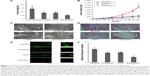
Tumor tissues were embedded in OCT and cut into 8 μm-thick frozen sections on plus adhesion glass slides, fixed in 4% neutral buffered formalin, air-dried, and stored at −80°C. Then the sections were thawed, rehydrated, blocked in 1% BSA, and incubated for 1 hour at 37°C with primary antibodies (rat antimouse CD31, 550274, BD Pharmingen San Diego, CA, USA) diluted in 1% BSA in PBS (1:100 dilution). After washing with PBS, the slides were incubated with secondary antibody (Dylight 488 goat anti-rat IgG [Heavy and Light Chain], 112-487-003, Jackson Immunoresearch, Lancaster, PA, USA) for 1 hour at 37°C. Finally, the slides were mounted and examined with a Nikon Eclipse TE2000-U microscope system. The results of measurement for vascular content from these tumors were determined by the average vessel density in 10 CD31-stained sections from each group. For the selection of 10 sections of each group, at least one section was selected from each tumor, and the others sections were chosen randomly within same group. Vessel density for every section was analyzed by NIH image software. The fluorescence intensity was quantified in a bar graph, relative to the control virus infected group.
Statistical analysis
Independent Student’s t-tests were used to determine if data of two groups were significantly different from each other, and P-values were calculated: *P<0.05; **P<0.01. Sample scores of immunohistochemical analysis for human tissues were analyzed by the Kruskal–Wallis test.
Results
Sema4D is highly expressed in human colorectal carcinomas
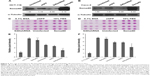
To determine whether Sema4D was expressed in human CRC, 66 samples were collected from 2011 to 2014, including 22 well differentiated, 30 moderately differentiated, and 14 poorly differentiated samples. All samples were obtained from paraffin-embedded sample arrays. Immunohistochemical analysis of tumor tissue and their matching normal control tissues was performed. Normal control tissues exhibited negative Sema4D expression, while colorectal carcinoma tissues demonstrated abundant Sema4D staining. Meanwhile, Sema4D showed cell surface-specific staining patterns of expression in most cases. In all, 84.85% of CRC samples analyzed exhibited moderate to strong Sema4D expression. The positive ratios of Sema4D staining for well, moderately, and poorly differentiated cancer were 77.27%, 96.67%, and 71.43%, respectively. These results are expressed graphically in Figure 1. Sample scores were analyzed by the Kruskal–Wallis test, with the positive ratio of Sema4D expression in moderately differentiated cancer obviously higher compared with all other groups (Figure 1). Meanwhile, we compared Sema4D expression levels between cancer nodules (+/−) and lymph node metastasis (+/−) group, and there was no significant difference between these two groups (Table 1). Our results suggest that Sema4D expression is a common feature in human colorectal carcinoma.
Sema4D is highly expressed in CRC cell lines, while VEGF level varies among these cell lines
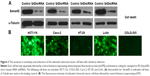
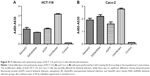
One clinical study has shown that higher Sema4D expression seems to be associated with poorer overall and disease-free survival.6 Tumor tissues from prostate demonstrate both significantly high-level and high-frequency expression of Sema4D.13,17 Sema4D is also expressed by some cancer cell lines. To investigate the expression level of different CRC cell lines, HCT-116, COLO-205, Caco-2, HT-29, and LoVo cell lines were selected to test for expression by immunoblot analysis. All CRC cell lines tested showed higher Sema4D protein levels than the normal human colon mucosal epithelial cell line NCM460 (Figure 2A). Plexin-B1, the receptor of Sema4D, was also highly expressed in CRC cell lines (Figure 2A), suggesting a potential selective advantage of this particular molecule. However, when compared with the normal colon mucosal epithelial cell line NCM460, the majority of CRC cell lines had higher VEGF protein levels, while HCT-116 had a lower VEGF level than NCM460, making it distinct from other CRC cell lines (Figure 2B). COLO-205 and LoVo cells showed a lower VEGFR2 level compared with the normal human colon mucosal epithelial cell line NCM460 and other CRC cell lines (Figure 2C). Meanwhile, the secreted Sema4D and VEGF were also detected by ELISA. All CRC cell lines tested showed relatively higher secreted Sema4D protein levels than the normal human colon mucosal epithelial cell line NCM460. However, the secreted VEGF protein level varied among indicated CRC cell lines (Figure 2D and E).
