Back to Journals » Risk Management and Healthcare Policy » Volume 13
The Role of Obstetric Factors, miRNA-30d and miRNA-181a in Postpartum Women with Pelvic Organ Prolapse
Authors Lin W , Lin L, Dong B, Chen L, Lei H , Gao Y, Chen Y , Sun P
Received 18 June 2020
Accepted for publication 15 September 2020
Published 28 October 2020 Volume 2020:13 Pages 2309—2316
DOI https://doi.org/10.2147/RMHP.S268235
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Marco Carotenuto
Wenyu Lin,1,2,* Liqing Lin,3,* Binhua Dong,2,4 Lihua Chen,1 Huifang Lei,1,2 Yuqin Gao,1,2 Yaojia Chen,1,2 Pengming Sun1,2,4
1Department of Gynecology, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, People’s Republic of China; 2Fujian Key Laboratory of Women and Children’s Critical Diseases Research, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, 350001, People’s Republic of China; 3Department of Women’s Health Care, Quanzhou Women’s and Children’s Hospital, Quanzhou, 362000, People’s Republic of China; 4Laboratory of Gynecologic Oncology, Department of Gynecology, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, 350001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Pengming Sun
Director of Laboratory of Gynecologic Oncology, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, No. 18 Daoshan Road, Fuzhou, Fujian 350001, People’s Republic of China
Email [email protected]
Background: The diagnosis of postpartum pelvic organ prolapse (POP) relies on symptoms combined with pelvic organ prolapse-quantification (POP-Q) and lacks serological indicators. The objective of this study was to assess serum elastin, type I collagen, miRNA-30d, and miRNA-181a in the early postpartum period to identify hematologic predictors of POP.
Material and Methods: The study included 1013 42- to 60-day-postpartum women who had delivered at Quanzhou Women’s and Children’s Hospital from October 1, 2016, to October 31, 2017. This study was performed in accordance with the Declaration of Helsinki. The pregnancy and childbirth characteristics and pelvic floor function were evaluated. Forty cases with and without POP were matched, and serum elastin and type I collagen were determined by enzyme-linked immunosorbent assay (ELISA). Reverse-transcription polymerase chain reaction (RT-PCR) was used to detect miRNA-30d and miRNA-181a in 15 pairs.
Results: Of the 1013 women recruited, 699 (69.00%) were diagnosed with POP. The mean age was 29.00 years old, and the mean body mass index (BMI) was 22.6 kg/m2. In the univariate analysis, age ≥ 35 years (OR, 1.449; 95% CI, 0.965, 2.298), postpartum BMI ≥ 24 (OR, 4.402; 95% CI, 2.657, 6.148), neonatal weight ≥ 4 kg (OR, 4.832; 95% CI, 1.373, 17.290) and vaginal delivery (OR, 2.751; 95% CI, 1.855, 4.081) were risk factors for postpartum POP. There were no significant differences in the concentrations of serum elastin and type I collagen between the groups (P=0.52; P=0.26). There were significant differences in the concentrations of miRNA-30d and miRNA-181a between the groups (P=0.004; P=0.003).
Conclusion: miRNA-30d and miRNA-181a tended to be increased in women with POP and could be potential clinical predictors.
Keywords: elastin, miRNA-30d, miRNA-181a, pelvic organ prolapse, type I collagen
Introduction
Pelvic organ prolapse (POP) is the downward displacement of pelvic organs that results in vaginal anterior wall bulging, vaginal posterior wall bulging and uterine prolapse. POP is the most common complication after childbirth, affecting up to 94% of women; the mean prevalence of POP is 19.7% in developing countries.1,2 Approximately 42% of women over the age of 50 have different degrees of POP.3 It has been suggested that women have an 11% risk of having a single operation due to POP in their lifetime.4 POP seriously affects the health and quality of life of women.5 However, few studies have investigated the incidence and risk factors of postpartum POP.
