Back to Journals » OncoTargets and Therapy » Volume 16
The Role of Neutrophil-to-Lymphocyte Ratio in Advanced EGFR-Mutant NSCLC Patients Treated with First-Line Osimertinib
Authors Chen KC , Huang YH, Hsu KH , Tseng JS , Chang GC, Yang TY
Received 5 February 2023
Accepted for publication 11 May 2023
Published 17 May 2023 Volume 2023:16 Pages 317—326
DOI https://doi.org/10.2147/OTT.S407301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Kuan-Chih Chen,1,* Yen-Hsiang Huang,1– 3,* Kuo-Hsuan Hsu,3,4 Jeng-Sen Tseng,1– 3,5,6 Gee-Chen Chang,6– 9 Tsung-Ying Yang1,3,10
1Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; 2College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan; 3Lung Cancer Comprehensive Care and Research Center, Taichung Veterans General Hospital, Taichung, Taiwan; 4Division of Critical Care and Respiratory Therapy, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; 5Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan; 6Institute of Biomedical Sciences, National Chung Hsing University, Taichung, Taiwan; 7Division of Pulmonary Medicine, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan; 8School of Medicine, Chung Shan Medical University, Taichung, Taiwan; 9Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan; 10Department of Life Sciences, National Chung Hsing University, Taichung, Taiwan
*These authors contributed equally to this work
Correspondence: Jeng-Sen Tseng, Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, No. 1650, Sect. 4, Taiwan Boulevard, Taichung, 407, Taiwan, Tel +886-4-23592525, ext. 3232, Fax +886-4-23741320, Email [email protected]
Purpose: Although serum neutrophil-to-lymphocyte ratio (NLR) is correlated with the outcome of various cancer types, its role in treatment-naïve, advanced, epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC) patients treated with osimertinib remains uncertain. We have the intention to use this biomarker to evaluate the outcomes in NSCLC.
Patients and Methods: Advanced EGFR-mutant NSCLC patients receiving osimertinib as the first-line treatment were included. We evaluated the prognostic role of baseline NLR and explored its association with patients’ characteristics. A high NLR was defined as pretreatment serum NLR ≥ 5.
Results: A total of 112 eligible patients were included. The objective response rate was 83.7%. The median progression-free survival (PFS) and overall survival (OS) were 20.5 months (95% CI 14.5– 26.5) and 47.3 months (95% CI 36.7– 58.2), respectively. A high NLR predicted an inferior PFS (HR 1.90 [95% CI 1.02– 3.51], P = 0.042) and OS (HR 3.85 [95% CI 1.39– 10.66], P = 0.009). Patients with stage IVB disease were more likely to have a high baseline NLR than those with stage IIIB-IVA (33.9% vs 15.1%, P = 0.029). Other patients’ characteristics did not correlate with the baseline NLR significantly. Patients with a high NLR had significantly more metastatic organs than those with a low NLR (2.5 ± 1.3 vs 1.8 ± 0.9, P = 0.012), particularly brain, liver, and bone metastasis. There was no significant association between NLR and intrathoracic metastasis.
Conclusion: Baseline serum NLR could act as an important prognostic marker for EGFR-mutant NSCLC patients receiving first-line osimertinib. A high NLR was associated with higher metastatic burden, more extrathoracic metastases, and therefore, a worse outcome.
Keywords: non-small cell lung cancer, epidermal growth factor receptor, osimertinib, neutrophil-to-lymphocyte ratio, treatment-naïve
Introduction
Lung cancer carries both a high incidence and a high mortality rate, and is the leading cause of cancer-related death worldwide.1 Epidermal growth factor receptor (EGFR) mutation is the most common genetic alteration among East Asian people with non-small cell lung cancer (NSCLC).2 Given the higher response rate, longer survival time, and better quality of life, EGFR-TKIs have emerged as the cornerstone therapy for advanced NSCLC patients harboring sensitizing EGFR mutations and are recommended as the standard first-line therapy.3,4 Three generations of EGFR-TKIs are clinically available. FLAURA is a double-blind, Phase 3 randomized controlled trial comparing the efficacy and safety of osimertinib and first-generation EGFR-TKIs among previously untreated, advanced EGFR-mutant (19del or L858R) NSCLC patients. The results suggest that osimertinib carries a favorable efficacy and lower rates of serious adverse events as compared with first-generation EGFR-TKIs.5 Currently, osimertinib serves as the preferred choice of first-line EGFR-TKIs.4
The serum neutrophil-to-lymphocyte ratio (NLR) is a highly available and easily measurable biomarker, which may represent the status of inflammation.6,7 In addition to its use in the treatment of critically ill patients receiving intensive care, infectious disease, and cardiovascular disease,8–10 NLR has been reported as an important prognostic factor among various cancer types, including lung cancer.11 Moreover, NLR is correlated with the efficacy of various antineoplastic treatments, including chemotherapy, immunotherapy, and targeted therapy.12–14 Consistently, a high NLR predicted an unfavorable outcome of treatment.
