Back to Journals » Nature and Science of Sleep » Volume 13
The Role of Perioperative Sleep Disturbance in Postoperative Neurocognitive Disorders
Authors Wang X, Hua D, Tang X, Li S, Sun R, Xie Z, Zhou Z, Zhao Y, Wang J, Li S , Luo A
Received 20 May 2021
Accepted for publication 21 July 2021
Published 6 August 2021 Volume 2021:13 Pages 1395—1410
DOI https://doi.org/10.2147/NSS.S320745
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Xuan Wang, Dongyu Hua, Xiaole Tang, Shan Li, Rao Sun, Zheng Xie, Zhiqiang Zhou, Yilin Zhao, Jintao Wang, Shiyong Li, Ailin Luo
Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, Hubei, People’s Republic of China
Correspondence: Shiyong Li; Ailin Luo Email [email protected]; [email protected]
Abstract: Postoperative neurocognitive disorder (PND) increases the length of hospital stay, mortality, and risk of long-term cognitive impairment. Perioperative sleep disturbance is prevalent and commonly ignored and may increase the risk of PND. However, the role of perioperative sleep disturbances in PND remains unclear. Nocturnal sleep plays an indispensable role in learning, memory, and maintenance of cerebral microenvironmental homeostasis. Hospitalized sleep disturbances also increase the incidence of postoperative delirium and cognitive dysfunction. This review summarizes the role of perioperative sleep disturbances in PND and elucidates the potential mechanisms underlying sleep-deprivation-mediated PND. Activated neuroinflammation and oxidative stress; impaired function of the blood-brain barrier and glymphatic pathway; decreased hippocampal brain-derived neurotrophic factor, adult neurogenesis, and sirtuin1 expression; and accumulated amyloid-beta proteins are associated with PND in individuals with perioperative sleep disorders. These findings suggest that the improvement of perioperative sleep might reduce the incidence of postoperative delirium and postoperative cognitive dysfunction. Future studies should further investigate the role of perioperative sleep disturbance in PND.
Keywords: perioperative sleep disturbance, cognitive impairment, postoperative delirium, postoperative cognitive dysfunction, neuroinflammation
Introduction
Nocturnal sleep plays an essential role in learning and memory through specific oscillations.1,2 As an indispensable part of the circadian rhythm, sleep promotes the removal of metabolic wastes to maintain cerebral microenvironmental homeostasis.3–5 The effects of sleep disruption on brain structure and cognitive performance have attracted increasing attention in recent years. The prevalence of sleep disturbance is 30.6–41.2% in the elderly at home6 and is much higher in hospitalized patients due to ward environment and patients’ primary diseases.7 Notably, sleep disturbance before admission has been proven to increase the risk of Alzheimer’s disease (AD),8 which would further promote the development of postoperative neurocognitive disorders in elderly patients.9,10 Contrary to the causes of sleep disturbance at home, perioperative sleep disturbance significantly increases the risk of postoperative complications, including postoperative delirium (POD) and postoperative cognitive dysfunction (POCD).11,12
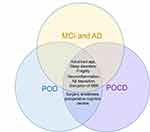
POD and POCD are common postoperative neurocognitive complications in the elderly and belong to perioperative cognitive dysfunction.13 In view of the extensive use of the terms “POCD” and “POD” to date, we use these terms in this review. POD and POCD share similar risk factors with preoperative neurocognitive disorders (Figure 1) and may co-exist following surgical procedures. POCD is more common in elderly patients with POD.14 POD and POCD would further cause long-term postoperative cognitive decline in elderly patients.15,16 Although extensive studies have focused on the potential mechanisms underlying postoperative neurocognitive disorders, to date, they remain unclear. In this review, we summarize the epidemiology, risk factors, potential mechanisms, current diagnosis, and treatments of preoperative and postoperative neurocognitive disorders (Table 1).
 |
Table 1 Epidemiology, Potential Mechanisms, Current Diagnosis and Treatments of Perioperative Neurocognitive Disorder |
Perioperative sleep disturbance may play a crucial role in postoperative neurocognitive disorders in light of the function of sleep in memory consolidation and the effect of sleep disturbance on cognition. However, little attention has been paid to investigating the role of perioperative sleep disturbances in postoperative neurocognitive disorders. Based on the role of sleep in memory and the role of sleep disruption in cognitive impairment, this review elaborates on the potential mechanisms by which perioperative sleep disturbance contributes to POD and POCD. The findings would shed light on the development of potential treatment strategies for POCD.
Role of Sleep in Learning and Memory
Normal Sleep and Its Physiological Role in Memory and Forgetting
According to electroencephalogram (EEG), sleep is divided into rapid-eye-movement (REM) sleep and non-rapid-eye-movement (NREM) sleep.17 NREM sleep accounts for 75–80% of the whole sleep and can be divided into four stages: N1, N2, N3, and N4;18 N3 and N4 are collectively known as slow-wave sleep (SWS).18 Electricity activities in NREM sleep include slow oscillations (SOs), spindles, and sharp-wave ripples;19 SOs originate in the neocortex,20 spindles generate from the thalamus, and sharp waves generate from the hippocampus.19,21 SOs, spindles, and hippocampal sharp waves couple to promote the transformation from information to long-term memory, indicating the dialogue of the neocortex-thalamus-hippocampus.22 Moreover, contrary to the alpha waves and beta waves during waking time, the dominating brain waves during sleep are delta waves and theta waves.19 As one of the hallmarks of SWS, delta waves (1–4 Hz) are similar to SOs (~1 Hz) with higher field potential rhythms.23 Delta waves have been proved to be beneficial to forgetting, while SOs is of advantage to memory preservation.23 Additionally, delta electrical stimulation of the neocortex enhances SOs, which strengthens the oscillation of spindles in the thalamus.5 These oscillations’ enhancement promotes the transformation from short-term memory stored in the hippocampus to long-term memory stored in the cortex.5 Moreover, REM sleep’s electricity activities consist of ponto-geniculo-occipital (PGO) waves and theta activities.24 During REM sleep, melanin-concentrating hormone-producing neurons (MCH neurons), which are located at the hypothalamus and project to the hippocampus densely, selectively promote the forgetting of hippocampal-dependent memory,2 and activation of MCH neurons impairs hippocampus-dependent memory.2 In summary, SOs mainly preserve memory by coupling spindles in NREM sleep,25 delta waves in NREM sleep and MCH neurons in REM sleep contribute to forgetting,2,25 and unnecessary memories can also be removed by synaptic remodeling to support new learning and memory based on the synaptic homeostasis hypothesis (SHY).26 Therefore, normal sleep ensures the function of learning and memory through the balance of information retention and forgetting.
Abundant studies on the brain network reveal that NREM sleep mainly facilitates consolidation of declarative memory, especially hippocampus-dependent memory, and REM sleep consolidates the memory of procedure and emotion.1 Moreover, the glial-lymphatic (glymphatic) pathway is active during sleep and facilitates the flow of cerebrospinal fluid (CSF) from perivascular spaces to the brain parenchyma through aquaporin 4 (AQP4) water channels.27 The convection velocity between interstitial fluid (ISF) and CSF increases significantly during natural sleep, which elevates the clearance of amyloid-beta (Aβ) protein in the brain of rodents.28 Additionally, a clinical study also suggests that during NREM sleep, CSF shows pulsed flow with large amplitude to enhance the clearance of cerebral metabolic wastes.3 Taken together, these findings prove the indispensable role of sleep in the formation and consolidation of memory and forgetting.
