Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
The Role of Recombinant Secretory Leukocyte Protease Inhibitor to CD163, FGF-2, IL-1 and IL-6 Expression in Skin Wound Healing
Authors Munadziroh E, Putri GA, Ristiana V, Agustantina TH, Nirwana I, Razak FA, Surboyo MDC
Received 1 February 2022
Accepted for publication 1 May 2022
Published 18 May 2022 Volume 2022:15 Pages 903—910
DOI https://doi.org/10.2147/CCID.S358897
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Elly Munadziroh,1 Giovani Anggasta Putri,2 Vera Ristiana,2 Titien Hary Agustantina,1 Intan Nirwana,1 Fathilah Abdul Razak,1,3 Meircurius Dwi Condro Surboyo4
1Department of Dental Materials, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, 60132, Indonesia; 2Bachelor of Dental Sciences, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, 60132, Indonesia; 3Department of Oral and Craniofacial Sciences, Faculty of Dentistry, Universiti Malaya, Kuala Lumpur, 50603, Malaysia; 4Department of Oral Medicine, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, 60132, Indonesia
Correspondence: Elly Munadziroh, Department of Dental Materials, Faculty of Dental Medicine, Universitas Airlangga, Jalan Prof. Dr. Moestopo No. 47, Surabaya, 60132, Indonesia, Tel +6231-5030255, Email [email protected]
Background: The wound healing process can be optimized through the addition of a biomaterial such as recombinant secretory leukocyte protease inhibitor (rSLPI). The SLPI is a non-glycosylated proteomic material that inhibits protease enzymes and has anti-inflammatory properties, thus accelerating wound healing. This study analyzed the administration of rSLPI doses 0.04 cc and 0.06 cc in skin wound healing on the CD163 expression of macrophages and cytokines such as interleukin 1 (IL-1), interleukin 6 (IL-6) and fibroblast growth factor 2 (FGF-2).
Materials and Methods: rSLPI produced from Escherichia coli TOP10 as the cloning host, BL21 (DE3) strains as the expression host and pET30a plasmids were used for the expression system construction. The wound was created on Wistar rat dorsal skin, then rSLPI 0.04 cc and 0.06 cc was administered. In the next four days, the back skin was biopsied and stained by immunohistochemistry to analyze the CD163, FGF-2, IL-1 and IL-6 expression.
Results: The administration of rSLPI increased CD163 and FGF-2 expression dependent on dose (p< 0.05). On the other hand, administration of rSLPI decreased IL-1 and IL-6 expression depending on dose (p < 0.05).
Conclusion: The administration of rSLPI is able to accelerate the wound healing process by increasing the CD163 and FGF-2 expression. The cytokines such as IL-1 and IL-6 decreased depending on rSLPI doses.
Keywords: recombinant secretory leukocyte protease inhibitor, wound healing, skin
Introduction
One of the problems in wounds that are still insurmountable is delayed healing. Delayed healing occurs because of the pathological extent of inflammation.1 It is necessary to be vigilant in order to overcome some possible complications that are caused by healing disorder.2 Wounds are divided into two categories, namely open wounds and closed wounds. Wound healing itself is a normal biological process that occurs in the human body that goes through several phases including hemostatic, inflammation, proliferation and remodeling.3,4 Wound healing is a complex process in which biocellular and biochemical activities occur continuously involving a series of complex reactions and interactions between cells and mediators,5 which aim to restore the integrity of damaged tissue and will begin immediately after the occurrence of tissue damage by going through several stages of mechanism or phase.6
Wound healing is characterized by an increase in the number of macrophages during inflammation phase. The function of macrophages in the wound healing process is to phagocytose and destroy necrotic tissue and foreign particles that enter the tissue.7 There are two macrophage phenotypes, namely M1 and M2.8 These two types of macrophages are distinguished by the cytokines they produce.9 The M2 expresses cluster of differentiation 163 (CD163),10 and produces an anti-inflammatory cytokine.11 The M1 produces pro-inflammatory cytokines such as tumor necrosis tumor (TNF), interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-12 (IL-12) and interleukin-17 (IL-17).12–16 Not only cytokines have an important role, one of the growth factors, namely fibroblast growth factor 2 (FGF-2), is also involved in the wound healing stage and can affect macrophage polarization into M2.17
Wound management has now developed in various ways and has focused on accelerating healing by an applied material. This is intended, because for example in the case of a diabetic wound,13,18 the wound will be difficult to heal and create delayed healing, so biomaterials are needed that are expected to accelerate the wound healing process.19 One of the materials to accelerate wound healing is the amnion membrane. This amnion membrane contains secretory leukocyte protease inhibitor (SLPI).20 Since the physiological concentration of SLPI of the amnion membrane is found in small quantities, thus it is necessary for an engineer to obtain it in large quantities, namely using recombinant with Escherichia coli strain. The entire process is called recombinant secretory leukocyte protease inhibitor (rSLPI). The rSLPI can express and produce SLPI.21 The SLPI itself is able to suppress monocytes, matrix-metallo-proteinase (MMP),20 contain growth factor,22 inhibit the proliferation of fibroblast and collagen to form a scar,23 and inhibit the inflammatory pathways through NF-kB.24 With this component the reepithelization process may occur immediately. Moreover, SLPI also has several functions including inhibiting protease, control of bacterial activity,25 anti-inflammatory,26 antibacterial27 and antivirus.28 However, the potential of rSLPI for wound healing has never been proven. Therefore, this study was conducted to analyze the potential of rSLPI in the wound healing process by analyzing the IL-1, IL-6, FGF-2 and CD163 expression.
