Back to Journals » Drug Design, Development and Therapy » Volume 12
The role of propranolol as a radiosensitizer in gastric cancer treatment
Authors Liao XH, Chaudhary P , Qiu G , Che XM, Fan L
Received 26 December 2017
Accepted for publication 24 February 2018
Published 28 March 2018 Volume 2018:12 Pages 639—645
DOI https://doi.org/10.2147/DDDT.S160865
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Xinhua Liao, Prakash Chaudhary, Guanglin Qiu, Xiangming Che, Lin Fan
General Surgery Department, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
Purpose: The National Comprehensive Cancer Network guidelines indicate that radiotherapy in gastric cancer shows limited effectiveness at reducing the growth of gastric cancer. Therefore, enhancing the sensitivity and effect of radiotherapy with propranolol, a β-adrenoceptor antagonist, could reduce tumor growth. The role of propranolol as a radiosensitizer has not been adequately studied; therefore, the purpose of the present study is to evaluate the effect of propranolol as a radiosensitizer against gastric cancer in vivo.
Methods: Sixty-four male nude mice bearing tumor xenografts were randomly divided into four groups. Cell culture was performed using the human gastric adenocarcinoma cell line SGC-7901. Mice with tumor xenografts were treated with propranolol, isoproterenol, and radiation. The data for tumor weight and volume were obtained for statistical analyses. Furthermore, the expression levels of COX-2, NF-κB, VEGF, and EGFR were examined using immunohistochemical techniques and Western blotting.
Results: The growth in the volume and weight of the tumor was lower in mouse models treated with propranolol and radiation therapy compared to the other groups. Decreased expression of NF-κB was also observed in treatment groups where both propranolol and radiation were used, leading to the reduction of COX-2, EGFR, and VEGF expression compared to that in the other groups.
Conclusion: The present study indicated that propranolol potentiates the antitumor effects of radiotherapy in gastric cancer by inhibiting NF-κB expression and its downstream genes: VEGF, EGFR, and COX-2.
Keywords: propranolol, radiosensitizer, gastric cancer, radiation therapy
Introduction
The global incidence of gastric cancer is the fourth highest, and the mortality of gastric cancer is the second highest of all cancers. Although the incidence and mortality of gastric cancer has dramatically decreased in the US and elsewhere over the past several decades, it remains a major public health problem.1 Additionally, gastric cancer places a huge burden on society and individuals.2 Recurrent tumors are often experienced, despite precise curative surgical resection, because diagnoses are typically made after the cancer is already at an advanced stage. Therefore, new therapies for advanced or late-stage gastric cancer are needed. National Comprehensive Cancer Network (NCCN) guidelines suggest radiotherapy as an important treatment for gastric cancer, but this therapy shows strong radiation resistance and a high risk of recurrence.3 The sensitivity of gastric cancer to radiotherapy is limited; therefore, a drug that increases the sensitivity of radiotherapy to gastric cancer must be identified.
Propranolol is a non-selective beta-adrenergic receptor (β-AR) blocker that is primarily used for the treatment of cardiovascular diseases, such as premature atrial and ventricular beats, sinus and ventricular tachycardia, atrial fibrillation, etc. Propranolol can also be used as secondary prevention to reduce the mortality rate of myocardial infarction, as a first-line drug for hypertension. Although mainly used for cardiovascular conditions, propranolol is also used in other conditions. Propranolol can be used for the management of postmenopausal osteoporosis.4 Further, propranolol also plays a role in tumor therapy, showing antitumor activity in neuroblastoma.5 In a previous in vitro study, propranolol was demonstrated as a radiotherapy sensitizer for gastric cancer.6 However, additional detailed research is needed to test the radiosensitizing effect of propranolol.
A number of studies have shown that COX-2, VEGF, and EGFR are key factors that influence the radiation sensitivity of the tumor7–11 and are associated with cellular differentiation, proliferation, and angiogenesis. NF-κB is also sensitive to radiation therapy in many tumors. Many studies have shown that NF-κB-mediated signaling pathways are associated with radiation resistance and adverse clinical outcomes in many cancers.12,13 In the present study, we examined the role of the β-AR antagonist propranolol as a radiosensitizer in gastric cancer treatment in vivo. This treatment may inhibit NF-κB expression and reduce the effects of EGFR, VEGF, and COX-2, which in turn will suppress the proliferation of gastric cancer cells. The aim of the present study is to determine whether propranolol has a radiosensitizing effect in vivo and increases the effectiveness of radiotherapy in restricting the proliferation of gastric cancer cells, to confirm the potential signaling pathway of this compound. It has not yet been demonstrated that propranolol could be used as a radiosensitizer in vivo.