CRC cell line HCT-116 and Caco-2 were selected for in vitro migration assay and in vivo tumor genesis
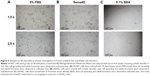
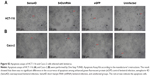
VEGF background varies among cancer cell lines. Meanwhile, the sensitivity of cells to lentivirus infection was also different.18–20 To select a suitable CRC cell line for our experiment, HCT-116, COLO-205, Caco-2, HT-29, and LoVo cell lines were infected by either GFP-expressing or Sema4D shRNA lentivirus. The result showed that HCT-116, Caco-2, and LoVo had a high percentage of GFP-positive cells after GFP-expressing lentivirus infection. Meanwhile, HCT-116, Caco-2, and LoVo also had a quite obvious RNAi effect for Sema4D expression after Sema4D shRNA lentivirus infection (Figure 3A). Meanwhile, these three cell lines had no apparent differences in cell morphology and vitality after viral infection. However, the other two CRC cell lines HT-29 and COLO-205 did not respond well to the same treatment since a large number of cells died after infection within 24 hours (Figure 3B).
HCT-116 and Caco-2 showed relatively higher Sema4D levels among the five CRC cell lines that we tested. On the other hand, HCT-116 showed the lowest VEGF level while Caco-2 showed the highest VEGF level among the five CRC cell lines, providing a distinctive VEGF background for detecting the potential phenotypic variance.
Based on the sensitivity of the cells to lentivirus infection and VEGF background, HCT-116 and Caco-2 were selected for the subsequent experiments to investigate the effects of tumor-produced Sema4D on angiogenesis. Before starting the following experiments, we performed a cell proliferation and cytotoxicity assay by Cell Counting Kit-8 (Beyotime, Shanghai, People’s Republic of China), and an apoptosis assay by One Step TUNEL Apoptosis Assay Kit (Beyotime). We observed that there was no large difference in the occurrence of apoptosis in the four treatment groups. However, we observed that lentiviral infection does affect the proliferation of cells, while there was no significant difference among enhanced green fluorescent protein control, Sema4D overexpressed, and Sema4D shRNA groups (Figures S1 and S2).
Sema4D induces tubulogenesis in endothelial cells
Cells cultured on BD Matrigel Basement Membrane Matrix can grow toward each other and form small tube-like structures that mimic blood vessel formation, a process called tubulogenesis.21 HUVEC cells were grown on 96-well plates coated with BD Matrigel Basement Membrane Matrix and cultured with serum-free media containing soluble Sema4D or not. Cell spreading and tubule formation were observed in 2.5 hours in soluble Sema4D treated HUVEC cells and 5% fetal bovine serum treated cells, but not in cells incubated with serum-free media. The result indicated that Sema4D could induce a proangiogenic response in HUVEC cells (Figure 4).
Production of Sema4D by CRC cell lines induces chemotaxis in endothelial cells
Tumor cells spontaneously shed Sema4D into the surrounding environment and exert long-range biological effects through diffusion to its targets.22 In order to investigate the effects of tumor-produced Sema4D on angiogenesis in vitro, we chose the CRC cell lines HCT-116 and Caco-2, with each designed to have four groups: uninfected, control virus infected, Sema4D shRNA-coding lentiviruses, and Sema4D-expressing lentiviruses infected groups. Infected cells exhibited reduced or enhanced levels of Sema4D protein compared with controls (Figure 5A and B).
When the conditioned media from these cells were used as chemoattractants for HUVEC in a migration assay, we observed that regardless of cell type (HCT-116 or Caco-2), media conditioned by CRC cells with downregulated Sema4D induced less endothelial cell migration when compared with uninfected and control virus infected groups. In contrast, CRC cells overexpressing Sema4D showed an enhanced ability to induce the migration of endothelial cells. No apparent distinction was found between uninfected and control infected groups, which had the same level of Sema4D (Figure 5C–F). Taken together, these results indicate that tumor-derived Sema4D can act as a potent chemoattractant in promoting HUVEC migration and silencing this factor could exert an inhibition on the in vitro proangiogenic effect. Compared with the observation in the four HCT-116 cell groups, relative HUVEC migration rate was slightly higher than that in all four Caco-2 cell groups. This may be attributed to the high VEGF background of the Caco-2 cell line since VEGF is definitely an inducer of angiogenesis.23–25 However, the result showed that Sema4D was able to exert its powerful proangiogenic effect over this VEGF background. There was still an apparent distinction between the four Caco-2 groups. The effect of Sema4D has not been masked under a high VEGF background.