Circadian Rhythms and Regulation of Sleep
Circadian rhythms are internal biological processes that occur regularly within a period of approximately 24 hours and are controlled by clock genes.29 Circadian rhythms have a feedback mechanism. The suprachiasmatic nucleus (SCN) in the hypothalamus serves as a primary pacemaker and controls peripheral cells as well as the circadian rhythms of thousands of downstream genes synchronously after receiving the optical signals from the retina.30 This process can be modulated by an autonomous transcription and translation feedback loop within the SCN to adapt to the external environment (including light, feeding, and temperature).31,32 Remarkably, circadian rhythms participate in memory, possibly by outputting gamma-aminobutyric acid from the SCN to learning target sites; this process is independent of sleep and indicates subregional crosstalk.33 The distribution of CSF and perivascular polarization of AQP4 are also under the control of circadian rhythm.34 Accordingly, the disturbance of circadian rhythm contributes to hippocampal-dependent cognitive impairment.35
As an essential part of circadian rhythm, sleep could be modulated by homeostatic drive, indicating that the body suffers from more pressure induced by adenosine (a degradation product of ATP) to fall into sleep due to sleep insufficiency.36 Moreover, subsequent NREM sleep after sleep deprivation is longer and deeper to meet homeostatic requirements.37 However, this homeostatic modulation would be attenuated after chronic sleep restriction.38 In addition, another modulation of sleep is circadian rhythm involving the periodic release of melatonin.37 Keeping in line with the pacemaker, the plasma level of melatonin is high during the night but low during the day.39 However, melatonin secretion is suppressed by even moderate light at night, which may be the precipitating factor of the high prevalence of sleep disorder in the hospital.40 Periodic release of melatonin not only consolidates clock genes’ expression patterns in the hippocampus but also enhances learning efficiency by modulating the synaptic plasticity during the daytime.41 These studies indicate the importance of normal circadian rhythm in maintaining sleep and awakening.
Role of Sleep Disruption in Cognitive Impairments
Growing evidence indicates that sleep disruption is associated with impairment of spatial memory, verbal fluency, attention, processing speed, and executive function.42–45 AD is the most prevalent type of major neurocognitive disorder in the elderly. A large proportion of AD victims suffer from sleep disorders,43 which in turn promotes the development of AD in the elderly.45 Particularly, insomnia is associated with poor cognitive performances and lower volumes of grey matter,46 and the atrophy of grey matter in the medial prefrontal cortex (mPFC) is associated with attenuated SOs-spindles coupling and impairment of hippocampal-dependent memory in the elderly.22 Remarkably, both insufficient sleep duration (less than 6 hours) and excessive sleep duration (more than 9 hours) are associated with cognitive impairment,47 and sleep fragmentation also accelerates the development of preoperative NCD.44
In addition, sleep deprivation also promotes deposition of Aβ protein in the hippocampus48,49 and increases the ratio of p-tau/total tau, thereby accelerating the occurrence and development of AD.50 Hence, management of sleep is expected to decrease the incidence of neurocognitive disorders in the elderly.51 Notably, except for specific effects of surgery and anesthesia on perioperative sleep, there are many similarities between perioperative sleep disturbance and sleep disruption at home.52–54 Based on the common characteristics of sleep disruption at home and perioperative sleep disturbance, we could provide more directions and choices for future studies investigating postoperative neurocognitive disorders.
Perioperative Sleep Disturbance and Postoperative Neurocognitive Disorders
In contrast to sleep disorders at home, perioperative sleep disturbance refers to disrupted sleep structure or decreased sleep duration at night after admission. Approximately 64% of hospitalized patients suffer from sleep disruption.7,55 The diagnosis of perioperative sleep disturbance depends on combined subjective questionnaires of sleep and objective examinations, such as eye and hand movement sensors, polysomnography, and EEG.7,56,57 As for treatments, present measures consist of perioperative psychological support58 and drugs including benzodiazepine hypnotics,59 melatonin receptor agonists,60 and analgesic and narcotic sedatives.61,62
Many studies on sleep disturbance at home and cognitive disorders have elucidated the role of perioperative sleep disturbance in POD and POCD.43 Except for certain effects of anesthesia and surgery, perioperative sleep disturbance may have some similarities with sleep disturbance at home and plays an important role in the development of neurocognitive disorders. Indeed, preoperative and postoperative sleep disturbances are associated with POD and POCD.12,52,63,64 Based on the role of sleep disruption at home in cognitive impairments and the impact of perioperative sleep disturbance on postoperative neurocognitive disorders, we summarize the potential mechanisms by which perioperative sleep disturbance promotes the development of postoperative neurocognitive disorders.
Impact of Surgery and Anesthesia on Perioperative Sleep
Perioperative sleep disturbances occurring in different periods may have various effects on postoperative neurocognitive disorders. To clarify this, we should first understand the impact of surgery and anesthesia on perioperative sleep and circadian rhythm. Surgery and anesthesia may accelerate the development of postoperative neurocognitive disorders.
Sleep disruption after admission is caused by noise, inadequate light, medical devices, and pain.7 The characteristics of EEG during anesthesia are different from those during physiological sleep, and the neural circuits involved in anesthetic sedation are also different from those in physiological sleep.65 Moreover, some general anesthetics, including isoflurane and propofol, disrupt the circadian rhythm of melatonin secretion.66 However, there are also many similarities between anesthesia and normal NREM sleep, including increased dominating slow waves and sleep spindles.67 These similarities may promote the recovery of preoperative sleep loss.68 Nevertheless, REM sleep is significantly reduced at the first night after surgery and would return to preoperative baseline at the third night after surgery.69 These findings indicate that superimposed effects of anesthetic and surgery, at least partially, contribute to postoperative sleep disorders in patients.
Furthermore, melatonin’s secretion is elevated by desflurane during the day and may be associated with stimulation of the sympathetic nerve.66 Interestingly, the use of isoflurane during the day causes significant suppression of circadian rhythm genes, including BMAL1, CLOCK, PER2, and CRY2, with only minimal inhibition at night;70 Three kinds of gas anesthetics all show no effect on the secretion of melatonin at night.71 Besides, circadian rhythm involving the circadian activity level has been proven to be severely disturbed after large surgery.72 For instance, melatonin concentration is reduced on the first night after surgery, and treatments of melatonin could prevent related sleep disturbances.73 These findings verify different effects of anesthesia and surgery on sleep during day and night, indicating that anesthesia and surgery during the night conform to the normal circadian rhythm and have smaller impacts on circadian rhythms.
Association Between Perioperative Sleep Disturbance and POD
Sleep disturbance at home is an independent risk factor for POD,74 and perioperative sleep disturbance increases the risk of POD11,75 (Table 2). Moreover, one study argued that perioperative sleep disturbance could be used as a predictor of POD in elderly patients in the ICU after heart surgery.76 Indeed, the improvement of sleep in elderly patients could reduce the risk of POD, further verifying the potential role of sleep disturbance in POD.77
 |
Table 2 Clinical Studies on Examining the Effect of Perioperative Sleep Disturbance on POD |
There are confounding factors for establishing a link between preoperative sleep disturbance and POD. Postoperative pain is inevitable following major surgery, and improper management of pain not only decreases the total sleep duration78 but also impacts the cognitive scores.79 The secretion of melatonin, an important regulator of circadian rhythm, is delayed during anesthesia and surgery,80–82 and the delay lasts up to the first night following surgery.73 Postoperative supplementation with melatonin prevents related sleep disturbances83,84 but fails to reduce the incidence of POD in elderly patients with nosocomial hip fractures.85 Interestingly, preoperative treatment with melatonin reduces the incidence of POD,86 indicating that early management of sleep disturbance after admission would be beneficial to postoperative cognitive outcomes.