Materials and methods
Cell culture
The experimental gastric cancer cell line SGC-7901, established by the Sixth Hospital in Shanghai China, was purchased from the Cell Bank, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM (high glucose; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% heat-inactivated FBS (Thermo Fisher Scientific) and streptomycin and penicillin (both are 100 U/mL; HPGC, Harbin, China) in an incubator (Thermo Election) at 37°C and 5% CO2, with routine passage by centrifugation.
Selection of animal samples
We obtained 64 4-week-old male nude mice weighing 20–25 g, from the Medical Research Center of Xi’an Jiaotong University (Xi’an, China). All of the animals were fed standard water and food in the Animal Laboratory Center of Xi’an Jiaotong University (specific pathogen free). The animals were maintained in cages at a constant temperature and a 12 h light/dark cycle. All experiments were approved by the Laboratory Animal Care Committee of Xi’an Jiaotong University.
Xenograft gastric tumor mouse model and intervention
All procedures were performed in accordance with the Principles of Laboratory Animal Care (National Institutes of Health, NIH) and guidelines of the laboratory animal care committee of Xi’an Jiaotong University. Rearing 4-week-old nude mice for 1 week. A 100 μL suspension containing 107 SGC-7901 cells was injected into the backs of the nude mice. When the tumor diameter reached 1 cm, the tumor block was cut into 1 mm3 using an ophthalmic instrument, and the small tumor block was injected into the back of 64 nude mice. Daily observations of the nude mice, including diet, activity, weight, and tumor growth, were performed. The following equation was used to calculate the tumor volume: volume =1/2 × length × width2. When the tumor volume reached 500 ± 50 mm3, 64 nude mice were divided into four groups by a random number table, with 16 nude mice in each group, followed by intervention. Intervention methods: the control group did not receive intervention and maintained feeding. The radiotherapy group received X-ray, 6 Gy, 200 cGy/min, 4 Mev (the source-skin distance was 100 cm) and the intervention was performed twice a week (X-ray Generator Precise Linear Accelerator, Elekta, UK). The propranolol combination radiotherapy group received an intraperitoneal injection 1 h prior to each radiotherapy session with 2 mg/kg propranolol (Sigma-Aldrich Co., St Louis, MO, USA), and the radiotherapy method was the same as that used for the radiotherapy group. The isoproterenol combination radiotherapy group received an intraperitoneal injection 5 min prior to each radiotherapy session with 0.1 mg/kg isoproterenol (Sigma-Aldrich Co.), and the radiotherapy method was the same as that used for the radiotherapy group. The drug concentration was selected according to previous studies.14–16 The nude mice were placed in a special, flexible operation box during radiotherapy. After 2 weeks the mice were sacrificed and the tumors were removed.
Histopathological analysis
The tumor tissue was fixed in 4% paraformaldehyde and then embedded in paraffin. The sections were cut, deparaffinized in xylene, dehydrated in graded alcohol, and finally hydrated in water. For antigen retrieval, the specimens were immersed in a new configuration of citrate buffer (pH = 6.0) and placed in a microwave oven (92°C) for 5 min × 2. Subsequently, 10% non-immunized rabbit serum was added, and the specimens were incubated for 45 min at room temperature to block the non-specific antigen. The primary antibody was added, at a dilution ratio of 1:100 for EGFR, VGEF, NF-κB, and COX-2 (Santa Cruz Biotechnology Inc., Dallas, TX, USA). The secondary antibody, labeled with biotin, was incubated in a box for 30 min at room temperature. Streptomyces antibiotin-peroxidase complex was added, followed by incubation at room temperature for 30 min. The color was developed in DAB-H2O2 liquid. Images were taken using a microscope (Olympus Corporation, Tokyo, Japan).