A POP diagnosis relies on symptoms combined with POP quantification (POP-Q); however, hand measurements of muscle strength are subjective. Thus, an objective serological index is urgently needed. Pelvic floor muscles, pelvic ligaments, endopelvic fascia, the fibromuscular wall of the vagina and the pelvic bone support the pelvic organs and protect them from prolapse.6 Type I collagen and elastin are the two most important components in the pelvic floor supporting ligament and fascia (type I collagen accounts for 80-99% of total collagen). Decreased collagen content, altered ratios of collagen types and altered morphologic collagen features in the supportive tissue of the pelvic floor among women with POP have been documented.7 Homeobox A11 (HOXA11) plays an important role in the development and maintenance of uterine ligaments by regulating collagen metabolism. Damage or abnormal collagen and elastin metabolism can lead to POP.8–10
Studies have shown that mature miRNAs degrade or repress target mRNA translation, with an apparent regulatory effect.11,12 There is increasing evidence that abnormally expressed miRNAs may promote the development of POP by regulating the expression of HOXA11.13 Microarray analysis showed that the expression of microRNA-30d and microRNA-181a were negatively correlated with HOXA11 expression, and microRNA-30d and microRNA-181a in uterine ligaments were over-expressed in patients with POP.14 Various basic studies have shown a correlation between POP and collagen, elastin and genetic material in tissue specimens, but few studies have investigated correlations in blood specimens.
POP results in a significant economic burden to women and healthcare systems. How to detect and prevent the occurrence and development of POP in the early stage and delay the progression of disease is particularly important. The objective of this study was to assess the risk factors involved in POP in the early postpartum period and investigate the potential role of serum elastin, type I collagen, miRNA-30d and miRNA-181a in the early postpartum period to identify clinical hematologic predictors of POP.
Materials and Methods
Study Population and Procedures
The study group included 42- to 60-day-postpartum women who underwent postpartum examination at Quanzhou Women's and Children's Hospital from October 1st, 2016, to October 31st, 2017 (Figure 1). All women provided informed consent for participation. The ethics committee of this hospital approved the study protocol. The inclusion criteria were as follows: (1) women with clean lochia at 42–60 days after delivery; and (2) women with no serious pregnancy complications. The exclusion criteria were as follows: (1) women with identified POP before pregnancy; (2) women with stress urinary incontinence (SUI) before pregnancy; (3) women who underwent previous pelvic floor function rehabilitation; (4) women with a history of pelvic surgery; and (5) women with severe urinary tract infection or tumor.
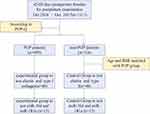 |
Figure 1 Flow chart of the patients included. |
Data regarding maternal age, height, weight, past history (including constipation, chronic cough, history of pelvic surgery or pelvic floor rehabilitation, etc.), prepregnancy POP status, pregnancy and postpartum SUI, number of pregnancies and births, mode of delivery, neonatal weight, time of first and second stage of labor, history of vaginal midwifery and perineal injury were collected.
Pelvic organ support was assessed by the POP-Q system at 42–60 days postpartum. The examination was performed with the women in the lithotomy position exerting maximum strain. Each distance was measured using a wooden spatula marked at 0.5-cm intervals. The POP-Q stage was established on the basis of the most prolapsed compartment, and the proportion of women with each POP-Q stage was calculated. Stage I was defined as protruding within 1 cm above the level of the hymen; stage II was defined as protruding within 1 cm proximal or distal to the plane of the hymen; stage III was defined as protruding more than 1 cm below the plane of the hymen with the vaginal length less than 2 cm; and stage IV was defined as the total protrusion of the uterus. POP-Q stages I–IV were recognized as POP. All the women who participated were fully informed about the study before enrollment and gave their consent. This study was performed in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of Fujian Provincial Maternity and Children’s Hospital, Affiliated Hospital of Fujian Medical University, and all patients provided written informed consent.
