Back to Journals » Journal of Inflammation Research » Volume 15
The Role of NLRP3 Inflammasome Signaling on Arrhythmias in Diabetes
Authors Zhang L, Liu HH, Li F, Yang F, Qian LL, Wang RX
Received 18 September 2022
Accepted for publication 16 December 2022
Published 29 December 2022 Volume 2022:15 Pages 6883—6889
DOI https://doi.org/10.2147/JIR.S390310
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam D Bachstetter
Lei Zhang,1,* Huan-Huan Liu,2,* Feng Li,1 Fan Yang,1 Ling-Ling Qian,1 Ru-Xing Wang1
1Department of Cardiology, Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi, People’s Republic of China; 2Wuxi School of Medicine, Jiangnan University, Wuxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ru-Xing Wang, Tel +86-510-85351593, Fax +86-510-85350555, Email [email protected]
Abstract: Diabetes is a significant risk factor for arrhythmias. However, the pathophysiology of diabetes-related arrhythmias still needs to be elucidated, presumably associated with structural and electrical remodeling. There is growing evidence that inflammation and arrhythmias are intimately associated, which has spurred significant interest in exploring the regulatory links in diabetes. Recent research findings have revealed a vital role for the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome signaling, and facilitated the occurrence of arrhythmias in diabetes, including NLRP3 inflammasome activation by multiple stressors and its downstream cytokines, interleukin-1β (IL-1β) and interleukin-18 (IL-18). This narrative review aims to summarize the complex interaction between NLRP3 inflammasomes signaling and diabetes-related arrhythmias. Articles regarding the role of NLRP3 inflammasome in diabetes-related arrhythmias and relevant mechanisms were selected. Relevant articles were selected from PubMed. The search terms were “NLRP3 inflammasome” and “diabetes” and “arrhythmia”. Important references from selected articles were also retrieved. The role of NLRP3 inflammasome signaling in diabetes-induced arrhythmias may provide a new option for the prevention and treatment diabetes-related arrhythmias.
Keywords: NLRP3, inflammasome, diabetes, arrhythmia
Introduction
Diabetes is a chronic metabolic disease characterized by hyperglycemia because of insulin resistance, insulin resistance, or both.1 Data from the International Diabetes Federation showed that 10.5% (536.6 million) of people worldwide suffered from diabetes in 2021, and the incidence is still rising.2 As a definite risk factor for cardiovascular disease, diabetes is significantly involved in the development of arrhythmias, including atrial arrhythmias,3 ventricular arrhythmias,4 and sudden death,5 accompanied by a significant global disease burden and increased mortality.6
Diabetes is associated with a chronic low-grade inflammatory state and can lead to negative remodeling of myocardial tissue. Advancing inflammatory response has been proven related to the development of arrhythmias.7,8 The NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome is a crucial mediator of the innate immune system and can promote the activation of caspase-1, which in turn leads to the maturation and secretion of the pro-inflammatory cytokines, interleukin-1β (IL-1β) and interleukin-18 (IL-18), triggering an inflammatory cascade and causing irreparable damage of target organs.9,10 In recent years, few reviews have summarized the role of NLRP3 inflammasome in diabetes and its complications.11,12 However, those reviews are limited to focusing on the association between NLRP3 inflammasome activation and arrhythmias. A growing number of recent studies have suggested that the increased expression of NLRP3, caspase-1, IL-1β, and IL-18 in the cardiac tissue drives electrical and structural remodeling and progresses the arrhythmogenic substrate to further enhance the development of arrhythmias in diabetes.7,13,14
This review aimed to summarize the contributing role of the NLRP3 inflammasome signaling, including NLRP3 inflammasome, caspase-1, IL-1β and IL-18, in arrhythmogenesis of diabetes. The underlying mechanisms might provide insight into the prevention and treatment of diabetes-related arrhythmias.