Western blot assay
The nitrocellulose membrane, BCA assay kit, and the chemiluminescence kit were obtained from EMD Millipore (Billerica, MA, USA) and Thermo Fisher Scientific. The procedures were performed in strict accordance with the standard protocols. The Bradford method was used to ensure that the amount of protein per sample was 20 mg. The proteins were subjected to 10% SDS-PAGE on a Bio-Rad Mini PROTEAN 3 System (Bio-Rad Laboratories Inc., Hercules, CA, USA) and subsequently electrotransferred onto nitrocellulose membranes (400 mA for 2 h; EMD Millipore). Wet transfer was used for EGFR protein, while semi-dry transfer was used for VEGF, COX-2, and NF-κB. TBS containing 10% milk powder and 10% Tween-20 was used to block the nitrocellulose membranes at 37°C for 4 h. Then, the nitrocellulose membranes were incubated in primary antibodies overnight at 4°C. The column dilution ratio for all primary antibodies, including VEGF, COX-2, and NF-κB, but not β-actin (1:500; Santa Cruz Biotechnology Inc.), was 1:200. According to the appropriate primary antibody, we selected different rabbit or mouse IgG antibodies as the secondary antibodies. We selected an enhanced chemiluminescence (Thermo Fisher Scientific) detection system to detect light strips and then transferred the images to X-ray film (Del DOC2000; Bio-Rad Laboratories Inc.).
Statistical analysis
Normally distributed data were evaluated with ANOVA. Origin V 7.5 and SPSS V 13.0 were used for the analysis of the experimental data and the experimental chart. p-values <0.05 were considered statistically significant.
Results
Changes in gastric tumor size after radiation therapy with propranolol treatment
To determine whether propranolol can sensitize gastric cancer to radiation, we examined the effect of radiation alone, isoproterenol with radiation, and a combination of propranolol with radiation on the growth of subcutaneous xenograft tumors. Based on tumor volume measurements on the 14th day after tumor cell implantation, we randomized animals into four groups, as described in “Materials and methods” section. The mice were treated with propranolol (2 mg/kg) or isoproterenol (0.1 mg/kg) prior to radiation once a week for 2 weeks. The tumor volume was determined and compared in all four groups. The tumor volumes of the control, isoproterenol with radiation, and radiation alone groups were larger than that of the propranolol with radiation treated group. Representative images of the tumor volume at this time are illustrated in Figure 1. The tumors treated with radiation and propranolol had significantly smaller average volumes when compared with tumors treated with radiation alone, isoproterenol with radiation, and the control groups. Therefore, tumors treated with propranolol with radiation displayed significant improvements in the biological effect of radiation. Reductions in tumor size were measured every 2 days in all four groups after different treatments (Figure 2).
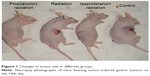  | Figure 1 Changes in tumor size in different groups. |
The effects of propranolol on radiation-induced genetic expression in gastric cancer xenografts
Figures 3 and 4 illustrate EGFR, VEGF, COX-2, and NF-κB immunohistochemical staining and Western blot analysis of the tumor xenografts, which were either treated with radiation alone or radiation in combination with either isoproterenol or propranolol, or left untreated (control). Immunohistochemical analysis and protein changes indicated that treatment with radiotherapy, in all tumors, reduced the expression of NF-κB, followed by a decrease in COX-2, VEGF, and EGFR expression, compared with that in the control group (Figures 3 and 4). The order of the genetic expression of these groups, from lowest to highest, was radiotherapy with propranolol, radiotherapy only, radiotherapy with isoproterenol, and control. Compared with the radiation only treatment group, the expression of COX-2, VEGF, EGFR, and NF-κB were significantly reduced after pretreatment with propranolol prior to radiation. In contrast, the experimental results of pre-treatment with isoproterenol were significantly different from those of the previously mentioned results, showing a reversal of the reduction of gene expression caused by radiation.
Discussion
The global incidence of gastric cancer is the fourth highest, and the mortality of gastric cancer is the second highest among all cancers. Radiotherapy is used as a combined treatment for gastric cancer patients. In previous cytological experiments, the β-AR has been associated with radiotherapy sensitivity of gastric cancer.6 Inhibiting the β-AR increases the radiotherapy sensitivity, whereas activating this receptor reduces the radiotherapy sensitivity of gastric cancer cells. In the last 10 years, researchers have continued to study the relationship between the β-AR and gastric cancer. Some clinical studies have demonstrated that adrenergic receptor blockers can improve the prognosis of patients with gastric cancer,17 and protect the normal population from the occurrence of gastric cancer.18 The activation of the adrenergic receptor is also responsible for the proliferation and drug resistance of gastric cancer cells. Lu et al showed that isoproterenol activates the adrenergic receptor and subsequently causes epithelial–mesenchymal transition changes to promote gastric cancer.19 Other studies have found that isoproterenol can promote the secretion of VEGF via the adrenergic receptor pathway to increase cell migration and tumor growth.20 Furthermore, the chemotherapy resistance of her-2 positive gastric cancer patients is caused by the activation of β2-AR.21 These studies have shown that the β-AR plays a role in the biology of gastric cancer and chemotherapy. Radiotherapy is an important part of gastric cancer treatment. However, previous studies have not explored the relationship between the β-AR and gastric cancer radiotherapy, and there is no related research on tumors other than gastric cancer. To further explain and confirm the relationship between β-AR and gastric cancer radiotherapy, we conducted animal experiments on the basis of previous cytological studies. In the present study, we assessed whether propranolol, a β-blocker antagonist, could inhibit the expression of NF-κB and down-regulate downstream genes to regulate the cell cycle and cell apoptosis in vivo.