Measurement of Serum Elastin and Type 1 Collagen
Three milliliters of whole blood samples were collected in biochemical tubes from participants. The samples were centrifuged at 1000×g for 20 minutes, and the supernatant was taken and stored it at −80°C. The total protein content was measured with the Lowry method. Serum elastin and type 1 collagen concentrations were measured using commercially available human-specific enzyme-linked immunosorbent assay (ELISA) (Elastin: Connetics Corp, Wuhan, China, SEB337Hu-96; Type 1 collagen: Connetics Corp, Wuhan, SEA571Hu-96T) kits according to the manufacturer’s protocol. Elastin standard curve, ranging from 0.25 to 2.5 ng/mL, type 1 collagen standard curve, ranging from 500 to 1500 pg/mL (supplement data).
Measurement of miRNA-30d and miRNA-181a
Total RNA was extracted by TRIzol reagent (Invitrogen). Add 1mL QIAzol lysate, 200μL chloroform (Sangon, Shanghai, China) to serum, and mix by inversion for 15s. Centrifuge at 12,000 x g for 15 minutes at 4°C. Add 700μL Buffer RWT (Sangon, Shanghai, China) and 500μL Buffer RPE (Sangon, Shanghai, China). Pipette the upper transparent liquid into a new tube, add 80% ethanol (Tiangen, Beijing, China) to the spin column, and centrifuge at 8000 x g for 2 minutes. Add 14μL of nucleic acid-free water and store it at −80°C. One microgram of DNase I-treated RNA samples was reverse transcribed to cDNA using a reverse transcription kit (Qiagen, Germany). The PCR primer sets were as follows: miRNA-30d:3ʹ-GAAGGUCAGCCCCUACAAAUGU-5ʹ; miRNA-181a:3ʹ-UGAGUGGCUGU CGCAACUUACAA-5ʹ; and β-actin sense 5ʹ- CGT ACC ACT GGC ATC TGA T-3ʹ. The reaction mixtures were preincubated at 95°C for 5 sec., followed by 40 cycles of denaturation at 95°C for 5 sec, annealing at 60°C for 34 sec. extension and at 95°C for 15 sec.; and final extension at 60°C for 1 min. and 95°C for 15 sec. The quantification of miRNAs was carried out using TaqMan miRNA assays (Qiagen, Germany) according to the manufacturer’s protocol. The samples were analyzed with a Roche LC 480 PCR system (Roche, Switzerland). All PCRs were performed in triplicate, and the specificity of each reaction was determined by melting curve analysis at the dissociation stage. The threshold cycle (Ct) method was used for quantification. Relative quantification of the genes was performed by using the 2−ΔΔCT method.
Statistical Analysis of the Data
All statistical analyses were performed using SPSS v 20.0. Statistical significance was set at P=0.05. Pearson’s chi-squared test was used to compare obstetrical categorical variables between groups, while Student’s t-test or the rank-sum test were used to compare the investigated markers. Logistic regression analysis was performed to clarify the impacts of the investigated markers on the prediction of POP.
Results
Demographic Characteristics
Patients were recruited from October 1, 2016, to October 31, 2017, and basic information about pregnancy and childbirth was collected. Of the 1013 recruited women, 699 (69%) were diagnosed with POP (Figure 1). The mean age was 29.69 (4.55) years, and the mean body mass index (BMI) was 23.19 (3.02) kg/m2. Table 1 shows the main characteristics of the 1013 included patients.
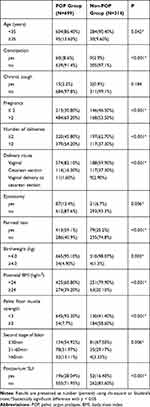 |
Table 1 Characteristics of Study Population |
Obstetrical Characteristics Between the POP and Non-POP Groups
Tables 1 and 2 show older age (age ≥35 years) (OR, 1.449; 95% CI, 0.965, 2.298) and postpartum overweight (postpartum BMI ≥ 24) (OR, 4.402; 95% CI, 2.657, 6.148) were risk factors for postpartum POP. Normal pelvic floor muscle strength (pelvic floor muscle strength ≥3) was a protective factor for postpartum POP (OR, 0.051; 95% CI, 0.034, 0.076). Urinary incontinence (OR, 1.920; 95% CI, 1.248, 2.953), neonatal weight ≥4 kg (OR, 4.832; 95% CI, 1.373, 17.290) and vaginal delivery (OR, 2.751; 95% CI, 1.855, 4.081) were risk factor for postpartum POP.