Materials and Methods
Animals
The research conducted was an experimental laboratory study using 18 Wistar rats (Rattus norvegicus), aged 2–3 months, conducted in September–November 2020. The experiments were performed in compliance with the guidelines of the Institutional Animal Care and Use Committee of Universitas Airlangga. The protocol of this research was approved by the ethics committee of the Faculty of Dentistry, Airlangga University, no. 538/HRECC. FODM/XII/2020.
rSLPI
The rSLPI was produced according to Munadziroh et al. 2017.21 This procedure has successfully produced a rSLPI. The SLPI encoding gene was inserted into pET-101/D-TOPO (Invitrogen, Carlsbad, CA, USA) as plasmid and cloned into BL21 Star (DE3) (Novagen, Darmstadt, Germany) as expression host. The entire system was inserted into Escherichia coli TOP10 (Invitrogen, Carlsbad, CA, USA) as cloning host. The rSLPI was produced as described in Figure 1.
 |
Figure 1 The rSLPI production generation. |
Wound Creation
The study was conducted in 18 male Rattus norvegicus, weighing 150–200 g, aged 2–3 months. The adaptation process lasted for a week in a cage measuring 60 cm by 65 cm by 80cm. Animals were anesthetized using ketamine at a dose of 22–44 mg/kg and diazepam 3–5 mg/kg by intra-muscular injection, before the incisional creation of the wound in the skin dorsal area using a round ended surgical blade, of 10 mm size and 5 mm depth. After the anesthesia, the dorsal skin of the animal was epilated, then the incision was made with a measurement of 10 mm long and depth of 1 mm using the round blade.
rSLPI Administration
The animals were divided into three groups and each group consisted of six animals. Each group was administered witha different dose of rSLPI. After this procedure the wound was closed by hypafix. The distribution of animals’ administration is described in Table 1. Four days after rSLPI administration, the animals were terminated using ketamine anesthesia intravenously. The wound tissue was collected, fixed using Whatman filter paper and then immersed in 10% formalin. Samples were then processed in histological preparation for immunohistochemistry staining.
 |
Table 1 The Animal Distribution |
The CD163, IL-1, IL-6 and FGF-2 Expression
The CD163, IL-1, IL-6 and FGF-2 expression was observed with indirect immunohistochemistry staining under light microscope with a magnification of 400x. The expression was visualized using AxioVision software to calculate the percentage area by single blinded operator in the five different fields. Antibody CD163 (antiCd163, mouse monoclonal, Santa Cruz biotechnology), IL-1 (antiIL1, mouse polyclonal, Santa Cruz biotechnology) and IL-6 (antiIL-6, mouse monoclonal, Santa Cruz biotechnology) and FGF-2 (antiFGF-2, mouse monoclonal, Santa Cruz biotechnology) were used.
Statistical Analysis
The differences in quantity of CD163, IL-1, IL-6, and FGF-2 expression were analyzed using One-way analysis of variance (ANOVA) and post hoc test Tukey High Significant Difference (Tukey-HSD) with significance value of p <0.05.