Sema4D contributes to tumor growth and vascularity in HCT-116 cells with low VEGF background
Since we observed a strongly proangiogenic response to Sema4D in vitro, we decided to examine whether Sema4D can augment tumor growth and vascularity in vivo. HCT-116 with low VEGF background was chosen, and again divided into four groups: uninfected and control-infected groups and Sema4D overexpressing and Sema4D shRNA groups, which were infected with the appropriate lentivirus and exhibited elevated or downregulated Sema4D protein levels compared with controls, respectively. Tumors derived from HCT-116 cells infected with Sema4D shRNA lentivirus weighed about ten times lighter than those of controls, while tumors composed of cells infected with Sema4D overexpressing lentivirus weighed heavier. Tumors that grew from uninfected HCT-116 cells were the largest among the four groups. It is possible that lentivirus transduction itself could influence HCT-116 cell long-term or in vivo vitality to a certain extent.18,19 This has been confirmed in our previous research. Tumor volumes also had a similar pattern (Figure 6A and B). Blood vessel density and structure were quite characteristic in the four groups (Figure 6C and D). In tumors from HCT-116 cells infected by Sema4D shRNA lentiviruses, the growth of blood vessels was attenuated and consisted merely of scattered tiny vessels inside the tumors with no large vessels, indicating that Sema4D downregulation in this case nearly abolished tumor formation and vascularity. In contrast, tumors composed of cells infected with Sema4D-expressing lentiviruses were slightly larger, with a much higher vessel density than the control group. Many very large, irregular blood vessels could be found spreading and winding all through the tumor tissue in a high density. The actual function and efficiency of this blood supply network are unclear. It has long been known that tumor blood vessels are morphologically abnormal, chaotic tortuous networks, lacking a normal hierarchical arrangement.26 Although tumors from the uninfected group reached the highest parameters among the four HCT-116 cell groups, vascular density only exceeded the Sema4D overexpressing groups by ~10%. The control and uninfected groups shared some similarities in vessel distribution pattern. Most blood vessels were evenly spaced and very similar in size, but lack large and irregular blood vessels inside the tumor tissue (Figure 6C–F).
Sema4D promotes tumor growth and vascularity in Caco-2 cells with high VEGF background
To examine whether Sema4D can augment tumor growth and vascularity in Caco-2 cells with high VEGF background, Caco-2 cells were divided into four groups again: uninfected, control-infected, Sema4D overexpressing, and Sema4D shRNA lentivirus infected groups. The results showed that Caco-2 cells grew into a fluid containing polycystic cystadenocarcinomas (Figure 7). Tumors comprised of Caco-2 cells infected with Sema4D shRNA-encoding lentiviruses were slightly smaller than controls. Tumors from the uninfected Caco-2 group grew almost to the same average size as controls, whereas tumors composed of cells overexpressing Sema4D reached the largest size during the same period of time. A very similar pattern could also be found in tumor weight (Figure 7A and B).
In addition, the internal structure of the Caco-2 cystadenocarcinomas was quite distinctive (Figure 7C and D). Tumors comprised of cells infected with lentiviruses coding for Sema4D shRNA had several large cysts filled with intraluminal fluid, while small cysts or microcysts in this group were rare. The wall was made mainly by a single layer of epithelial cells lining the inner side of cysts. The control infected and uninfected groups shared very similar internal structure, as both were composed of multiple, fluid-filled, round, or oval cysts with smooth margins. There were several large cysts but small cysts were also found around them. In contrast, tumors from cells overexpressing Sema4D had the maximum amount of small cysts crowded between or around the larger ones, almost covering them. In this group, the internal cell density was the greatest. Tumor cells were densely compacted in multiple layers, almost filling entire cysts, with fluid containing areas being quite rare. Sema4D shRNA-coding lentivirus dramatically reduced blood vessel content of tumors when compared with control and uninfected groups. No apparent difference could be identified between the uninfected and the control infected groups. However, overexpression of Sema4D exhibited a great effect in increasing tumor vascularity, with large vessels in the cyst walls and internal spaces crowded with dense tumor cells. These results are shown graphically in Figure 7E and F.