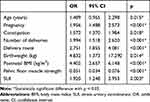 |
Table 2 Logistic Regression Analysis of Association Between Possible Risk Factors and Anatomic Pelvic Organ Prolapse (aPOP) at 6 Weeks Postpartum |
Comparisons of the Investigated Markers Between the POP and Non-POP Groups
The range of elastin in the non-POP group was 2.28ng/mL to 2.88ng/mL, while that in the POP group was 2.35ng/mL to 2.28ng/mL. Nevertheless, the difference was not significant (P>0.05) (Table 3) (Figure 2).
 |
Table 3 Comparison of Elastin, Type I Collagen, miRNA-30d and miRNA-181a Expression Between POP and No POP Group at 6 Weeks Postpartum |
The range of type I collagen in the non-POP group was 232.00 pg/mL to 599.68 pg/mL, while that in the POP group was 163.41 pg/mL to 756.40 pg/mL. According to the rank-sum test, the median (interquartile range) of type I collagen in the non-POP group was 350.28 (300.21, 400.20) pg/mL, and the median (interquartile range) of type I collagen in the POP group was 352.85 (290.24, 375.13) pg/mL. The difference was not statistically significant (P>0.05) (Figure 3).
The serum miRNA-30d levels between the groups showed significant differences (P = 0.004), with miRNA-30d tending to be higher in the POP group (2.05 (1.80,3.33)) pg/mL than in the non-POP group (3.63 (2.71, 5.18)) pg/mL. Serum miRNA-181a levels were increased in the POP (3.36 (2.62, 4.80)) pg/mL group compared with those in the non-POP group (2.08 (2.00, 2.93)) pg/mL. Significant differences in the serum miRNA-30d and miRNA-181a levels between the groups were noted (P = 0.003).
Discussion
POP is the most common complication of childbirth and can seriously affect the health and quality of life of women. The results of this study showed that the incidence of POP was 69.0%; the prevalence of anterior wall prolapse was 63.9% (highest), of posterior wall prolapse was 28.7%, of uterine prolapse was 26.6%, and of POP combined SUI was 28.0%. Related reports showed that the incidence of POP in developing countries was approximately 3.4%-56.7%,15–17 and the incidence increased to 33%-79% from 6 weeks to 1 year after delivery.18 The prevalence in this study was higher than the rates reported in the literature. One possible reason is that other studies assessing POP post-delivery at 3–6 months post-delivery. In China, the postpartum follow-up time is in 42–60 days. Postpartum POP could be self-improvement over time. Another reason is that in China, a postpartum physical examination is not included in the medical insurance policy, so women without symptoms generally do not visit hospitals, leading to an increasing proportion of women with POP. According to the three zones anatomical theory, the pelvic floor structure is divided into 3 compartments, anterior compartment, middle compartment, and posterior compartment. In 1990, Petros proposed Integral Theory, that is, different chambers and different vaginal support axes together constitute an anatomical and functional whole. The anterior vaginal wall belongs to the anterior pelvic cavity, which is closely related to the urethra and bladder. Therefore, POP is often associated with SUI. The posterior pelvic organs are fixed by the posterior levator ani muscle plate and bilateral puborectalis muscles. The posterior pelvic organs have less activity than the anterior and middle pelvis, and the risk of pregnancy and childbirth injury is decreased. In summary, the incidence of vaginal posterior wall bulging is decreased. Although pregnancy and childbirth are natural physiological processes, some postpartum injuries can be actively healed. However, some injuries are gradually aggravated with age and lifestyle changes, which may affect the quality of life.