Although no definitive conclusion has been reached, we speculate that preoperative sleep disturbance would promote the occurrence of POD and further increase the long-term cognitive impairment in patients after discharge.74,87 With regard to the relationship between perioperative sleep disturbance and POD, mutual crosstalk rather than a causal link is more likely.
Association Between Perioperative Sleep Disturbance and POCD
Acute sleep deprivation caused by the ward environment or patients’ diseases is very common in the hospital and leads to damaged sleep structure and decreased sleep duration.88,89 Nevertheless, only a few studies have investigated the role of perioperative sleep disturbance in POCD. In view of the high incidence and harmful outcomes of POCD, more attention should be paid to the role of perioperative sleep disturbance in POCD.
Due to the absence of unified diagnostic criteria for POCD, there are few clinical studies directly pertaining to perioperative sleep disturbance and POCD (Table 3). Some scholars consider postoperative sleep disturbance a phenotype of POCD.90,91 POCD is associated with increased perioperative awakening times and subjective poor sleep quality in hospitalized patients.92 The occurrence of perioperative sleep disturbance may be partly due to altered rhythm of melatonin secretion.93 However, there is a lack of clinical evidence to support the role of melatonin in perioperative sleep disturbance-related POCD.
 |
Table 3 Clinical Studies on Exploring the Role of Perioperative Sleep Disturbance in POCD |
Moreover, during normal aging, the duration of total sleep and slow-wave sleep is decreased, but sleep fragmentation and the difficulty of falling asleep are increased.94 Advanced age is a known risk factor for POCD,95 suggesting potential undiagnosed sleep disturbance in the elderly before admission. Sleep quality is worse in hospitals, thus inducing overlapping pathophysiological pathways.
Potential Mechanisms Underlying Perioperative Sleep Disturbance-Associated POCD
A wealth of preclinical studies have indicated a close association between perioperative sleep disturbance and POCD. However, the specific mechanisms require further exploration. In this section, we summarize preclinical evidence on the adverse effect of perioperative sleep disturbance on cognition (Table 4) and the potential mechanism of perioperative sleep disturbance in POCD (Table 5). We describe anatomical changes in the brain and illustrate potential mechanisms associated with perioperative sleep disturbance-induced POCD.
 |
Table 4 Preclinical Studies on Investigating the Association Between Sleep Disturbance and Cognitive Impairments |
 |
Table 5 Preclinical Studies on Exploring the Association Between Perioperative Sleep Disturbance and Postoperative Neurocognitive Disorder |
Perioperative Sleep Disturbance-Induced Structural Changes
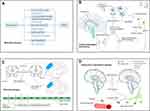
In parallel with peripheral inflammation, the integrity and function of the blood-brain barrier are impaired by sleep restriction and surgery,96 which are known to be involved in POCD.97 According to MRI findings, sleep fragmentation induces hippocampal atrophy due to decreased neurogenesis in the dentate gyrus (DG) and neuronal loss in the cornu ammonis (CA),98 which is thought to be the origin of slow waves. MPFC atrophy during aging has been used to predict the impairment of slow-wave activity and NREM-associated and hippocampal-dependent memory.22 Furthermore, sleep disturbance decreases hippocampal-prefrontal cortex functional connectivity and thus impairs memory consolidation.22,99 Strikingly, individuals with inadequate sleep duration exhibit decreased white matter integrity and cognitive impairment.100 Moreover, as mentioned above, perioperative sleep disturbance includes changes in structure and duration. Therefore, perioperative sleep disturbances may induce extensive abnormalities in the brain.
The glymphatic pathway is a recently discovered cerebral structure, and its function is similar to that of the peripheral lymphatic system.101 Its clearing activity in protein waste products is dependent on the exchange of CSF-ISF and polarized expression of AQP4 on astrocytic endfeet;27 the glymphatic pathway is most active during NREM sleep3,102 and is enhanced during general anesthesia.103 As a noxiously neurotoxic substance, Aβ is mainly cleared through the glymphatic system.3 The level of Aβ in the ISF is elevated in the dark, and the amplitude of elevation is inversely proportional to the NREM sleep time.32 Additionally, sleep deprivation inhibits the inflow of apolipoprotein E (APOE) in CSF and clearance of APOE in ISF,104 suggesting that sleep deprivation reduces the removal of Aβ in CSF. Notably, it was reported that even the deprivation of one-night sleep would significantly increase cerebral Aβ levels in middle-aged men;49 Excessive cerebral Aβ increases the wakefulness of mice, which in turn affects the sleep-wake cycle.105 Moreover, Aβ plaques also trigger the mislocalization of AQP4 and decrease CSF influx, thus forming a vicious circle.104 Notably, Aβ accumulation in the brain is associated with neuropathological changes that contribute to cognitive dysfunction in MCI, AD, and POCD.106–108 Based on these findings, we hypothesize that perioperative sleep disorder contributes to POCD by impairing the waste protein clearance of the glymphatic system. However, it was reported that general anesthesia, especially high-dose agents, could inhibit the glymphatic system; thus, further investigation is needed to determine whether perioperative sleep disturbance triggers original changes in the glymphatic system and whether appropriate anesthetics promote glymphatic influx.
Taken together, these findings suggest that perioperative sleep disturbances induce structural changes in the brain and possibly contribute to cognitive impairment. Future studies are needed to ascertain whether there is a causal link between anatomical abnormalities in the brain and perioperative sleep disturbance-associated POCD.
Potential Signaling Pathways
Morphological changes in the brain detected by imaging methods are usually caused by pathological abnormalities at the cellular and molecular levels. With regard to pathological changes in POCD, neuronal injury and overactivated microglia and astrocytes have been observed in animal models.109,110 Coincidentally, sleep deprivation upregulates the expression of proinflammatory cytokines in the hippocampus,111 facilitates microglial activation by suppressing melatonin secretion,112 promotes Aβ deposition,113 and increases the expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.114 Furthermore, sleep deprivation impairs the microglial activity of synaptic pruning, which is essential to form the functional neural circuits in learning.115 Overactive neuroinflammation is usually considered the culprit of POCD.109,110 Studies examining the effect of sleep disturbance on POCD have revealed that acute preoperative sleep disruption increases the level of proinflammatory cytokines, which consequently impairs cognitive performance.52,64 Moreover, surgical procedures upregulate proinflammatory cytokines in the peripheral plasma and hippocampus and induce or exaggerate neuroinflammation.97,116–118 Furthermore, the inflammatory environments, in turn, deteriorate sleep quality.119 In other words, a vicious circle is formed. Additionally, sleep deprivation activates neurotoxic complement components C3a and C5a, which disturb the hippocampal brain-derived neurotrophic factor (BDNF) pathway and adult neurogenesis, eventually impairing spatial memory.120 Considering the complicated effect of general anesthetics on immune function,121 it is a great challenge to distinguish which factor (sleep disturbance, surgical procedures, or general anesthetics) is the last straw to prime the neuroinflammatory cascade.
In addition, astrocytic ATP and adenosine A1 receptor activity contribute to sleep deprivation-induced deficits in hippocampal synaptic plasticity and hippocampus-dependent spatial memory.122 Interestingly, the astrocyte circadian clock regulates inflammatory Chi3l1 induction and further reduces the phagocytosis of microglia in the context of AD neuropathogenesis.123 Microglia-derived factors could induce the transition of astrocytes to neurotoxic phenotype A1 astrocytes.124 In light of the complicated crosstalk between microglia and astrocytes, it is difficult to identify the initial changes in sleep deprivation-induced cognitive impairment. In summary, perioperative sleep disturbance, particularly acute sleep deprivation, may induce overactivated microglia and astrocytes, thus resulting in postoperative cognitive impairment.