Results
CD163 Expression
Immunohistochemistry staining on skin wound showed an CD163 expression as brown color (Figure 2A–C). The CD163 expression in the group administered with rSLPI 0.06 (12.83±3.31) is higher than group administered with rSLPI 0.04 (10.50±1.52) and control (3.17±1.60) (p = 0.000 and p = 0.023) (Table 2).
 |
Table 2 The CD163, IL-1, and IL-6 Expression in Skin Wound Tissue |
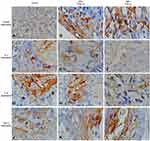 |
Figure 2 The cytokine expression in the skin wound. (A–C) IL-6 expression; (D–F) FGF-2 expression; (G–I) CD163 expression; (J–L) IL-1 expression. Microscopic feature with magnification 1000x. |
IL-1 Expression
Immunohistochemistry staining on skin wound showed an IL-1 expression as brown color (Figure 2D–F). The IL-1 expression in the group administered with rSLPI 0.06 (8.00±1.24) is lower than control (14.00±2.45) (p = 0.000). The IL-1 expression in the group administered with rSLPI 0.04 (11.33±1.86) is lower than control (14.00±2.45) (p = 0.000) (Table 2).
IL-6 Expression
Immunohistochemistry staining on skin wound showed an IL-6 expression as brown color (Figure 2G–I). The IL-6 expression in the group administered with rSLPI 0.06 (4.38±1.47) is lower than group administered with rSLPI 0.04 (8.67±1.75) and control (2.33±3.08) (p = 0.023 and, p = 0.000, respectively). The IL-6 expression in the group administered with rSLPI 0.04 (8.67±1.75) is lower than control (2.33±3.08) (p = 0.03) (Table 2).
FGF-2 Expression
Immunohistochemistry staining on skin wound showed an FGF-2 expression as brown color (Figure 2J–L). The FGF-2 expression in the group administered with rSLPI 0.06 (15.67±3.67) is higher than group administered with rSLPI 0.04 cc (11.17±1.95) and control (6.17±2.48) (p = 0.035 and p = 0.000, respectively). The FGF-2 expression in the group administered with rSLPI 0.04 (11.17±1.95) is higher than control (6.17±2.48) (p = 0.019) (Table 2).
Discussion
rSLPI is a SLPI obtained through replication using bacteria.21 The generation of rSLPI has been successfully performed using cloning using Escherichia coli.21 SLPI is a constituent of amnion membrane that is able to inhibit protease, control bacterial activity, is anti-inflammatory and antiretroviral, and able to suppress matrix metalloproteinase that can cause tissue damage. Administration of rSLPI on experimental animal wounds showed increased CD163 expression. The CD163 expression also depended on rSLPI doses. The CD163 is expressed on macrophages and becomes a determinant of macrophage polarization to become dominant to M1. This is due to the rSLPI mechanism that functions as an anti-inflammatory and inhibits the production of IFN-y induced by NF-kB.29 rSLPI can bind to the annexin II receptor on the surface of macrophage cells and can inhibit the downstream activation of NF-kB by protecting the degradation inhibitor of kappa (IKb) from degradation by the ubiquitin-proteasome pathway.29 In addition, rSLPI can compete with NF-kB for binding to the NF-kB binding site in the nucleus.30–32 Thus, rSLPI prevents the secretion of pro-inflammatory cytokines such as TNF-α, IL-1 and nitric oxide.33 It is also possible that rSLPI enhances macrophage recruitment of the factor plasmin, cathepsins and sialidases. This is consistent with an in vitro study by Sano et al., 2000 showing that the addition of exogenous SLPI can increase macrophage recruitment.34
Inhibition of IFN-y can increase the production of IL-4.35 This increase in IL-4 production affects the polarization of macrophages to become dominant in M2 compared with M1.36 M2 then releases anti-inflammatory mediators such as IL-10 and growth factors. In addition, M2 can also produce precursors for fibroblast activation and collagen synthesis which is necessary for wound healing, namely FGF-2.37
The FGF-2 expression also increased after administration of rSLPI in the wound area. The FGF-2 expression also depends on rSLPI doses, as observed in CD163 expression. FGF-2 is produced by keratinocytes, fibroblasts, endothelial cells, smooth muscle cells, chondrocytes and mast cells.38 Research has shown a significant increase in FGF-2 expression with the administration of rSLPI. Another study conducted earlier, explained that bovine sponge amnion containing rSLPI is able to control the activity of TGF-β thus stimulating fibroblast activity that causes an increase in fibroblasts.39 This explains that the amnion membrane containing SLPI can increase fibroblasts and increased the FGF-2. It is also in accordance with studies that the increase in FGF-2 will lead to increased proliferation of fibroblasts so as to accelerate wound healing.40