The present study indicated that age, postpartum BMI, neonatal weight, and vaginal delivery were independently associated with an increased risk of POP after vaginal birth. Vaginal delivery and high neonatal weight involve excessive stretching of the muscles during vaginal birth to allow the passage of the newborn. Vaginal delivery also involves the fetal head descending rapidly through the birth canal, and spontaneous vaginal delivery does not allow time for the normal accommodation of the muscles. The pelvic floor structure and tissue relaxation can lead to avulsion of the pelvic floor neuromuscular junction, which can disrupt pelvic floor function and thus lead to postpartum POP and SUI. These mechanisms may lead to an increase in injury and could explain why both factors are associated with POP after delivery.
Pelvic floor muscle injury caused by vaginal delivery mainly occurs in the second stage of labor.19 The pelvic floor muscles and surrounding soft tissues are exposed during the whole process. Continuous mechanical compression and expansion and connective tissue fractures may occur in severe cases.20 Cesarean section can avoid injuries associated with vaginal delivery, especially those caused by midwifery instruments and perineal incision, which can result in irreversible damage to the pelvic floor support tissue. Selective cesarean section can prevent the loss of certain pelvic floor functions, but cesarean section after trial production does not have this effect.21,22 The two different modes of delivery have effects on pelvic floor muscle strength. The effect of vaginal delivery on pelvic floor muscle strength is greater than that of selective cesarean section, but cesarean section does not reduce the risk of SUI. Although the results of this study indicated that cesarean section was a protective factor for POP, the results of the study were limited to the analysis of the incidence of POP in 42- to 60-day-postpartum women. Vaginal delivery may mask other factors’ effects on POP occurrence; thus, it is not possible to recommend cesarean section to reduce the occurrence of POP.23
Decreased collagen content, altered ratios of collagen types and altered morphologic collagen features in the supportive tissue of the pelvic floor among women with POP have also been documented. In summary, collagen amounts, elastin amounts, structural characteristics, metabolic abnormalities, etc., can lead to POP, but the experimental materials in previous studies included tissue specimens to study collagen or elastin in the lesion24. Serum relaxin and pelvic floor function are the most closely related factors.16 Type I collagen has a relatively large diameter and a high antitension effect. Type III collagen has a fine diameter and is related to elasticity.25 The lack of studies on the use of blood specimens may be related to the distribution of collagen and elastin in the blood. Serum samples were used in this study. The results showed that there was no significant difference in the serum elastin and type I collagen concentrations between the case group and the control group. The reason may be that the distribution level and metabolism of these two proteins in blood and tissues are inconsistent. Another reason may be that the endocrine level changed postpartum. Therefore, it is necessary to expand the sample size, eliminate interfering factors and increase the time of detection (eg, 3 months, 6 months, and 12 months after delivery) for dynamic observation to clearly understand postpartum changes and provide new methods for predicting pelvic dysfunction.
The levels of miRNA-30d and miRNA-181a in the case group were higher than those in the control group, consistent with the results of Myung Jae, Jeon et al miRNAs are a class of endogenous noncoding RNAs that are approximately 20–25 nucleotides in length. They are immediately encapsulated by proteins and are not easily degraded by RNase. They can be consistently present in body fluids, such as blood and urine, so they are easily removed from fresh tissue. They are ideal biomarkers in paraffin-embedded tissues and serum samples. The target of the miRNA-30 family is connective tissue growth factor (a powerful inducer of extracellular matrix synthesis).9 Therefore, excessive levels of miRNA-30d may inhibit the expression of connective tissue growth factor and HOXA11. Limiting the synthesis of collagen, which affects the homeostasis of collagen in uterosacral ligaments (USLs), promotes the occurrence of POP.9 In addition, miRNA-30d and 181a are known to play important roles in apoptosis, and miRNA-30d acts as a proapoptotic inducer.10 miRNA-181a directly inhibits B-cell lymphoma-2 (a type of mitochondria) interaction with antiapoptotic proteins,26 thereby promoting apoptosis. Studies have shown that increased mitochondrial apoptosis and decreased expression of B-cell lymphoma-2 have been reported in pelvic support tissues in POP patients.13,14 Therefore, the detection of levels of these two miRNAs in the early postpartum period may be beneficial to prevent or promptly intervene in POP progression in women. Reducing the treatment of miRNA-30d and 181a may be beneficial to prevent or restore the reduction in collagen in the USLs.14
In conclusion, this study provides preliminary evidence of an association between POP and the following obstetric factors: serum elastin, type I collagen, miRNA-30d, and miRNA-181a. The detection of risk factors during pregnancy and the detection of miRNA levels may help establish a prediction model for postpartum POP, providing a theoretical basis for early prevention and treatment of POP. Women should avoid the above-mentioned risk factors during the perinatal period, and postpartum pelvic floor screening should prolong for follow-up, such as reassessment after 3 or 6 months to follow up the dynamic changes, and commit to maternal health. Further studies are required to investigate the significance of this finding and improve the prognosis of symptomatic prolapse later in life in women with support defects in the postpartum period.