In addition to the indirect modulation effect of microglia and astrocytes, sleep disruption can directly impair neuronal excitability and synaptic plasticity.18 Specifically, acute sleep deprivation reduces dendritic spine density in the hippocampal neurons and results in long-term memory impairment, while recovery sleep ameliorates spine loss and memory impairment.125 Moreover, sleep deprivation induces abnormal hippocampal neuronal autophagy and apoptosis, which contribute to cognitive impairment.126 Reduced neurogenesis and BDNF mediated by overactivated microglia are associated with sleep disruption-induced spatial memory impairment.111 Therefore, direct and indirect neuronal injuries are involved in sleep deprivation-induced hippocampal-dependent learning and memory.
Sleep deprivation also causes oxidative stress by downregulating the α7 nicotinic acetylcholine receptor and downstream PI3K/AKT/GSK-3β in the hippocampus.127 In addition, as anti-inflammatory and anti-oxidative factors, Nrf2 and HO-1 are also downregulated by sleep deprivation.127 The PI3K/AKT/Nrf2/HO-1 pathway has been confirmed to be involved in POCD.128 Moreover, activation of the Nrf2/HO-1 pathway improves cognitive impairments induced by sleep deprivation,129 and the elevated expression of NADPH oxidase in the hippocampus and cortex induced by sleep fragmentation promotes hippocampal memory impairments.130 NADPH oxidase in the central nervous system produces redundant reactive oxygen species (ROS), thus promoting the development of POCD.114
BDNF is involved in hippocampus-dependent memory formation and consolidation by regulating synaptic plasticity and hippocampal neurogenesis.131 On the one hand, the BDNF signaling pathway plays a key role in maintaining homeostasis of REM sleep without sex difference.132–134 On the other hand, abnormal sleep reduces the expression of BDNF. One study suggests that the reduction of peripheral BDNF possibly contributes to cognitive impairment in insomnia patients whose sleep duration is less than 6 hours.132 Notably, the expression level of BDNF in the hippocampus, but not in the neocortex, is lower in chronic sleep restriction and sleep deprivation models.135,136 Considering the role of the abnormal BDNF pathway in POCD,137–139 abnormal changes in the BDNF signaling pathway induced by sleep disturbances, especially preoperative sleep disturbances, are possibly further exaggerated by other perioperative stress and contribute to cognitive impairment following surgery.
Sirtuin 1 (SIRT1) in the hippocampus is reduced after total sleep deprivation or surgery.140,141 Interestingly, melatonin enhances memory by restoring the activity of SIRT1 after total sleep deprivation.140 Our study and other studies found that the reduction of SIRT1 was associated with postoperative cognitive impairment by activating the neuroinflammatory pathway and inhibiting neurotrophic factors.141,142 Considering our previous findings that SIRT1 mediated abnormal tau modification in an aged POCD model,143 it is plausible that SIRT1 is involved in sleep disturbance-related POCD. Future studies are needed to validate this hypothesis.
Based on sleep disturbance-induced neuropathogenesis ranging from morphological changes to abnormal signaling pathways, we conclude that perioperative sleep disturbance induces extensive changes that possibly contribute to POCD. The potential mechanisms are shown in Figure 2.
Conclusions
Perioperative sleep disturbances are prevalent among surgical individuals. Clinical findings indicate that perioperative sleep disturbances increase the risk of POD and POCD, especially in the elderly population. We summarize the potential mechanisms underlying perioperative sleep-disturbance-associated neurocognitive disorders. There are extensive overlapping neuropathological changes, such as neuroinflammation, ROS generation, and reduction in BDNF and SIRT1, between sleep disturbance-induced cognitive impairments and POCD. Some preclinical studies have shown that restoring sleep could alleviate the injury and improve cognitive function. These findings indicate that early screening and timely intervention of perioperative sleep disturbances would be a far-reaching way to prevent POCD. Moreover, appropriate operative time and anesthetic medication may also contribute to maintain perioperative circadian rhythm and reduce the risk of postoperative neurocognitive disorders.
Abbreviations
Aβ, amyloid-beta; AD, Alzheimer’s disease; APOE, apolipoprotein E; AQP4, aquaporin 4; BDNF, brain-derived neurotrophic factor; CA, cornu ammonis; CSF, cerebrospinal fluid; DG, dentate gyrus; EEG, electroencephalogram; ISF, interstitial fluid; MCH, melanin-concentrating hormone; mPFC, medial prefrontal cortex; NADPH, nicotinamide adenine dinucleotide phosphate; NREM, non-rapid-eye-movement; POD, postoperative delirium; POCD, postoperative cognitive dysfunction; PND, perioperative cognitive dysfunction; PGO, ponto-geniculo-occipital; REM, rapid-eye-movement; ROS, reactive oxygen species; SCN, suprachiasmatic nucleus; SHY, synaptic homeostasis hypothesis; SOs, slow oscillations; SIRT1, Sirtuin1; SWS, slow-wave sleep.
Acknowledgments
The present work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81771159 and 81571047 to Ai-lin Luo) and China National Key R&D (Program No. 2020YFC2009002 to Ai-lin Luo). Additionally, we would like to thank Editage (www.editage.cn) for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13(5):309–321. doi:10.1016/j.smrv.2008.08.002
2. Izawa S, Chowdhury S, Miyazaki T, et al. REM sleep-active MCH neurons are involved in forgetting hippocampus-dependent memories. Science. 2019;365(6459):1308–1313. doi:10.1126/science.aax9238
3. Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366(6465):628–631. doi:10.1126/science.aax5440
4. Raven F, Van der Zee EA, Meerlo P, Havekes R. The role of sleep in regulating structural plasticity and synaptic strength: implications for memory and cognitive function. Sleep Med Rev. 2018;39:3–11. doi:10.1016/j.smrv.2017.05.002
5. Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. doi:10.1038/nature05278
6. Lu L, Wang S-B, Rao W, et al. The prevalence of sleep disturbances and sleep quality in older chinese adults: a comprehensive meta-analysis. Behav Sleep Med. 2019;17(6):683–697. doi:10.1080/15402002.2018.1469492
7. Wesselius HM, van den Ende ES, Alsma J, et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018;178(9):1201–1208. doi:10.1001/jamainternmed.2018.2669
8. Shi L, Chen S-J, Ma M-Y, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. doi:10.1016/j.smrv.2017.06.010
9. Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg. 2018;127(2):496–505. doi:10.1213/ANE.0000000000003514
10. Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249(1):173–178. doi:10.1097/SLA.0b013e31818e4776
11. Evans JL, Nadler JW, Preud’homme XA, et al. Pilot prospective study of post-surgery sleep and EEG predictors of post-operative delirium. Clin Neurophysiol. 2017;128(8):1421–1425. doi:10.1016/j.clinph.2017.05.004
12. Song J, Chu S, Cui Y, et al. Circadian rhythm resynchronization improved isoflurane-induced cognitive dysfunction in aged mice. Exp Neurol. 2018;306:45–54. doi:10.1016/j.expneurol.2018.04.009
13. Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121(5):1005–1012. doi:10.1016/j.bja.2017.11.087
14. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316–323. doi:10.1093/bja/aex130
15. Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi:10.1056/NEJMoa1112923
16. Monk TG, Weldon B, Garvan C, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. doi:10.1097/01.anes.0000296071.19434.1e
17. Kilduff TS, Lein ES, de la Iglesia H, et al. New developments in sleep research: molecular genetics, gene expression, and systems neurobiology. J Neurosci. 2008;28(46):11814–11818. doi:10.1523/JNEUROSCI.3768-08.2008
18. Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol. 2013;23(17):R774–788. doi:10.1016/j.cub.2013.07.025
19. De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7(5):423–440. doi:10.1053/smrv.2002.0252
20. Molle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J Neurosci. 2002;22(24):10941–10947. doi:10.1523/jneurosci.22-24-10941.2002
21. Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31(3):551–570. doi:10.1016/0306-4522(89)90423-5
22. Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16(3):357–364. doi:10.1038/nn.3324
23. Ngo HV, Born J. Sleep and the balance between memory and forgetting. Cell. 2019;179(2):289–291. doi:10.1016/j.cell.2019.09.007
24. Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi:10.1038/nrn2762
25. Kim J, Gulati T, Ganguly K. Competing roles of slow oscillations and delta waves in memory consolidation versus forgetting. Cell. 2019;179(2):514–526 e513. doi:10.1016/j.cell.2019.08.040
26. Li W, Ma L, Yang G, Gan WB. REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci. 2017;20(3):427–437. doi:10.1038/nn.4479
27. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–1024. doi:10.1016/S1474-4422(18)30318-1
28. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi:10.1126/science.1241224
29. Panda S. The arrival of circadian medicine. Nat Rev Endocrinol. 2019;15(2):67–69. doi:10.1038/s41574-018-0142-x
30. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35(1):445–462. doi:10.1146/annurev-neuro-060909-153128
31. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291(5503):490–493. doi:10.1126/science.291.5503.490
32. Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi:10.1126/science.1180962
33. Nf R, Hwang CE, Wessells C, et al. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci U S A. 2008;105(40):15593–15598. doi:10.1073/pnas.0808259105
34. Hablitz LM, Plá V, Giannetto M, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11(1):4411. doi:10.1038/s41467-020-18115-2
35. Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 2011;108(4):1657–1662. doi:10.1073/pnas.1018375108
36. Peng W, Wu Z, Song K, et al. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science. 2020;369(6508):eabb0556. doi:10.1126/science.abb0556
37. Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204.