NLRP3 Inflammasome Signaling
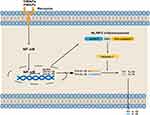
Inflammasomes, a key actor of innate inflammatory signaling, serve as the first defense response to danger signals such as pathogens (viruses, bacteria) or molecules. Inflammasomes are classified into the canonical inflammasomes that activate caspase-1 and the noncanonical inflammasomes that activate caspase-4/5/11.15 NLRP3 inflammasome is a canonical inflammasome typically composed of NLRP3, apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspase-1.16 Typically, a two-step process is required for the activation of NLRP3 inflammasome that involves priming and activation (Figure 1). External stimuli are sensed by cells, such as exogenous pathogen-associated molecular patterns (PAMPs) or endogenous damage-associated molecular patterns (DAMPs), and then the activation of NLRP3 inflammasome is initiated, leading to the increasing of NLRP3, pro-IL-1β, and pro-IL-18 transcription through nuclear factor kB (NF-κB); activation refers to the binding of the pyrin domain (PYD) domain of NLRP3 and ASC, which in turn recruits the effector molecule pro-caspase-1 via CARD-CARD interactions, resulting in the assembly of NLRP3 inflammasome.17,18 Currently, it is considered that DAMPs include self-derived, including soluble uric acid, cholesterol crystals, high mobility group box one, and so on, and foreign-derived, including nanoparticles, carbon nanotubes, aluminum hydroxide, etc. The PAMPs are composed of several microorganisms and microbial products, such as lipolysaccharide, peptidoglycan, etc.19,20
However, these PAMPs and DAMPs barely activate NLRP3 inflammasome directly, often through some changes at cellular level. There are three main reasons that contribute to the activation of NLRP3 inflammasome from initiation: (1) changes in intracellular or extracellular ion concentrations. These include intracellular K+ efflux, Ca2+ efflux, and Na+ efflux and Cl− efflux; (2) rupture of intracellular lysosomes; (3) excessive production of reactive oxygen species (ROS) by damaged mitochondria.10
Previous studies have showed that the maturation and secretion of the cytokines, IL-1β and IL-18, are depended on the caspases after the activation of NLRP3 inflammasome.21 After activation of NLRP3 inflammasome, the pro-caspase-1 is induced into caspase-1, a cysteine protease, subsequently activating pro-IL-1β and pro-IL-18 to their mature forms, which can generate pro-inflammatory effects. Meanwhile, caspase-1 can also cleave gasdermin-D, and then the gasdermin-N domain of gasdermin-D subsequently leads to the formation of membrane pores to drive the release of pro-inflammatory cytokine and pyroptosis.17
The NLRP3 inflammasome signaling consisting of NLRP3 inflammasome and its downstream inflammatory factors, IL-1β and IL-18, is a key regulator leading to an inflammatory cascade of reactions ultimately for the purpose of restoring organismal homeostasis. IL-1β, which is secreted extracellularly, is central to mediate the pro-inflammatory response and can contribute to the activation of other inflammatory cytokines, such as interleukin-6 (IL-6), leading to tissue damage.22 The IL-18 is also involved in immune response regulation, causing inflammatory damage to tissues and organs. Previous studies showed that the β-adrenergic agonist isoprenaline (ISO) can induce the increase of NLRP3 inflammasome-dependent IL-18 expression in myocardium, inducing sequentially macrophage infiltration and myocardial fibrosis. Whereas after incubation with IL-18 neutralizing antibodies for 1 hour, macrophage infiltration, myocardial fibrosis and cardiac diastolic dysfunction all showed significant improvement.23 Similar outcomes were confirmed that the activation of NLRP3 inflammasome signaling in cardiomyocytes, in turn can act on cardiomyocytes and non-cardiomyocytes to produce pro-inflammatory factors such as TNF-α and IL-6, forming a positive feedback loop that leads to myocardial remodeling under stress.24 In addition, a recent review has showed that exercise is closely linked to the activation of NLRP3 inflammasome signaling, and exercise training could attenuate its activation in diabetic cardiomyopathy.25
Activation and Regulation of NLRP3 Inflammasome Signaling in the Heart with Diabetes
The pathogenic mechanisms of diabetes and its complications are initiated as a low-grade chronic inflammatory state. Currently, the upregulated expression and activation of NLRP3 inflammasome and the downstream targets, including IL-1β and IL-18, have been observed in the heart of diabetes.26–28 Zaharieva et al showed elevated levels of IL-1β and IL-18 in the serum of diabetic patients.29 Similarly, the overexpressed above were also demonstrated in cardiac tissue in type 1 diabetic patients.30 The heart is a complex multicellular organ consisting of cardiomyocytes, fibroblasts, immune cells etc. Monnerat et al suggested that the IL-1β produced by cardiac macrophages through NLRP3 inflammasome signaling can act on cardiomyocytes, causing electrical remodeling in diabetes.14 Zhang et al showed that high glucose could also induce NLRP3 inflammasome activation in cardiac fibroblasts, causing cardiac fibrosis.31 Furthermore, in cultured cardiomyocytes with high glucose, NLRP3 inflammasome signaling was activated similarly.32 The above results showed that diabetes activates NLRP3 inflammasome signaling (including NLRP3, caspase-1, IL-18, IL-1β) in cardiomyocytes and non-myocytes.