Previous research has shown that rSLPI meets the criteria as a biomaterial that can heal wounds efficiently on vascular endothelial growth factor (VEGF) and collagen.41 In this study we analyzed that the IL-6 expression observed in all groups is decreased, and is dose-dependent. This indicates that the administration of rSLPI fluid has an influence on the wound healing process. This is in accordance with the theory of wound healing process where in the inflammatory process IL-6 acting as a pro-inflammatory cytokine will increase, because IL-6 is an inflammatory mediator that appears after surgery, acute trauma and infection.42 Damage associated molecular pattern (DAMP) produced by damaged cells will bind pattern recognition receptor (PRR) contained in cell membrane. The existence of this bond will trigger signaling that will increase the expression of NF-kB. NF-kB which acts as an inflammatory initiator will work to trigger the synthesis process of inflammatory-related proteins. In the inflammatory phase, macrophages tend to polarize to M1 and produce pro-inflammatory cytokines that will trigger inflammation of the tissues. Pro-inflammatory cytokines produced TNF-α, IL-1 and IL-6 to stimulate Toll-like receptor (TLR). TLR is found on the surface of macrophages and stimulates pro-inflammatory cytokines and activates the signal pathway to activate the NF-kB.43,44
SLPI plays a role in inhibiting NF-kB binding in IL-6 region promoters.43 The research found that there was a significant decrease in IL-6 expression with the administration of rSLPI. This is because rSLPI binds to annexin II receptors located on the surface of macrophages and can inhibit the activation of NF-kB through inhibitors of nuclear factor kappa B (I-kB). I-kB kinase is part of a multiprotein complex consisting of subunits IKK-α and IKK-B, and is important in cytokine-induced IKB phosphorylation. Activation of the IKK complex resulted in phosphorylation and degradation of IKBα and was subsequently followed by the release of NF-kB. Inhibition of NF-kB will inhibit the secretion of pro-inflammatory cytokines such as TNF-α, IL1, IL-6 and nitric oxide (NO). Inhibition of NF-kB will cause decreased production of IL-6 and IL-1 which are pro-inflammatory cytokines, thus accelerating the wound healing process.45
Conclusion
The administration of rSLPI is able to accelerate the wound healing process by increasing CD163 and FGF-2 expression. Cytokines such as IL-1 and IL-6 decreased dependent on rSLPI doses.
Funding
All author declares no funding.
Disclosure
All author declares no conflicts of interest.
References
1. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12):2085. doi:10.3390/ijms17122085
2. Politis C, Schoenaers J, Jacobs R, Agbaje JO. Wound healing problems in the mouth. Front Physiol. 2016;2(7):1–13.
3. Guo S, DiPietro LA. Critical review in oral biology & medicine: factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi:10.1177/0022034509359125
4. Nosrati H, Khodaei M, Alizadeh Z, Banitalebi-Dehkordi M. Cationic, anionic and neutral polysaccharides for skin tissue engineering and wound healing applications. Int J Biol Macromol. 2021;192:298–322. doi:10.1016/j.ijbiomac.2021.10.013
5. Schreml S, Szeimies R-M, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. J Am Acad Dermatol. 2010;63(5):866–881. doi:10.1016/j.jaad.2009.10.048
6. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. Int J Med Res. 2009;37(5):1528–1542. doi:10.1177/147323000903700531
7. Surboyo MDC, Mahdani FY, Ernawati DS, Sarasati A, Rezkita F. The macrophage responses during diabetic oral ulcer healing by liquid coconut shell smoke: an immunohistochemical analysis. Eur J Dent. 2020;14(03):410–414. doi:10.1055/s-0040-1712776
8. Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The metabolic signature of macrophage responses. Front Immunol. 2019;10:1–16. doi:10.3389/fimmu.2019.01462
9. Vogel DYS, Heijnen PDAM, Breur M, et al. Macrophages migrate in an activation-dependent manner to chemokines involved in neuroinflammation. J Neuroinflammation. 2014;11(1):23. doi:10.1186/1742-2094-11-23
10. Jayasingam SD, Citartan M, Thang TH, Mat Zin AA, Ang KC, Ch’ng ES. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: technicalities and challenges in routine clinical practice. Front Oncol. 2020;9:1–9. doi:10.3389/fonc.2019.01512
11. Peng H, Xian D, Liu J, Pan S, Tang R, Zhong J. Regulating the polarization of macrophages: a promising approach to vascular dermatosis. J Immunol Res. 2020;2020:1–13.