38. Skorucak J, Weber N, Carskadon MA, et al. Homeostatic response to sleep restriction in adolescents. Sleep. 2021. doi:10.1093/sleep/zsab106
39. Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev. 2005;9(1):5–9. doi:10.1016/j.smrv.2004.11.004
40. Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi:10.1111/j.1469-7793.2000.00695.x
41. Jilg A, Bechstein P, Saade A, et al. Melatonin modulates daytime-dependent synaptic plasticity and learning efficiency. J Pineal Res. 2019;66(3):e12553. doi:10.1111/jpi.12553
42. Zhu B, Dong Y, Xu Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48(3):348–355. doi:10.1016/j.nbd.2012.06.022
43. Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–1032. doi:10.5665/sleep.2802
44. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. doi:10.1016/S1474-4422(14)70172-3
45. Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi:10.1001/jama.2011.1115
46. Grau-Rivera O, Operto G, Falcón C, et al. Association between insomnia and cognitive performance, gray matter volume, and white matter microstructure in cognitively unimpaired adults. Alzheimers Res Ther. 2020;12(1):4. doi:10.1186/s13195-019-0547-3
47. Ramos AR, Tarraf W, Daviglus M, et al. Sleep duration and neurocognitive function in the hispanic community health study/study of Latinos. Sleep. 2016;39(10):1843–1851. doi:10.5665/sleep.6166
48. Ju YS, Ooms SJ, Sutphen C, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain. 2017;140(8):2104–2111. doi:10.1093/brain/awx148
49. Ooms S, Overeem S, Besse K, et al. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71(8):971–977. doi:10.1001/jamaneurol.2014.1173
50. Guisle I, Gratuze M, Petry S, et al. Circadian and sleep/wake-dependent variations in tau phosphorylation are driven by temperature. Sleep. 2019;43(4):zsz266. doi:10.1093/sleep/zsz266
51. Xu W, Tan CC, Zou JJ, Cao XP, Tan L. Sleep problems and risk of all-cause cognitive decline or dementia: an updated systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91(3):236–244. doi:10.1136/jnnp-2019-321896
52. Ni P, Dong H, Zhou Q, et al. Preoperative sleep disturbance exaggerates surgery-induced neuroinflammation and neuronal damage in aged mice. Mediators Inflamm. 2019;2019:8301725. doi:10.1155/2019/8301725
53. Bellesi M, de Vivo L, Chini M, et al. Sleep loss promotes astrocytic phagocytosis and microglial activation in mouse cerebral cortex. J Neurosci. 2017;37(21):5263–5273. doi:10.1523/JNEUROSCI.3981-16.2017
54. Pak VM, Onen S-H, Bliwise DL, et al. Sleep disturbances in MCI and AD: neuroinflammation as a possible mediating pathway. Front Aging Neurosci. 2020;12:69. doi:10.3389/fnagi.2020.00069
55. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. doi:10.1093/sleep/18.6.425
56. Rosenberg J, Wildschiødtz G, Pedersen MH, von Jessen F, Kehlet H. Late postoperative nocturnal episodic hypoxaemia and associated sleep pattern. Br J Anaesth. 1994;72(2):145–150. doi:10.1093/bja/72.2.145
57. Chung F, Liao P, Yang Y, et al. Postoperative sleep-disordered breathing in patients without preoperative sleep apnea. Anesth Analg. 2015;120(6):1214–1224. doi:10.1213/ane.0000000000000774
58. Scarpa M, Pinto E, Saraceni E, et al. Randomized clinical trial of psychological support and sleep adjuvant measures for postoperative sleep disturbance in patients undergoing oesophagectomy. Br J Surg. 2017;104(10):1307–1314. doi:10.1002/bjs.10609
59. Whitehead C, Sanders L, Appadurai I, et al. Zopiclone as a preoperative night hypnotic: a double-blind comparison with temazepam and placebo. Br J Anaesth. 1994;72(4):443–446. doi:10.1093/bja/72.4.443
60. Bourne RS, Mills GH. Melatonin: possible implications for the postoperative and critically ill patient. Intensive Care Med. 2006;32(3):371–379. doi:10.1007/s00134-005-0061-x
61. Treggiari-Venzi M, Borgeat A, Fuchs-Buder T, Gachoud JP, Suter PM. Overnight sedation with midazolam or propofol in the ICU: effects on sleep quality, anxiety and depression. Intensive Care Med. 1996;22(11):1186–1190. doi:10.1007/bf01709334
62. Lee A, O’Loughlin E, Roberts LJ. A double-blinded randomized evaluation of alfentanil and morphine vs fentanyl: analgesia and sleep trial (DREAMFAST). Br J Anaesth. 2013;110(2):293–298. doi:10.1093/bja/aes362
63. Hou J, Shen Q, Wan X, et al. REM sleep deprivation-induced circadian clock gene abnormalities participate in hippocampal-dependent memory impairment by enhancing inflammation in rats undergoing sevoflurane inhalation. Behav Brain Res. 2019;364:167–176. doi:10.1016/j.bbr.2019.01.038
64. Lu B, Liu RJ, Meng B, et al. [Effect of fragmented sleep on postoperative cognitive function and central neuroinflammation]. Zhonghua Yi Xue Za Zhi. 2020;100:1341–1344. Chinese. doi:10.3760/cma.j.cn112137-20191215-02734
65. Brown EN, Lydic R, Schiff ND, Schwartz RS. General anesthesia, sleep, and coma. N Engl J Med. 2010;363(27):2638–2650. doi:10.1056/NEJMra0808281
66. Naguib M, Gottumukkala V, Goldstein PA. Melatonin and anesthesia: a clinical perspective. J Pineal Res. 2007;42(1):12–21. doi:10.1111/j.1600-079X.2006.00384.x
67. Nelson LE, Franks NP, Maze M. Rested and refreshed after anesthesia? Overlapping neurobiologic mechanisms of sleep and anesthesia. Anesthesiology. 2004;100(6):1341–1342. doi:10.1097/00000542-200406000-00003
68. Benveniste H, Heerdt PM, Fontes M, Rothman DL, Volkow ND. Glymphatic system function in relation to anesthesia and sleep states. Anesth Analg. 2019;128(4):747–758. doi:10.1213/ane.0000000000004069
69. Krenk L, Jennum P, Kehlet H. Sleep disturbances after fast-track hip and knee arthroplasty. Br J Anaesth. 2012;109(5):769–775. doi:10.1093/bja/aes252
70. Gökmen N, Baris I, Ocmen E, et al. Day-time isoflurane administration suppresses circadian gene expressions in both the brain and a peripheral organ, liver. Turk J Anaesthesiol Reanim. 2017;45(4):197. doi:10.5152/TJAR.2017.68466
71. Ocmen E, Erdost HA, Duru LS, et al. Effect of day/night administration of three different inhalational anesthetics on melatonin levels in rats. Kaohsiung J Med Sci. 2016;32(6):302–305. doi:10.1016/j.kjms.2016.04.016