Abbreviations
BMI, body mass index; ELISA, enzyme-linked immunosorbent assay; HOXA11, Homeobox A11; POP, pelvic organ prolapse; POP-Q, pelvic organ prolapse-quantification; RT-PCR, Reverse-transcription polymerase chain reaction; SUI, stress urinary incontinence.
Ethical Approval
The study was approved by our institutional review board (No: 2017-016).
Acknowledgments
Special thanks to the assistants and Quanzhou Women's and Children's Hospital for obtaining the samples. We would like to thank the participants for their patience and kindness.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Walker GJ, Gunasekera P. Pelvic organ prolapse and incontinence in developing countries: review of prevalence and risk factors. Int Urogynecol J. 2011;22(2):127–135. doi:10.1007/s00192-010-1215-0
2. Thompson JF, Roberts CL, Currie M, Ellwood DA. Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Birth. 2002;29(2):83–94. doi:10.1046/j.1523-536X.2002.00167.x
3. Rogo-Gupta L, Baxter ZC, Le NB, Raz S, Rodriguez LV. 1188 Long-term durability of the distal urethral polypropylene sling procedure for stress urinary incontinence: minimum 10-year follow up. J Urol. 2012;188(5):1822–1827. doi:10.1016/j.juro.2012.07.033
4. Lawrence JM, Lukacz ES, Nager CW, Hsu JWY, Luber KM. Prevalence and co-occurrence of pelvic floor disorders in community-dwelling women. Obstet Gynecol. 2008;111(3):678. doi:10.1097/AOG.0b013e3181660c1b
5. Dietz HP, Clarke B. The urethral pressure profile and ultrasound imaging of the lower urinary tract. Int Urogynecol J. 2001;12(1):38–41. doi:10.1007/s001920170092
6. Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;106(3):615. doi:10.1097/01.AOG.0000175832.13266.bb
7. Ferrari MM, Rossi G, Biondi ML, Viganò P, Dell’Utri C, Meschia M. Type I collagen and matrix metalloproteinase 1, 3 and 9 gene polymorphisms in the predisposition to pelvic organ prolapse. Arch Gynecol Obstet. 2012;285(6):1581–1586. doi:10.1007/s00404-011-2199-9
8. Connell KA, Guess MK, Heidi C, Vaagn A, Richard B, Taylor HS. HOXA11 is critical for development and maintenance of uterosacral ligaments and deficient in pelvic prolapse. J Clin Invest. 2008;118(3):1050–1055.
9. Ma Y, Guess M, Datar A, et al. Knockdown of Hoxa11 in vivo in the uterosacral ligament and uterus of mice results in altered collagen and matrix metalloproteinase activity. Biol Reprod. 2011;86(4):100.