Several molecular mechanisms link diabetes to the activation of NLRP3 inflammasome signaling: (1) As epicardial adipose tissue accumulates in obese patients with type 2 diabetes, adipokines secreted by adipose tissue, like IL-1β, contribute to worsening the activation of NLRP3 inflammasome signaling and also directly elicit detrimental effects on the heart;33 (2) high glucose directly leads to the upregulation of nuclear factor-κB (NF-κB) through multiple pathways that were increasing the expression of the components in NLRP3 inflammasome signaling;24,26 (3) upon the activation of the renin-angiotensin-aldosterone system (RAAS), accumulation of advanced glycation end-products (AGEs) in diabetes, the toll-like receptors (TLRs) pathway is then activated, which leads to NLRP3 inflammasome signaling activation and mediates myocardial inflammation;34,35 (4) with the increasing of oxidative stress and excessive ROS production from prolonged exposure to hyperglycemia, the NLRP3 inflammasome/caspase-1/IL-1β/IL-18 signaling is activated, as a result, that can lead to increased cellular oxidative stress in turn, forming a positive feedback loop;10 (5) diabetes is also associated with a disorder of non-coding RNAs. Recently, it had shown that miR-141 was downregulated in myocardial tissue during diabetes, activating NLRP3 inflammasome signaling and leading to myocardial fibrosis.36 Multiple diabetes-related metabolic disorders may function as NLRP3-inducing stimuli, resulting in the activation of the NLRP3 inflammasome/caspase-1/IL-1β/IL-18 signaling. These changes may involve the development of myocardial electrical and structural remodeling.
NLRP3 Inflammasome Signaling in Diabetes-Related Arrhythmias
Given that different stimulation has been reported in diabetes and the subsequent activation of NLRP3 inflammasome signaling, a large amount of the pro-inflammatory factors will be released.9 Those pro-inflammatory factors will further increase myocardial inflammation, and contribute to myocardial fibrosis, ion channel remodeling, and gap junction remodeling, which could be a mechanism for increased susceptibility to arrhythmias due to diabetes (Figure 2). This process will eventually predispose to arrhythmias including but not limited to atrial fibrillation, conduction block, and ventricular arrhythmia. Nonetheless, most studies focused on atrial fibrillation and ventricular arrhythmia up to now. The present review focuses on these aspects of atrial fibrillation and ventricular arrhythmia in diabetes.
Atrial Fibrillation
Diabetes is one of the major known risk factors for atrial fibrillation (AF), and the association has been well established in many reviews.37 Still, no validated measures exist to prevent and treat AF in diabetes.
A recent study by Yao et al established that NLRP3 inflammasome was increased in atrial myocytes from patients with paroxysmal and long-standing persistent AF, and cardiac-specific knock-in mice expressing constitutively active NLRP3 can develop spontaneous premature atrial beats or increase induction of AF.38 However, after the use of MCC950 (a specific NLRP3 inhibitor) or NLRP3 homozygous knockout, the mouse showed a reduced incidence of AF. Meanwhile, the study also demonstrated that NLRP3 inflammasome activation could affect calcium homeostasis through ryanodine receptor 2 (RyR2) and increase the ultra-rapid delayed rectifier K+ current (IKur) and acetylcholine-regulated K+ current (Ik, Ach), leading to a shortened effective refractory period, which both together promoted the development of AF. In addition, the role of NLRP3 inflammasome in the genesis of AF was also observed in obese models.39
The IL-1β and IL-18, as major downstream molecules of the NLRP3 inflammasome, link NLRP3 inflammasome with multiple downstream events in the innate immune response. These are implicated in the development and maintenance of AF in diabetes.9 Wu et al showed NLRP3 inflammasome, IL-1β, and IL-18 were upregulated in diabetic rabbit atrial tissues and serum.40 Furthermore, the study also demonstrated that the conduction inhomogeneity and slower epicardial conduction velocity in diabetic rabbits could be reversed by glibenclamide, an NLRP3-inflammasome inhibitor. Likewise, during the follow-up in patients without a history of AF who underwent open-heart surgery, the results exhibited a more vital expression of the NLRP3-inflammasome signaling in atrial whole-tissue homogenates and cardiomyocytes in postoperative AF. Moreover, after acute application of IL-1β upon addition of the Ca2+/calmodulin-dependent protein kinase-II (CaMKII) inhibitor KN-93, spontaneous SR Ca2+-release events were attenuated in both postoperative AF cardiomyocytes and HL-1 cardiomyocytes. IL-1β can also cause NLRP3 inflammasome signaling activation and CaMKII-dependent RyR2 hyperphosphorylation in HL-1 cardiomyocytes.41 The results above revealed that CaMKII might act as a critical molecular between NLRP3 inflammasome signaling and AF. In addition, the release of IL-1β from cardiomyocytes due to the activation of NLRP3 inflammasome can activate non-myocytes in the heart, such as cardiac myofibroblasts and macrophages, promoting the release of inflammatory cytokines like IL-1β and TNFα, and sequentially contributing to abnormal cardiac remodeling. These inflammatory factors in turn activate the formation of active NLRP3 inflammasome signaling in cardiomyocytes at the same time.42 This positive feedback would perpetuate the pathological process of atrial fibrillation in diabetes. However, the downstream effectors of the NLRP3 inflammasome signaling in diabetes-related AF remain unclear.