72. Gogenur I, Bisgaard T, Burgdorf S, van Someren E, Rosenberg J. Disturbances in the circadian pattern of activity and sleep after laparoscopic versus open abdominal surgery. Surg Endosc. 2009;23(5):1026–1031. doi:10.1007/s00464-008-0112-9
73. Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Melatonin secretion after surgery. Lancet. 2000;356(9237):1244–1245. doi:10.1016/S0140-6736(00)02795-1
74. Todd OM, Gelrich L, MacLullich AM, et al. Sleep disruption at home as an independent risk factor for postoperative delirium. J Am Geriatr Soc. 2017;65(5):949–957. doi:10.1111/jgs.14685
75. Leung JM, Sands LP, Newman S, et al. Preoperative sleep disruption and postoperative delirium. J Clin Sleep Med. 2015;11(8):907–913. doi:10.5664/jcsm.4944
76. Simeone S, Pucciarelli G, Perrone M, et al. Delirium in ICU patients following cardiac surgery: an Observational Study. J Clin Nurs. 2018;27(9–10):1994–2002. doi:10.1111/jocn.14324
77. Kratz T, Heinrich M, Schlauss E, Diefenbacher A. Preventing postoperative delirium. Dtsch Arztebl Int. 2015;112:289–296. doi:10.3238/arztebl.2015.0289
78. Miller A, Roth T, Roehrs T, Yaremchuk K. Correlation between sleep disruption on postoperative pain. Otolaryngol Head Neck Surg. 2015;152(5):964–968. doi:10.1177/0194599815572127
79. Strutz PK, Kronzer V, Tzeng W, et al. The relationship between obstructive sleep apnoea and postoperative delirium and pain: an Observational Study of a surgical cohort. Anaesthesia. 2019;74(12):1542–1550. doi:10.1111/anae.14855
80. Scholtens RM, van Munster BC, van Faassen M, et al. Plasma melatonin levels in hip fracture patients with and without delirium: a Confirmation Study. Mech Ageing Dev. 2017;167:1–4. doi:10.1016/j.mad.2017.08.016
81. Mahanna-Gabrielli E, Miano TA, Augoustides JG, et al. Does the melatonin receptor 1B gene polymorphism have a role in postoperative delirium? PLoS One. 2018;13(11):e0207941. doi:10.1371/journal.pone.0207941
82. Karkela J, Vakkuri O, Kaukinen S, Huang WQ, Pasanen M. The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta Anaesthesiol Scand. 2002;46(1):30–36. doi:10.1034/j.1399-6576.2002.460106.x
83. Riemersma-van DL. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299(22):2642–2655. doi:10.1001/jama.299.22.2642
84. Nishimura S, Fujino Y, Shimaoka M, et al. Circadian secretion patterns of melatonin after major surgery. J Pineal Res. 1998;25(2):73–77. doi:10.1111/j.1600-079x.1998.tb00542.x
85. de Jonghe A, van Munster BC, Goslings JC, et al. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ. 2014;186(14):E547–556. doi:10.1503/cmaj.140495
86. Sultan SS. Assessment of role of perioperative melatonin in prevention and treatment of postoperative delirium after hip arthroplasty under spinal anesthesia in the elderly. Saudi J Anaesth. 2010;4(3):169–173. doi:10.4103/1658-354X.71132
87. Lingehall HC, Smulter NS, Lindahl E, et al. Preoperative cognitive performance and postoperative delirium are independently associated with future dementia in older people who have undergone cardiac surgery: a Longitudinal Cohort Study. Crit Care Med. 2017;45(8):1295–1303. doi:10.1097/CCM.0000000000002483
88. Simini B. Patients‘ perceptions of intensive care. Lancet. 1999;354(9178):571–572. doi:10.1016/S0140-6736(99)02728-2
89. Young JS, Bourgeois JA, Hilty DM, Hardin KA. Sleep in hospitalized medical patients, part 2: behavioral and pharmacological management of sleep disturbances. J Hosp Med. 2009;4(1):50–59. doi:10.1002/jhm.397
90. Krenk L, Rasmussen LS, Kehlet H. New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesthesiol Scand. 2010;54(8):951–956. doi:10.1111/j.1399-6576.2010.02268.x
91. Rosenberg J. Sleep disturbances after non-cardiac surgery. Sleep Med Rev. 2001;5(2):129–137. doi:10.1053/smrv.2000.0121
92. Gogenur I, Middleton B, Burgdorf S, et al. Impact of sleep and circadian disturbances in urinary 6-sulphatoxymelatonin levels, on cognitive function after major surgery. J Pineal Res. 2007;43(2):179–184. doi:10.1111/j.1600-079X.2007.00460.x
93. Yin YQ, Luo AL, Guo XY, Li LH, Huang YG. Postoperative neuropsychological change and its underlying mechanism in patients undergoing coronary artery bypass grafting. Chin Med J (Engl). 2007;120(22):1951–1957. doi:10.1097/00029330-200711020-00003
94. Moraes W, Piovezan R, Poyares D, et al. Effects of aging on sleep structure throughout adulthood: a population-based study. Sleep Med. 2014;15(4):401–409. doi:10.1016/j.sleep.2013.11.791
95. Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344(6):395–402. doi:10.1056/NEJM200102083440601
96. He J, Hsuchou H, He Y, et al. Sleep restriction impairs blood-brain barrier function. J Neurosci. 2014;34(44):14697–14706. doi:10.1523/JNEUROSCI.2111-14.2014
97. Skvarc DR, Berk M, Byrne LK, et al. Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev. 2018;84:116–133. doi:10.1016/j.neubiorev.2017.11.011
98. Joo EY, Kim H, Suh S, Hong SB. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep. 2014;37(7):1189–1198. doi:10.5665/sleep.3836
99. Maingret N, Girardeau G, Todorova R, Goutierre M, Zugaro M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat Neurosci. 2016;19(7):959–964. doi:10.1038/nn.4304
100. Grumbach P, Opel N, Martin S, et al. Sleep duration is associated with white matter microstructure and cognitive performance in healthy adults. Hum Brain Mapp. 2020;41(15):4397–4405. doi:10.1002/hbm.25132
101. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi:10.1038/nature14432
102. Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76(6):845–861. doi:10.1002/ana.24271
103. Gakuba C, Gaberel T, Goursaud S, et al. General anesthesia inhibits the activity of the “glymphatic system”. Theranostics. 2018;8(3):710–722. doi:10.7150/thno.19154
104. Peng W, Achariyar TM, Li B, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–225. doi:10.1016/j.nbd.2016.05.015
105. Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115–119. doi:10.1038/nrneurol.2013.269
106. Shankar GM, Li S, Mehta TH, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–842. doi:10.1038/nm1782
107. Xie Z, McAuliffe S, Swain CA, et al. Cerebrospinal fluid aβ to tau ratio and postoperative cognitive change. Ann Surg. 2013;258(2):364–369. doi:10.1097/SLA.0b013e318298b077