The present results indicated that tumors grew significantly less in mice treated with propranolol and radiation compared to the mice in the other three treatment groups. Therefore, the combination of propranolol and radiotherapy had a more significant anti-proliferation effect than radiotherapy alone in SGC-7901 gastric cancer cells. We also determined that there was at least an additive effect of propranolol in combination with radiotherapy. Although individual radiotherapy inhibited tumor growth, the effect of propranolol significantly enhanced this effect. In contrast, isoproterenol had the opposite effect of propranolol, inducing an anti-radiotherapy effect and improving the survival rate of the cells after radiotherapy. These results show that propranolol can affect the sensitivity of gastric cancer to radiotherapy through β-AR and may represent an effective drug sensitizer for gastric cancer. Therefore, propranolol combined with radiotherapy may be an effective treatment for patients with advanced or recurring gastric cancer.
Some studies have shown that the role of NF-κB in radiation, including its activation in radiation and cell protection, demonstrates its resistance to radiation. NF-κB can be induced in cancer cells by radiation therapy, which decreases the radiosensitivity of cells.22–24 Cellular stress activates NF-κB and then regulates the expression of genes involved in cell proliferation and apoptosis, and NF-κB continues entering the nucleus to regulate the transcription process.25–26 The present study showed that treating SGC-7901 cells with propranolol as a radiosensitizer down-regulated the level of NF-κB, suggesting an increase in cell radiosensitivity by propranolol-induced NF-κB inhibition. NF-κB and its downstream genes EGFR, COX-2, and VEGF are closely related to regulating angiogenesis and apoptosis.27 The COX-2 inhibitor enhances tumor responses to radiation through an enzyme that converts arachidonic acid to prostaglandin. COX-2 genes are widely present in different tumor cells and are involved in the development and metastasization of tumors.28,29 Thus, the COX-2 antagonist acts as a radiosensitizer in cancer.7
The combination of EGFR with EGF or TGF-α activates intracellular tyrosine kinase for the regulation of the cell cycle. High-level EGFR expression was associated with radiotherapy resistance and poor prognosis. This phenomenon has been reported in some tumors, particularly head and neck squamous carcinoma.9 Additionally, the combination of EGFR and ligands triggers a signaling process. This process is closely related to tumor proliferation, cell migration, angiogenesis, and apoptosis, and is directly involved in tumor development. Therefore, inhibiting HER1/EGFR activity could effectively block downstream signaling events and, consequently, tumorigenesis.30 An EGFR antagonist has been administered as a radiosensitizer in many carcinomas, such as cutaneous or head and neck squamous carcinomas.31,32
The important function of VEGF was to participate in angiogenesis and regulate endothelial permeability. A recent study showed that inhibiting tumor angiogenesis increases the effectiveness of coadministered radiotherapy.33,34 According to the function of VEGF, this treatment could inhibit the formation of blood vessels in the tumor, thus causing hypoxia, which might enhance the radiosensitivity of tumor cells and drug penetration.35 NF-κB is the upstream target for the regulation of VEGF, COX-2, and EGFR expression levels.36–38 Changes in VEGF, COX-2, and EGFR signaling molecules were associated with propranolol radiotherapy sensitization. These results showed that propranolol could be used as a radiotherapy-sensitizing agent for gastric cancer, and its effect was achieved by influencing NF-κB and regulating VEGF, COX-2, and EGFR. The present study demonstrated that propranolol, a β-blocker, could increase the radiotherapy sensitivity of gastric cancer.