Ventricular Arrhythmias
Limited data to date are available about the relationship between the NLRP3 inflammasome signaling and ventricular arrhythmias in diabetes. Hyperglycemia can prompt the activation of NLRP3 inflammasome in ventricular macrophages.14 This process increased immune cell recruitment and cytokine production; moreover, the upregulation of macrophage-secreted inflammatory cytokines is closely related to ion channel remodeling, gap junctional remodeling and myocardial fibrosis in the ventricle, inducing the development of ventricular arrhythmias.43
In type 1 diabetes, Monnerat et al reported that enhanced IL-1β production in cardiac macrophage via NLRP3 inflammasome activation induced an increase in oxidation and phosphorylation of CaMKII, and calcium sparks and inhibited the transient outward potassium currents (Ito) in cardiomyocytes, therefore prolonging action potential duration (APD) and increasing the incidence of ventricular arrhythmias.14 Furthermore, these results were reversed by following treatment with MCC-950, anakinra (an IL-1 receptor antagonist), or knockdown of either NLRP3, caspase-1 or IL-1 receptor (IL-1R). Moreover, the increasing level of cardiac IL-1β protein can produce mitochondrial reactive oxygen species in a dose-dependent manner in type 2 diabetes mice, which sequentially increases oxidation of RyR2, sarcoplasmic reticulum Ca2+ leak, thus causing the presentation of ventricular arrhythmia. Whereas the use of anakinra ameliorated electrical remodeling and ventricular arrhythmias risk.44 Dysfunction of cardiomyocytes, immune cells, and fibroblasts in diabetic hearts, which participate in the process of diabetes-related complications through intercellular communication.45 Currently, IL-18 on ventricular arrhythmias in diabetes remains elusive. In general, these results identified that NLRP3 inflammasome signaling could provide new insights into the pathogenesis of diabetes-related ventricular arrhythmias.
Conclusion
This review highlights the pivotal role of the NLRP3 inflammasome signaling in diabetes-related arrhythmias. The activation of NLRP3 inflammasome signaling can promote the secretion of proinflammatory factors, as well as regulate other regulators (CaMKII, RyR2, etc), all of which cause electrical and structural remodeling of the myocardium leading to a predisposition to the development of arrhythmias. Taken together, the NLRP3 inflammasome signaling could be a potential therapeutic target for diabetes-related arrhythmias. Yet, the exact mechanism whereby the NLRP3 inflammasome signaling regulates abnormal cardiac remodeling in diabetes is yet to be determined. In addition, the majority of studies on the association between arrhythmias and the NLRP3 inflammasome signaling in diabetes describe evidence for atrial fibrillation and ventricular arrhythmias but less is known for other forms of arrhythmias. Further research in this area could pave the way to control and prevent arrhythmias in patients with diabetes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ceriello A, Prattichizzo F, Phillip M, et al. Glycaemic management in diabetes: old and new approaches. Lancet Diabetes Endocrinol. 2022;10(1):75–84. doi:10.1016/S2213-8587(21)00245-X
2. Sun H, Saeedi P, Karuranga S, et al. IDF diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2021;183:109119. doi:10.1016/j.diabres.2021.109119
3. Echouffo-Tcheugui JB, Shrader P, Thomas L, et al. Care patterns and outcomes in atrial fibrillation patients with and without diabetes: ORBIT-AF Registry. J Am Coll Cardiol. 2017;70(11):1325–1335. doi:10.1016/j.jacc.2017.07.755