108. Zhang J, Zhu S, Jin P, et al. Graphene oxide improves postoperative cognitive dysfunction by maximally alleviating amyloid beta burden in mice. Theranostics. 2020;10(26):11908–11920. doi:10.7150/thno.50616
109. Vacas S, Degos V, Feng X, Maze M. The neuroinflammatory response of postoperative cognitive decline. Br Med Bull. 2013;106(1):161–178. doi:10.1093/bmb/ldt006
110. Luo A, Yan J, Tang X, et al. Postoperative cognitive dysfunction in the aged: the collision of neuroinflammaging with perioperative neuroinflammation. Inflammopharmacology. 2019;27(1):27–37. doi:10.1007/s10787-018-00559-0
111. Wadhwa M, Prabhakar A, Ray K, et al. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation. 2017;14(1):222. doi:10.1186/s12974-017-0998-z
112. Huang CT, Chiang RP, Chen CL, Tsai YJ. Sleep deprivation aggravates median nerve injury-induced neuropathic pain and enhances microglial activation by suppressing melatonin secretion. Sleep. 2014;37(9):1513–1523. doi:10.5665/sleep.4002
113. Pan XD, Zhu Y-G, Lin N, et al. Microglial phagocytosis induced by fibrillar beta-amyloid is attenuated by oligomeric beta-amyloid: implications for Alzheimer’s disease. Mol Neurodegener. 2011;6(1):45. doi:10.1186/1750-1326-6-45
114. Qiu -L-L, Ji M-H, Zhang H, et al. NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav Immun. 2016;51:109–118. doi:10.1016/j.bbi.2015.08.002
115. Tuan LH, Lee LJ. Microglia-mediated synaptic pruning is impaired in sleep-deprived adolescent mice. Neurobiol Dis. 2019;130:104517. doi:10.1016/j.nbd.2019.104517
116. Terrando N, Eriksson LI, Kyu Ryu J, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70(6):986–995. doi:10.1002/ana.22664
117. Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68(3):360–368. doi:10.1002/ana.22082
118. Hu J, Feng X, Valdearcos M, et al. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth. 2018;120(3):537–545. doi:10.1016/j.bja.2017.11.096
119. Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. doi:10.1152/physrev.00010.2018
120. Kessler R, Knutson KL, Mokhlesi B, et al. Sleep and activity patterns in older patients discharged from the hospital. Sleep. 2019;42(11):zsz153. doi:10.1093/sleep/zsz153
121. Stollings LM, Jia L-J, Tang P, et al. Immune modulation by volatile anesthetics. Anesthesiology. 2016;125(2):399–411. doi:10.1097/ALN.0000000000001195
122. Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J Neurosci. 2011;31(19):6956–6962. doi:10.1523/JNEUROSCI.5761-10.2011
123. Lananna BV, McKee CA, King MW, et al. Chi3l1/YKL-40 is controlled by the astrocyte circadian clock and regulates neuroinflammation and Alzheimer’s disease pathogenesis. Sci Transl Med. 2020;12(574):eaax3519. doi:10.1126/scitranslmed.aax3519
124. Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi:10.1038/nature21029
125. Havekes R, Park AJ, Tudor JC, et al. Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. Elife. 2016;5:e13424. doi:10.7554/eLife.13424
126. Cao Y, Yang Y, Wu H, et al. Stem-leaf saponins from Panax notoginseng counteract aberrant autophagy and apoptosis in hippocampal neurons of mice with cognitive impairment induced by sleep deprivation. J Ginseng Res. 2020;44(3):442–452. doi:10.1016/j.jgr.2019.01.009
127. Xue R, Wan Y, Sun X, et al. Nicotinic mitigation of neuroinflammation and oxidative stress after chronic sleep deprivation. Front Immunol. 2019;10:2546. doi:10.3389/fimmu.2019.02546
128. Fan J, Li L, Qu P, Diao Y, Sun Y. Kappaopioid receptor agonist U50488H attenuates postoperative cognitive dysfunction of cardiopulmonary bypass rats through the PI3K/AKT/Nrf2/HO1 pathway. Mol Med Rep. 2021;23(4):293. doi:10.3892/mmr.2021.11933
129. Wang W, Yang L, Liu T, et al. Ellagic acid protects mice against sleep deprivation-induced memory impairment and anxiety by inhibiting TLR4 and activating Nrf2. Aging (Albany NY). 2020;12(11):10457–10472. doi:10.18632/aging.103270
130. Nair D, Zhang SXL, Ramesh V, et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184(11):1305–1312. doi:10.1164/rccm.201107-1173OC
131. Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi:10.1016/s0092-8674(03)00035-7
132. Fan T-T, Chen WH, Shi L, et al. Objective sleep duration is associated with cognitive deficits in primary insomnia: BDNF may play a role. Sleep. 2018;42:zsy192. doi:10.1093/sleep/zsy192
133. Garner JM, Chambers J, Barnes AK, Datta S. Changes in brain-derived neurotrophic factor expression influence sleep-wake activity and homeostatic regulation of rapid eye movement sleep. Sleep. 2018;41(2):zsx194. doi:10.1093/sleep/zsx194
134. Kumar D, Koyanagi I, Carrier-Ruiz A, et al. Sparse activity of hippocampal adult-born neurons during REM sleep is necessary for memory consolidation. Neuron. 2020;107(3):552–565 e510. doi:10.1016/j.neuron.2020.05.008
135. Zielinski MR, Kim Y, Karpova SA, et al. Chronic sleep restriction elevates brain interleukin-1 beta and tumor necrosis factor-alpha and attenuates brain-derived neurotrophic factor expression. Neurosci Lett. 2014;580:27–31. doi:10.1016/j.neulet.2014.07.043
136. Guzman-Marin R, Ying Z, Suntsova N, et al. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol. 2006;575(3):807–819. doi:10.1113/jphysiol.2006.115287
137. Qiu LL, Pan W, Luo D, et al. Dysregulation of BDNF/TrkB signaling mediated by NMDAR/Ca(2+)/calpain might contribute to postoperative cognitive dysfunction in aging mice. J Neuroinflammation. 2020;17(1):23. doi:10.1186/s12974-019-1695-x
138. Tian X-S, Tong Y-W, Li Z-Q, et al. Surgical stress induces brain-derived neurotrophic factor reduction and postoperative cognitive dysfunction via glucocorticoid receptor phosphorylation in aged mice. CNS Neurosci Ther. 2015;21(5):398–409. doi:10.1111/cns.12368
139. Wyrobek J, LaFlam A, Max L, et al. Association of intraoperative changes in brain-derived neurotrophic factor and postoperative delirium in older adults. Br J Anaesth. 2017;119(2):324–332. doi:10.1093/bja/aex103
140. Chang HM, Wu UI, Lan CT. Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats. J Pineal Res. 2009;47(3):211–220. doi:10.1111/j.1600-079X.2009.00704.x
141. Yan J, Luo A, Gao J, et al. The role of SIRT1 in neuroinflammation and cognitive dysfunction in aged rats after anesthesia and surgery. Am J Transl Res. 2019;11:1555–1568.