12. Xiao T, Yan Z, Xiao S, Xia Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res Ther. 2020;11(1):1–9. doi:10.1186/s13287-020-01755-y
13. Surboyo MDC, Arundina I, Rahayu RP, Mansur D, Bramantoro T. Potential of distilled liquid smoke derived from coconut (cocos nucifera L) shell for traumatic ulcer healing in diabetic rats. Eur J Dent. 2019;13(02):271–279. doi:10.1055/s-0039-1693527
14. Surboyo MDC, Ernawati DS, Radithia D, et al. Distilled liquid smoke coconut shell attenuates the cytokine profile of macrophages in oral ulcer in experimental model of diabetes mellitus. J Appl Pharm Sci. 2021;11(08):62–69.
15. Surboyo MDC, Ernawati DS, Arundina I, et al. The potential of liquid smoke as an oral ulcer remedies: a proposed mechanism based on systematic review. J Pharm Pharmacogn Res. 2021;9(6):905–920.
16. Contassot E, Beer H, French L. Interleukin-1, inflammasomes, autoinflammation and the skin. Swiss Med Wkly. 2012;142:1–10.
17. Im JH, Buzzelli JN, Jones K, et al. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nat Commun. 2020;11(1):4064. doi:10.1038/s41467-020-17914-x
18. Surboyo MDC, Ernawati DS, Arundina I, Rahayu RP. Oral ulcer healing after treatment with distilled liquid smoke of coconut shell on diabetic rats. J Krishna Inst Medical Sci Univ. 2019;8(2):70–79.
19. Kasuya A, Tokura Y. Attempts to accelerate wound healing. J Dermatol Sci. 2014;76(3):169–172. doi:10.1016/j.jdermsci.2014.11.001
20. Ashcroft GS, Lei K, Jin W, et al. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med 2000;6(10):1147–1153. doi:10.1038/80489
21. Munadziroh E, Purnamasari S, Puspaningsih NNT, Rubianto M, Ismaya WT. Generation of a soluble and active recombinant human secretory leukocyte protease inhibitor. Biotecnol Apl. 2017;34(2):1271–1274.
22. Indrawati DW, Munadziroh E, Sulisetyawati TIB, El Fadhlallah PM. Sponge amnion potential in post tooth extraction wound healing by interleukin-6 and bone morphogenetic protein-2 expression analysis: an animal study. Dent Res J (Isfahan). 2019;16(5):283–288. doi:10.4103/1735-3327.266089
23. Sumi Y, Muramatsu H, Hata K, Ueda M, Muramatsu T. Secretory leukocyte protease inhibitor is a novel inhibitor of fibroblast-mediated collagen gel contraction. Exp Cell Res. 2000;256(1):203–212. doi:10.1006/excr.2000.4815
24. Mikami Y, Iwase T, Komiyama Y, Matsumoto N, Oki H, Komiyama K. Secretory leukocyte protease inhibitor inhibits expression of polymeric immunoglobulin receptor via the NF-κB signaling pathway. Mol Immunol. 2015;67(2):568–574. doi:10.1016/j.molimm.2015.07.021
25. Gomez SA, Argüelles CL, Guerrieri D, et al. Secretory leukocyte protease inhibitor. Am J Respir Crit Care Med. 2009;179(3):247–253. doi:10.1164/rccm.200804-615OC
26. Subramaniyam D, Hollander C, Westin U, Erjefält J, Stevens T, Janciauskiene S. Secretory leukocyte protease inhibitor inhibits neutrophil apoptosis. Respirology. 2011;16(2):300–307. doi:10.1111/j.1440-1843.2010.01901.x
27. King AE. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol Hum Reprod. 2000;6(2):191–196. doi:10.1093/molehr/6.2.191
28. Majchrzak-Gorecka M, Majewski P, Grygier B, Murzyn K, Cichy J. Secretory leukocyte protease inhibitor (SLPI), a multifunctional protein in the host defense response. Cytokine Growth Factor Rev. 2016;28:79–93. doi:10.1016/j.cytogfr.2015.12.001
29. Geraghty P, Greene CM, O’Mahony M, O’Neill SJ, Taggart CC, McElvaney NG. Secretory leucocyte protease inhibitor inhibits interferon-γ-induced cathepsin S expression. J Biol Chem. 2007;282(46):33389–33395. doi:10.1074/jbc.M706884200