4. Weidner K, Behnes M, Schupp T, et al. Type 2 diabetes is independently associated with all-cause mortality secondary to ventricular tachyarrhythmias. Cardiovasc Diabetol. 2018;17(1):125. doi:10.1186/s12933-018-0768-y
5. Lynge TH, Svane J, Pedersen-Bjergaard U, et al. Sudden cardiac death among persons with diabetes aged 1–49 years: a 10-year nationwide study of 14 294 deaths in Denmark. Eur Heart J. 2020;41(28):2699–2706. doi:10.1093/eurheartj/ehz891
6. Powers MA, Bardsley J, Cypress M, et al. Diabetes self-management education and support in type 2 diabetes: a joint position statement of the American diabetes association, the American association of diabetes educators, and the academy of nutrition and dietetics. Diabetes Care. 2015;38(7):1372–1382. doi:10.2337/dc15-0730
7. Karam BS, Chavez-Moreno A, Koh W, et al. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16(1):120. doi:10.1186/s12933-017-0604-9
8. Grune J, Yamazoe M, Nahrendorf M. Electroimmunology and cardiac arrhythmia. Nat Rev Cardiol. 2021;18(8):547–564. doi:10.1038/s41569-021-00520-9
9. Varghese B, Feldman DI, Chew C, et al. Inflammation, atrial fibrillation, and the potential role for colchicine therapy. Heart Rhythm O2. 2021;2(3):298–303. doi:10.1016/j.hroo.2021.03.011
10. Bai Y, Mu Q, Bao X, et al. Targeting NLRP3 inflammasome in the treatment Of diabetes and diabetic complications: role of natural compounds from herbal medicine. Aging Dis. 2021;12(7):1587–1604. doi:10.14336/AD.2021.0318
11. Sharma A, Tate M, Mathew G, et al. Oxidative stress and NLRP3-inflammasome activity as significant drivers of diabetic cardiovascular complications: therapeutic implications. Front Physiol. 2018;9:114. doi:10.3389/fphys.2018.00114
12. Menini S, Iacobini C, Vitale M, et al. The Inflammasome in chronic complications of diabetes and related metabolic disorders. Cells. 2020;9(8):1812. doi:10.3390/cells9081812
13. Sokolova M, Ranheim T, Louwe MC, et al. NLRP3 inflammasome: a novel player in metabolically induced inflammation-potential influence on the myocardium. J Cardiovasc Pharmacol. 2019;74(4):276–284. doi:10.1097/FJC.0000000000000704
14. Monnerat G, Alarcon ML, Vasconcellos LR, et al. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat Commun. 2016;7:13344. doi:10.1038/ncomms13344
15. Li Y, Huang H, Liu B, et al. Inflammasomes as therapeutic targets in human diseases. Signal Transduct Target Ther. 2021;6(1):247. doi:10.1038/s41392-021-00650-z
16. Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13(4):333–342. doi:10.1038/ni.2237
17. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of pro-IL-beta. Mol Cell. 2002;10(2):417–426. doi:10.1016/s1097-2765(02)00599-3
18. Kanneganti TD. The inflammasome: firing up innate immunity. Immunol Rev. 2015;265(1):1–5. doi:10.1111/imr.12297
19. Ratsimandresy RA, Dorfleutner A, Stehlik C. An update on PYRIN domain-containing pattern recognition receptors: from immunity to pathology. Front Immunol. 2013;4:440. doi:10.3389/fimmu.2013.00440
20. Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. doi:10.1038/s41577-019-0165-0
21. Monteleone M, Stow JL, Schroder K. Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine. 2015;74(2):213–218. doi:10.1016/j.cyto.2015.03.022
22. Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122(12):1722–1740. doi:10.1161/CIRCRESAHA.118.311362
23. Xiao H, Li H, Wang JJ, et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur Heart J. 2018;39(1):60–69. doi:10.1093/eurheartj/ehx261
24. Enni JBA, Ninh V, Miyamoto S, et al. NLRP3 inflammasome products IL-1beta and IL-18 have a direct effect on cardiomyocytes. FASEB J. 2020;34(Suppl 1):1. doi:10.1096/fasebj.2020.34.s1.06628