142. Shi J, Zou X, Jiang K, Wang F. SIRT1 mediates improvement of cardiac surgery-induced postoperative cognitive dysfunction via the TLR4/NF-κB pathway. World J Biol Psychiatry. 2019;21(10):757–765. doi:10.1080/15622975.2019.1656820
143. Yan J, Luo A, Sun R, et al. Resveratrol mitigates hippocampal tau acetylation and cognitive deficit by activation SIRT1 in aged rats following anesthesia and surgery. Oxid Med Cell Longev. 2020;2020:4635163. doi:10.1155/2020/4635163
144. Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51–58. doi:10.1001/jamainternmed.2016.6807
145. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi:10.1016/s0140-6736(17)31363-6
146. Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18(7):419–434. doi:10.1038/nrn.2017.48
147. Lazarov O, Hollands C. Hippocampal neurogenesis: learning to remember. Prog Neurobiol. 2016;138–140:1–18. doi:10.1016/j.pneurobio.2015.12.006
148. Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364(23):2227–2234. doi:10.1056/NEJMcp0910237
149. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161–172. doi:10.1016/S1474-4422(08)70019-X
150. Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312(23):2551–2561. doi:10.1001/jama.2014.13806
151. Shimada H, Makizako H, Doi T, et al. Effects of combined physical and cognitive exercises on cognition and mobility in patients with mild cognitive impairment: a randomized clinical trial. J Am Med Dir Assoc. 2018;19(7):584–591. doi:10.1016/j.jamda.2017.09.019
152. American Psychiatric Pub. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub; 2013.
153. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi:10.1016/S0140-6736(13)60688-1
154. Zhu C, Wang B, Yin J, et al. Risk factors for postoperative delirium after spinal surgery: a systematic review and meta-analysis. Aging Clin Exp Res. 2020;32(8):1417–1434. doi:10.1007/s40520-019-01319-y
155. Xie Z, Swain CA, Ward SA, et al. Preoperative cerebrospinal fluid β-Amyloid/Tau ratio and postoperative delirium. Ann Clin Transl Neurol. 2014;1(5):319–328. doi:10.1002/acn3.58
156. van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375(9716):773–775. doi:10.1016/S0140-6736(09)61158-2
157. Cerejeira J, Nogueira V, Luis P, Vaz-Serra A, Mukaetova-Ladinska EB. The cholinergic system and inflammation: common pathways in delirium pathophysiology. J Am Geriatr Soc. 2012;60(4):669–675. doi:10.1111/j.1532-5415.2011.03883.x
158. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21:1190–1222. doi:10.1016/j.jagp.2013.09.005
159. Young J, Murthy L, Westby M, et al. Guidelines diagnosis, prevention, and management of delirium: summary of NICE guidance. Br Med J. 2010;341(jul28 2):c3704–c3704. doi:10.1136/bmj.c3704
160. Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73–81. doi:10.1001/jama.2012.6857
161. Wang W, Li H-L, Wang D-X, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial*. Crit Care Med. 2012;40(3):731–739. doi:10.1097/CCM.0b013e3182376e4f
162. Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(Suppl 1):i41–46. doi:10.1093/bja/aep291
163. Litaker D, Locala J, Franco K, Bronson DL, Tannous Z. Preoperative risk factors for postoperative delirium. Gen Hosp Psychiatry. 2001;23(2):84–89. doi:10.1016/s0163-8343(01)00117-7
164. Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of post-operative cognitive dysfunction. Lancet. 1998;351(9106):857–861. doi:10.1016/S0140-6736(97)07382-0
165. Zywiel MG, Prabhu A, Perruccio AV, Gandhi R. The influence of anesthesia and pain management on cognitive dysfunction after joint arthroplasty: a systematic review. Clin Orthop Relat Res. 2014;472(5):1453–1466. doi:10.1007/s11999-013-3363-2
166. Kim J, Shim JK, Song JW, Kim EK, Kwak YL. Postoperative cognitive dysfunction and the change of regional cerebral oxygen saturation in elderly patients undergoing spinal surgery. Anesth Analg. 2016;123(2):436–444. doi:10.1213/ANE.0000000000001352
167. Liu Y-H, Wang D-X, Li L-H, et al. The effects of cardiopulmonary bypass on the number of cerebral microemboli and the incidence of cognitive dysfunction after coronary artery bypass graft surgery. Anesth Analg. 2009;109(4):1013–1022. doi:10.1213/ane.0b013e3181aed2bb
168. Wan YJ, Xu J, Meng F, et al. Cognitive decline following major surgery is associated with gliosis, beta-amyloid accumulation, and tau phosphorylation in old mice. Crit Care Med. 2010;38(11):2190–2198. doi:10.1097/CCM.0b013e3181f17bcb
169. Zhang X, Dong H, Li N, et al. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J Neuroinflammation. 2016;13(1):127. doi:10.1186/s12974-016-0592-9
170. Rudolph JL, Schreiber KA, Culley DJ, et al. Measurement of post-operative cognitive dysfunction after cardiac surgery: a systematic review. Acta Anaesthesiol Scand. 2010;54(6):663–677. doi:10.1111/j.1399-6576.2010.02236.x
171. Yildizeli B, Oğuzhan Özyurtkan M, Batırel HF, et al. Factors associated with postoperative delirium after thoracic surgery. Ann Thorac Surg. 2005;79(3):1004–1009. doi:10.1016/j.athoracsur.2004.06.022
172. Fadayomi AB, Ibala R, Bilotta F, Westover MB, Akeju O. A systematic review and meta-analysis examining the impact of sleep disturbance on postoperative delirium. Crit Care Med. 2018;46(12):e1204–e1212. doi:10.1097/ccm.0000000000003400
173. Cho MR, Song SK, Ryu CH. Sleep disturbance strongly related to the development of postoperative delirium in proximal femoral fracture patients aged 60 or older. Hip Pelvis. 2020;32(2):93–98. doi:10.5371/hp.2020.32.2.93
174. Fan Y, Yuan L, Ji M, Yang J, Gao D. The effect of melatonin on early postoperative cognitive decline in elderly patients undergoing hip arthroplasty: a randomized controlled trial. J Clin Anesth. 2017;39:77–81. doi:10.1016/j.jclinane.2017.03.023
175. Hansen MV, Madsen MT, Andersen LT, et al. Effect of melatonin on cognitive function and sleep in relation to breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Int J Breast Cancer. 2014;2014:416531. doi:10.1155/2014/416531
176. Wadhwa M, Prabhakar A, Anand JP, et al. Complement activation sustains neuroinflammation and deteriorates adult neurogenesis and spatial memory impairment in rat hippocampus following sleep deprivation. Brain Behav Immun. 2019;82:129–144. doi:10.1016/j.bbi.2019.08.004
177. Vacas S, Degos V, Maze M. Fragmented sleep enhances postoperative neuroinflammation but not cognitive dysfunction. Anesth Analg. 2017;124(1):270–276. doi:10.1213/ANE.0000000000001675
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.