According to these results, we concluded that propranolol has a radiotherapy sensitization effect on the radiation resistance of tumors and can reduce radiation side effects on surrounding tissues. Furthermore, this effect is more pronounced in gastric cancer cells. Propranolol, a β-blocker, plays a definite role in the radiation treatment of gastric cancer. The results showed that blocking the β-AR pathway can improve the radiation sensitivity of gastric cancer cells, and the molecular pathways may be through influencing NF-κB and regulating VEGF, COX-2, and EGFR. Moreover, β-AR agonists have the opposite effect on gastric cancer cells. These results suggest that by blocking the β-adrenoceptor receptor-signaling pathway, the effect of radiotherapy on gastric cancer can be improved.
Conclusion
In conclusion, the results show that the β-receptor antagonist propranolol has a definite radiotherapy sensitization effect on gastric cancer. In addition, this effect is caused by the regulation of NF-κB and its downstream genes VEGF, COX-2, and EGFR.
Acknowledgments
The authors would like to thank Dr Xuqi Li of the General Surgery Department of First Affiliated Hospital of Xi’an Jiaotong University for providing expert opinions on methods of our study and his useful comments. This work was financially supported by a grant from the Natural Basic Research Program of Shaanxi Province, China (no 2014JQ4123).
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–1145. | ||
Soerjomataram I, Lortet-Tieulent J, Parkin DM, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380(9856):1840–1850. | ||
Glinski K, Wasilewska-Tesluk E, Rucinska M, et al. Clinical outcome and toxicity of 3D-conformal radiotherapy combined with chemotherapy based on the Intergroup SWOG 9008/INT0116 study protocol for gastric cancer. J BUON. 2015;20(2):428–437. | ||
Khajuria DK, Razdan R, Mahapatra DR, Bhat MR. Osteoprotective effect of propranolol in ovariectomized rats: a comparison with zoledronic acid and alfacalcidol. J Orthop Sci. 2013;18(5):832–842. | ||
Wolter JK, Wolter NE, Blanch A, et al. Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget. 2014;5(1):161–172. | ||
Liao XH, Che XM, Zhao W, et al. Effects of propranolol in combination with radiation on apoptosis and survival of gastric cancer cells in vitro. Radiation Oncology. 2010;5:98. | ||
Salehifar E, Hosseinimehr SJ. The use of cyclooxygenase-2 inhibitors for improvement of efficacy of radiotherapy in cancers. Drug Discov Today. 2016;21(4):654–662. | ||
Karagkounis G, DeVecchio J, Ferrandon S, Kalady MF. Simvastatin enhances radiation sensitivity of colorectal cancer cells. Surg Endosc. 2018;32(3):1533–1539. | ||
Shaghaghi Z, Hosseinimehr SJ. Synergistic effect of epidermal growth factor receptor inhibitors and ionization radiation in cancer treatment. Recent Pat Anticancer Drug Discov. 2007;12(4):323–339. | ||
Laube M, Kniess T, Pietzsch J. Development of antioxidant COX-2 inhibitors as radioprotective agents for radiation therapy—a hypothesis-driven review. Antioxidants (Basel). 2016;15(2):E14. | ||
Zhang N, Gao X, Zhao Y, et al. Rationally combining anti-VEGF therapy with radiation in NF2 schwannoma. J Rare Dis Res Treat. 2016;1(2):51–55. | ||
Li G, Wang Z, Chong T, et al. Curcumin enhances the radiosensitivity of renal cancer cells by suppressing NF-κB signaling pathway. Biomed Pharmacother. 2017;94:974–981. | ||
Hsu FT, Liu YC, Liu TT, Hwang JJ. Curcumin sensitizes hepatocellular carcinoma cells to radiation via suppression of radiation-induced NF-κB activity. Biomed Res Int. 2015;2015:363671. | ||
Tatsuta M, Iishi H, Yamamura H, Taniguchi H. Enhancement by propranolol of the inhibitory effect of tetragastrin on gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Res. 1987;47(1):111–114. | ||
Iishi H, Tatsuta M, Baba M, et al. Promotion by the alpha-adrenoceptor agonist phenylephrine, but not by the beta-adrenoceptor agonist isoproterenol, of gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. 1998;122(1–2):61–65. | ||