Discussion
Semaphorin–plexin interactions have already been implicated in a variety of responses, including epithelial cell contact modification and branching morphogenesis, regulation of pathological angiogenesis, tissue organization during development, and immune responses.4,5
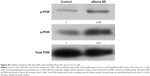
Our observations suggest that overexpression of Sema4D may provide tumors with the potential to support much denser cell populations, thus limiting the space for large fluid-filled cysts, and favoring the formation of much smaller fluid-filled cysts. Sema4D downregulation in these populations made the same cell lines unable to form tumors with dense cell populations. This may be explained by higher Sema4D levels enhancing angiogenesis through an increased blood supply, providing more resources for the dividing cells within that space. Tumors with lower Sema4D were less capable of driving neoangiogenesis in the tumor microenvironment, with an insufficient blood supply failing to support a large amount of cells in a limited amount of space. However, it is also possible that Sema4D/Plexin-B1 signaling might contribute to the proliferation and invasiveness of tumor cells directly, through coupling with the tyrosine kinase receptor Met, which functions as a receptor for hepatocyte growth factor.27 Sema4D–Plexin-B1 binding has been found to induce Met phosphorylation and result in promotion of tumor invasion and metastasis.28 Evidence from the literature indicates that tumors overexpressing Sema4D are highly aggressive, very difficult to control during therapy, and often show poor prognosis, even without a special emphasis on angiogenesis. That may help to explain why Plexin-B1 is also highly expressed among CRC cell lines, as activation of this signaling pathway might provide tumor cells with a certain selective advantage (Figure S3).
The study also suggested that although Sema4D makes its unique contribution to tumor growth rate and blood vessel density, the extent of such influence was undoubtedly affected by other growth factors and angiogenic-inducing agents, especially the endogenous VEGF level of that cell line. In both CRC cell lines infected by Sema4D shRNA lentivirus, the Caco-2 group did not show as much of a dramatic reduction in tumor size and weight as seen in the HCT-116 group. There was a 90% decrease in the HCT-116 group but only a 40% decrease in the Caco-2 group. We can at least partly attribute these results to the fact that the Caco-2 group had a relatively higher level of endogenous VEGF than HCT-116. This factor could well support the formation of blood supply system at a basic level.
Drugs or antibodies targeting VEGF or its receptors may successfully inhibit the growth of many rodent tumors.29 However, these strategies have been proved to be less successful in human cancer patients,30–32 and on average extend patient survival only for a few months.2,33 In fact, increasing evidence has suggested that many other angiogenic factors can be upregulated in tumors following therapy, which targets the VEGF–VEGFR axis only, giving tumors more than one path to overcome inhibition of angiogenesis. Only a subset of tumor blood vessels require VEGF for their development or maintenance, whereas others may have initial or acquired VEGF independence.1,2,23,34 Combination therapies targeting multiple angiogenic agents might be one consideration leading to more effective treatment.
Fast growing tumor cells could easily overwhelm the ability of the existing vasculature to meet their high demands for metabolism and proliferation. Antiangiogenic interventions could decrease or damage the tumor blood supply, driving cells toward necrosis. Cancer cells may have more than one strategy to solve this crisis, but one crafty choice could involve expressing Sema4D at higher levels in order to drive neoangiogenesis and generate a tumor blood supply. Other investigations have shown that anti-VEGF therapy could increase Sema4D levels in tumor tissues as well as in cell culture, thus providing evidence that upregulation of Sema4D could possibly play a significant role in tumor angiogenesis and vascularity. On one hand, Sema4D may cooperate with VEGF to induce angiogenesis and contribute to tumor progression. On the other, Sema4D can exert significant angiogenic effects when it functions alone.9,13,22,35 This can help to explain what might possibly happen during anti-VEGF treatment. Thus, targeting Sema4D could be an option for some cancer patients when anti-VEGF treatment has failed, or combining this approach with anti-VEGF therapy might exert a more powerful antiangiogenic effect. Anti-Sema4D therapy is also predicted to be safe, as Sema4D/Plexin-B1 signaling is not strictly required during normal vascular development.35,36
Conclusion
In conclusion, tumors may have more than one strategy to evade or work against current antiangiogenic medicines, one of which could depend on the proangiogenic function of Sema4D. The results of this study suggest that Sema4D can play a significant role in tumor angiogenesis. Interference or blocking of the Sema4D signaling pathway could be a valuable companion to anti-VEGF or other antiangiogenic therapies. In the future, if we are to develop more effective and personalized antiangiogenic therapies, we need to further decipher the complexities of tumor vascularization.