25. Sun Y, Ding S. NLRP3 inflammasome in diabetic cardiomyopathy and exercise intervention. Int J Mol Sci. 2021;22(24):13228. doi:10.3390/ijms222413228
26. Frati G, Schirone L, Chimenti I, et al. An overview of the inflammatory signaling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc Res. 2017;113(4):378–388. doi:10.1093/cvr/cvx011
27. Qiu Z, Lei S, Zhao B, et al. NLRP3 inflammasome activation-mediated pyroptosis aggravates myocardial ischemia/reperfusion injury in diabetic rats. Oxid Med Cell Longev. 2017;2017:9743280. doi:10.1155/2017/9743280
28. Zhang D, He Y, Ye X, et al. Activation of autophagy inhibits nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation and attenuates myocardial ischemia-reperfusion injury in diabetic rats. J Diabetes Investig. 2020;11(5):1126–1136. doi:10.1111/jdi.13235
29. Zaharieva E, Kamenov Z, Velikova T, et al. Interleukin-18 serum level is elevated in type 2 diabetes and latent autoimmune diabetes. Endocr Connect. 2018;7(1):179–185. doi:10.1530/EC-17-0273
30. Niu J, Gilliland MG, Jin Z, et al. MCP-1and IL-1β expression in the myocardia of two young patients with Type 1 diabetes mellitus and fatal diabetic ketoacidosis. Exp Mol Pathol. 2014;96(1):71–79. doi:10.1016/j.yexmp.2013.11.001
31. Zhang X, Fu Y, Li H, et al. H3 relaxin inhibits the collagen synthesis via ROS- and P2X7R-mediated NLRP3 inflammasome activation in cardiac fibroblasts under high glucose. J Cell Mol Med. 2018;22(3):1816–1825. doi:10.1111/jcmm.13464
32. Luo B, Li B, Wang W, et al. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One. 2014;9(8):e104771. doi:10.1371/journal.pone.0104771
33. Ernault AC, Meijborg VMF, Coronel R. Modulation of cardiac arrhythmogenesis by epicardial adipose tissue: JACC state-of-The-art review. J Am Coll Cardiol. 2021;78(17):1730–1745. doi:10.1016/j.jacc.2021.08.037
34. Yan SF, Ramasamy R, Naka Y, et al. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93(12):1159–1169. doi:10.1161/01.RES.0000103862.26506.3D
35. Sciarretta S, Paneni F, Palano F, et al. Role of the renin-angiotensin-aldosterone system and inflammatory processes in the development and progression of diastolic dysfunction. Clin Sci. 2009;116(6):467–477. doi:10.1161/01.RES.0000103862.26506.3D
36. Che H, Wang Y, Li H, et al. Melatonin alleviates cardiac fibrosis via inhibiting lncRNA MALAT1/miR-141-mediated NLRP3 inflammasome and TGF-β1/Smads signaling in diabetic cardiomyopathy. FASEB J. 2020;34(4):5282–5298. doi:10.1096/fj.201902692R
37. Wang A, Green JB, Halperin JL, Piccini JP
38. Yao C, Veleva T, Scott L
39. Scott Jr L
40. Wu X, Liu Y, Tu D, et al. Role of NLRP3-inflammasome/caspase-1/galectin-3 pathway on atrial remodeling in diabetic rabbits. J Cardiovasc Transl Res. 2020;13(5):731–740. doi:10.1007/s12265-020-09965-8
41. Heijman J, Muna AP, Veleva T, et al. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ Res. 2020;127(8):1036–1055. doi:10.1161/CIRCRESAHA.120.316710
42. Li N, Brundel B. Inflammasomes and proteostasis novel molecular mechanisms associated with atrial fibrillation. Circ Res. 2020;127(1):73–90. doi:10.1161/CIRCRESAHA.119.316364
43. Chen M, Li X, Wang S, et al. The role of cardiac macrophage and cytokines on ventricular arrhythmias. Front Physiol. 2020;11:1113. doi:10.3389/fphys.2020.01113
44. Liu H, Zhao Y, Xie A, et al. Interleukin-1beta, Oxidative stress, and abnormal calcium handling mediate diabetic arrhythmic risk. JACC Basic Transl Sci. 2021;6(1):42–52. doi:10.1016/j.jacbts.2020.11.002
45. Tian CJ, Zhang JH, Liu J, et al. Ryanodine receptor and immune-related molecules in diabetic cardiomyopathy. ESC Heart Fail. 2021;8(4):2637–2646. doi:10.1002/ehf2.13431
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.