30. Lentsch AB, Yoshidome H, Warner RL, Ward PA, Edwards MJ. Secretory leukocyte protease inhibitor in mice regulates local and remote organ inflammatory injury induced by hepatic ischemia/reperfusion. Gastroenterology. 1999;117(4):953–961. doi:10.1016/S0016-5085(99)70355-0
31. Doumas S, Kolokotronis A, Stefanopoulos P. Anti-inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect Immun. 2005;73(3):1271–1274. doi:10.1128/IAI.73.3.1271-1274.2005
32. Zhong QQ, Wang X, Li YF, Peng LJ, Jiang ZS. Secretory leukocyte protease inhibitor promising protective roles in obesity-associated atherosclerosis. Exp Biol Med. 2017;242(3):250–257. doi:10.1177/1535370216672747
33. Choi BD, Jeong SJ, Wang G, et al. Temporal induction of Secretory Leukocyte Protease Inhibitor (SLPI) in odontoblasts by lipopolysaccharide and wound infection. J Endod. 2009;35(7):997–1002. doi:10.1016/j.joen.2009.04.008
34. Sano C, Shimizu T, Sato K, Kawauchi H, Tomioka H. Effects of secretory leucocyte protease inhibitor on the production of the anti-inflammatory cytokines, IL-10 and transforming growth factor-beta (TGF=β), by lipopolysaccharide-stimulated macrophages. Clin Exp Immunol. 2000;121(1):77–85. doi:10.1046/j.1365-2249.2000.01269.x
35. Kak G, Raza M, Tiwari BK. Interferon-gamma (IFN-γ): exploring its implications in infectious diseases. Biomol Concepts. 2018;9(1):64–79. doi:10.1515/bmc-2018-0007
36. Reeves ARD, Spiller KL, Freytes DO, Vunjak-Novakovic G, Kaplan DL. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials. 2015;73:272–283. doi:10.1016/j.biomaterials.2015.09.027
37. Santos-Vizcaino E, Salvador A, Vairo C, et al. Overcoming the inflammatory stage of non-healing wounds: in vitro mechanism of action of negatively charged microspheres (NCMS). Nanomaterials. 2020;10(6):1–14. doi:10.3390/nano10061108
38. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi:10.1111/j.1524-475X.2008.00410.x
39. Faadhila T, Valentina M, Munadziroh E, Nirwana I, Soekartono H, Surboyo MC. Bovine sponge amnion stimulates socket healing: a histological analysis. J Adv Pharm Technol Res. 2021;12(1):99. doi:10.4103/japtr.JAPTR_128_20
40. Nagayasu-Tanaka T, Anzai J, Takaki S, et al. Action mechanism of Fibroblast Growth Factor-2 (FGF-2) in the promotion of periodontal regeneration in beagle dogs Matsumoto T, editor. PLoS One. 2015;10(6):e0131870. doi:10.1371/journal.pone.0131870
41. Indrawati DW, Munadziroh E, Indah T, Sulisetyawati B, Mahardhika P, Fadhlallah E. Sponge amnion potential in post tooth extraction wound healing by interleukin - 6 and bone morphogenetic protein - 2 expression analysis: an animal study. Dent Res J. 2019;16:283–288.
42. MK Noh, Jung M, SH Kim, et al. Assessment of IL-6, IL-8 and TNF-α levels in the gingival tissue of patients with periodontitis. Exp Ther Med. 2013;6(3):847–851. doi:10.3892/etm.2013.1222
43. Svensson D, Aidoukovitch A, Anders E, Jönsson D, Nebel D, Nilsson B-O. Secretory leukocyte protease inhibitor regulates human periodontal ligament cell production of pro-inflammatory cytokines. Inflamm Res. 2017;66(9):823–831. doi:10.1007/s00011-017-1062-2
44. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:e17023. doi:10.1038/sigtrans.2017.23
45. Murata E, Sharmin S, Shiota H, Shiota M, Yano M, Kido H. The effect of topically applied secretory leukocyte protease inhibitor on the eosinophil response in the late phase of allergic conjunctivitis. Curr Eye Res. 2003;26(5):271–276.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