Acknowledgments
We would like to thank Dr John R Basile of the University of Maryland, Dental School, Department of Oncology and Diagnostic Sciences for providing plasmids, technical assistance, and critical reading and reviewing of our manuscript. We also would like to thank Junying Chen and Yue Pan from the Institute of Medical Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, for collection of the sample and technical assistance. This research was funded by the National Natural Science Foundation of China (grant no 81171946), the National “Major New Drug Discovery” Technology Major Projects, Ministry of Science and Technology of China (grant no 2012ZX09104-302), the Natural Science Foundation of Yunnan Province (grant nos 2009ZC187M, 2011CA016 and 2012FB188), and the Special Research Fund for the Doctoral Program of Higher Education of China (grant no 20111106120055).
Disclosure
The authors report no conflicts of interest in this work.
References
Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14(20):6371–6375. | ||
Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8(4):299–309. | ||
Zhou H, Binmadi NO, Yang YH, Proia P, Basile JR. Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis. 2012;15(3):391–407. | ||
Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75(7):1389–1399. | ||
Roney K, Holl E, Ting J. Immune plexins and semaphorins: old proteins, new immune functions. Protein Cell. 2013;4(1):17–26. | ||
Ch’ng E, Tomita Y, Zhang B, et al. Prognostic significance of CD100 expression in soft tissue sarcoma. Cancer. 2007;110(1): 164–172. | ||
Klagsbrun M, Eichmann A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev. 2005;16(4–5):535–548. | ||
Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436(7048):193–200. | ||
Torres-Vazquez J, Gitler AD, Fraser SD, et al. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7(1):117–123. | ||
Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64(15):5212–5224. | ||
Sakurai A, Gavard J, Annas-Linhares Y, et al. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 2010;30(12):3086–3098. | ||
Zhou H, Yang YH, Basile JR. The Semaphorin 4D-Plexin-B1-RhoA signaling axis recruits pericytes and regulates vascular permeability through endothelial production of PDGF-B and ANGPTL4. Angiogenesis. 2014;17(1):261–274. | ||
Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci U S A. 2006;103(24):9017–9022. | ||
Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93(21):11382–11388. | ||
Naldini L, Blomer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–267. | ||
Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25(16):6889–6898. | ||
Wong OG, Nitkunan T, Oinuma I, et al. Plexin-B1 mutations in prostate cancer. Proc Natl Acad Sci U S A. 2007;104(48): 19040–19045. | ||
Cao F, Xie X, Gollan T, et al. Comparison of gene-transfer efficiency in human embryonic stem cells. Mol Imaging Biol. 2010;12(1):15–24. | ||
Lafitte M, Rousseau B, Moranvillier I, et al. In vivo gene transfer targeting in pancreatic adenocarcinoma with cell surface antigens. Mol Cancer. 2012;11:81. | ||
Boucher DL, Chen JQ, Cherry SR, Borowsky AD. Establishment of clonal MIN-O transplant lines for molecular imaging via lentiviral transduction & in vitro culture. PLoS One. 2012;7(6):e39350. | ||
Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107(4):1589–1598. | ||
Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem. 2007;282(9):6899–6905. | ||
Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312(5):549–560. | ||
Ferrara N. Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev. 2010;21(1):21–26. | ||
Nagy JA, Vasile E, Feng D, et al. VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb Symp Quant Biol. 2002;67:227–237. | ||
Dudley AC. Tumor endothelial cells. Cold Spring Harb Perspect Med. 2012;2(3):a006536. | ||
Giordano S, Corso S, Conrotto P, et al. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4(9):720–724. | ||
Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23(30):5131–5137. | ||
Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29(6 Suppl 16):10–14. | ||
Ellis LM, Rosen L, Gordon MS. Overview of anti-VEGF therapy and angiogenesis. Part 1: Angiogenesis inhibition in solid tumor malignancies. Clin Adv Hematol Oncol. 2006;4(1):suppl 1–10; quiz 11–12. | ||
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. | ||
Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3(1):24–40. | ||
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. | ||
Crawford Y, Kasman I, Yu L, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15(1):21–34. | ||
Worzfeld T, Swiercz JM, Senturk A, et al. Genetic dissection of plexin signaling in vivo. Proc Natl Acad Sci U S A. 2014;111(6):2194–2199. | ||
Fazzari P, Penachioni J, Gianola S, et al. Plexin-B1 plays a redundant role during mouse development and in tumour angiogenesis. BMC Dev Biol. 2007;7:55. |
Supplementary materials
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.