Luna SL, Neuman S, Aguilera J, Brown DI, Lara HE. In vivo β-adrenergic blockade by propranolol prevents isoproterenol-induced polycystic ovary in adult rats. Horm Metab Res. 2012;44(9):676–681. | ||
Takahashi K, Kaira K, Shimizu A, et al. Clinical significance of β2-adrenergic receptor expression in patients with surgically resected gastric adenocarcinoma. Tumour Biol. 2016;37(10):13885–13892. | ||
Chang PY, Huang WY, Lin CL, et al. Propranolol reduces cancer risk: a population-based cohort study. Medicine (Baltimore). 2015;94(27):e1097. | ||
Lu YJ, Geng ZJ, Sun XY, et al. Isoprenaline induces epithelial-mesenchymal transition in gastric cancer cells. Mol Cell Biochem. 2015;408(1–2):1–13. | ||
Lu Y, Xu Q, Zuo Y, et al. Isoprenaline/β2-AR activates Plexin-A1/VEGFR2 signals via VEGF secretion in gastric cancer cells to promote tumor angiogenesis. BMC Cancer. 2017;17(1):875. | ||
Wei B, Sun X, Geng Z, et al. Isoproterenol regulates CD44 expression in gastric cancer cells through STAT3/MicroRNA373 cascade. Biomaterials. 2016;105:89–101. | ||
Kalita B, Ranjan R, Singh A, et al. A combination of podophyllotoxin and rutin attenuates radiation induced gastrointestinal injury by negatively regulating NF-κB/p53 signaling in lethally irradiated mice. PLoS One. 2016;11(12):e0168525. | ||
Pordanjani SM, Hosseinimehr SJ. The role of NF-kB inhibitors in cell response to radiation. Curr Med Chem. 2016;23(34):3951–3963. | ||
Zhang W, Kang M, Zhang T, et al. Triptolide combined with radiotherapy for the treatment of nasopharyngeal carcinoma via NF-κB-related mechanism. Int J Mol Sci. 2016;17(12):E2139. | ||
Park M, Yoon HJ, Kang MC, Kwon J, Lee HW. PTK7 regulates radioresistance through nuclear factor-kappa B in esophageal squamous cell carcinoma. Tumour Biol. 2016;37(10):14217–14224. | ||
Adams J. The proteasome, structure, function, and role in the cell. Cancer Treat Rev. 2003;29 Suppl 1:3–9. | ||
Lin J, Wu H, Shi H, et al. Combined inhibition of epidermal growth factor receptor and cyclooxygenase-2 leads to greater anti-tumor activity of docetaxel in advanced prostate cancer. PLoS One. 2013;8(10):e76169. | ||
Pang LY, Hurst EA, Argyle DJ. Cyclooxygenase-2: a role in cancer stem cell survival and repopulation of cancer cells during therapy. Stem Cells Int. 2016;2016:2048731. | ||
Yu T, Lao X, Zheng H. Influencing COX-2 activity by COX related pathways in inflammation and cancer. Mini Rev Med Chem. 2016;16(15):1230–1243. | ||
Komposch K, Sibilia M. EGFR signaling in liver diseases. Int J Mol Sci. 2015;17(1):E30. | ||
Cuneo KC, Nyati MK, Ray D, Lawrence TS. EGFR targeted therapies and radiation: optimizing efficacy by appropriate drug scheduling and patient selection. Pharmacol Ther. 2015;154:67–77. | ||
Li C, Huang S, Armstrong EA, et al. Antitumor effects of MEHD7945A, a dual-specific antibody against EGFR and HER3, in combination with radiation in lung and head and neck cancers. Mol Cancer Ther. 2015;14(9):2049–2059. | ||
Jiang X, Engelbach JA, Yuan L, et al. Anti-VEGF antibodies mitigate the development of radiation necrosis in mouse brain. Clin Cancer Res. 2014;20(10):2695–2702. | ||
Gao X, Zhao Y, Stemmer-Rachamimov AO, et al. Anti-VEGF treatment improves neurological function and augments radiation response in NF2 schwannoma model. Proc Natl Acad Sci U S A. 2015;112(47):14676–14681. | ||
Matsumoto K, Ema M. Roles of VEGF-A signalling in development, regeneration, and tumours. J Biochem. 2014;156(1):1–10. | ||
Sclabas GM, Uwagawa T, Schmidt C, et al. Nuclear factor κB activation is a potential target for preventing pancreatic carcinoma by aspirin. Cancer. 2005;103(12):2485–2490. | ||
Takada Y, Kobayashi Y, Aggarwal BB. Evodiamine abolishes constitutive and inducible NF-κB activation by inhibiting InBa kinase activation, thereby suppressing NF-κB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J Biol Chem. 2005;280(17):17203–17212. | ||
Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24(46):6957–6969. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