10. Connell KA, Guess MK, Chen HW, Tara L, Richard B, Taylor HS. HOXA11 promotes fibroblast proliferation and regulates p53 in uterosacral ligaments. Reproductive Sci. 2009;16(7):694. doi:10.1177/1933719109334260
11. Corney DC, Chang-Il H, Andres M, et al. Frequent downregulation of miR-34 family in human ovarian cancers. Clinical Cancer Res Official J Am Association Cancer Res. 2010;16(4):1119–1128. doi:10.1158/1078-0432.CCR-09-2642
12. Pecot CV, Rajesha R, Da Y, et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun. 2013;4(9):2427. doi:10.1038/ncomms3427
13. Moisés M, Alonso CR. The regulation of Hox gene expression during animal development. Development. 2013;140(19):3951–3963. doi:10.1242/dev.068346
14. Jeon MJ, Kim EJ, Lee M, et al. MicroRNA-30d and microRNA-181a regulate HOXA11 expression in the uterosacral ligaments and are overexpressed in pelvic organ prolapse. J Cell Mol Med. 2015;19(2):501–509. doi:10.1111/jcmm.12448
15. Dragoo J, Padrez K, Lindsey D R. The effect of relaxin on the female anterior cruciate ligament: analysis of mechanical properties in an animal model. Knee. 2009;16(1):69–72. doi:10.1016/j.knee.2008.09.005
16. Marie-Andrée H, Johnston SL, Davies GAL. Mid-trimester serum relaxin concentrations and post-partum pelvic floor dysfunction. Acta Obstet Gynecol Scand. 2011;87(12):1315–1321.
17. King JK, Freeman RM. Is antenatal bladder neck mobility a risk factor for postpartum stress incontinence? Br J Obstet Gynaecol. 2010;105(12):1300–1307. doi:10.1111/j.1471-0528.1998.tb10009.x
18. Guzmán Rojas RA, Salvesen K, Aring VI. Anal sphincter defects and fecal incontinence 1524 years after first delivery: a cross-sectional study. Ultrasound Obstet Gynecol. 2018;51:677–683. doi:10.1002/uog.18827
19. Caudwell-Hall J, Kamisan AI, Martin A, et al. Intrapartum predictors of maternal levator ani injury. Acta Obstet Gynecol Scand. 2017;96(4):426. doi:10.1111/aogs.13103
20. González MS, Garriga JC, Capel CD, Roda OP, Capó JP, Saladich IG. Is obstetric anal sphincter injury a risk factor for levator ani muscle avulsion in vaginal delivery? Ultrasound Obstet Gynecol. 2017;49(2):257–262. doi:10.1002/uog.15847
21. Mira L. Prevention of urinary and anal incontinence: role of elective cesarean delivery. Curr Opin Obstet Gynecol. 2003;15(5):439. doi:10.1097/00001703-200310000-00014
22. Vswift MK. The effect of pregnancy and mode of delivery on the prevalence of urinary and fecal incontinence. Am J Obstet Gynecol. 2005;193(2):512–517. doi:10.1016/j.ajog.2005.03.056
23. Hannah ME, Hannah WJ, Hodnett ED, et al. Outcomes at 3 months after planned cesarean vs planned vaginal delivery for breech presentation at term: the international randomized Term Breech Trial. JAMA. 2002;287(14):1822–1831. doi:10.1001/jama.287.14.1822
24. Chen YS, Wang XJ, Feng W, Hua KQ. Advanced glycation end products decrease collagen I levels in fibroblasts from the vaginal wall of patients with POP via the RAGE, MAPK and NF-κB pathways. Int J Mol Med. 2017;40:4. doi:10.3892/ijmm.2017.3097
25. Tola EN, Koroglu N, Yıldırım GY, Koca HB. The role of ADAMTS-2, collagen type-1, TIMP-3 and papilin levels of uterosacral and cardinal ligaments in the etiopathogenesis of pelvic organ prolapse among women without stress urinary incontinence. European J Obstet Gynecol Reproductive Biol. 2018;231:158–163. doi:10.1016/j.ejogrb.2018.10.043
26. Ouyang YB, Lu Y, Yue S, Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012;12(2):213–219. doi:10.1016/j.mito.2011.09.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