With regard to lung cancer targeted therapy, many studies revealed that an elevated serum NLR was associated with lower disease control rate and inferior survival time for EGFR-mutant NSCLC patients undergoing EGFR-TKI treatment.13,15,16 However, both the EGFR mutation subtypes and the EGFR-TKI regimens were heterogeneous in most of these studies. Studies focusing on osimertinib remain limited.17 Herein, we evaluated the prognostic role of baseline serum NLR in treatment-naïve EGFR-mutant NSCLC patients treated with osimertinib and explored the association between NLR and patients’ characteristics.
Materials and Methods
Study Population
This was a retrospective cohort study. In this study, we analyzed patients with lung cancer diagnosed and treated at Taichung Veterans General Hospital from November 2017 to May 2022. To be eligible for this study, patients were required to have cytologically or pathologically confirmed NSCLC, inoperable stage III to IVB disease, osimertinib as the first-line monotherapy, sensitizing EGFR mutations, precise data records, and clear survival follow-up. Since EGFR exon 20 T790M-mutant tumors are sensitive to osimertinib, patients who harbored a primary T790M mutation could be included in this study.18 Patients were excluded if they had mixed components of small cell carcinoma, had stage III disease treated with attempted curative local therapy, had another active malignancy, or had incomplete data records.
Data Records for Analysis
Clinical data in the analysis included patients’ age, gender, smoking status, the histological classification, the Eastern Cooperative Oncology Group performance status (ECOG PS), EGFR mutation status, tumor stage, metastatic organs, treatment response, survival status, and pretreatment serum NLR. In the case of NLR, the highest NLR value between the diagnosis and the prescription of first dose of osimertinib was recorded and we avoid recording the value detected during acute infection episode or corticosteroid usage. Lung cancer TNM (tumor, node, and metastases) staging was conducted according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system.19 Unidimensional measurements as defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 were used in this study.20 This study was approved by the Institutional Review Board of Taichung Veterans General Hospital (IRB No. CF12019 and CF20175), which complied with the Declaration of Helsinki. Written informed consent for clinical data records and genetic testing was obtained from all patients.
EGFR Mutation Analysis
The EGFR mutation status of all patients included in this study was determined using tumor specimens by either matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS) as previously described or by commercialized cobas® EGFR Mutation Test v2 platform.21,22
Statistical Analysis
Fisher’s exact test was used to evaluate the association between categorized variables. Kaplan–Meier method was used to estimate the survival time. Progression-free survival is defined as the length of time from starting osimertinib treatment to disease progression or death of any cause and overall survival (OS) is defined as the length of time from starting osimertinib treatment to death of any cause. Association between baseline NLR and numbers of metastatic organs was assessed by Student’s t-test. Cox proportional hazard model was performed for univariate and multivariate analyses of the prognostic factors of survival outcomes. In the present study, we defined high NLR as pretreatment serum NLR ≥ 5, which was consistent with prior studies.11,23 All statistical tests were carried out using SPSS 15.0 (SPSS Inc., Chicago, IL, USA). Two-tailed tests with P values <0.05 were considered statistically significant.
Results
Patients and Their Demographic Data
From November 2017 to May 2022, there were 118 patients treated with osimertinib in the first-line setting. Five patients were excluded because they were also receiving chemotherapy simultaneously, and one patient who harbored the EGFR complex mutation with exon 20 insertion was also excluded. Finally, a total of 112 patients were included in the analysis. The baseline characteristics and demographic data are summarized in Table 1. The median age was 62.5 years. Of them, 59.8% were females, while 69.6% were never smokers. Adenocarcinoma comprised the major histological type (97.3%). Fifty-nine patients (52.7%) had stage IVB disease and baseline ECOG PS was 0–1 in 88.4% of our patients. Exon 19 deletion (19Del) (73.2%) and exon 21 L858R (23.2%) accounted for the most common EGFR mutation types.
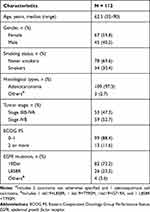 |
Table 1 Patients’ Characteristics and Demographic Data |
Efficacy of First-Line Osimertinib Treatment
After exclusion of 14 patients without measurable lesions, one patient achieved a complete response, while 81 patients achieved a partial response. Moreover, 8 patients had stable disease and 8 patients had progressive disease. The objective response rate and disease control rate were 83.7% and 91.8%, respectively.
The median follow-up time of our patients was 20.0 months (95% CI 18.0–20.0). The data cutoff date was December 16, 2022. The PFS was 20.5 months (95% CI 14.5–26.5). The median OS was 47.3 months (95% CI 36.7–58.2).
Association Between Patients’ Characteristics and Survival Time
The results of the univariate analysis of patients’ characteristics and survival time are summarized in Table 2. In the PFS analysis, EGFR 19Del predicted a longer PFS as compared with other mutation types (HR 0.40 [95% CI 0.22–0.74], P = 0.004). By contrast, patients with stage IVB disease and a high baseline NLR experienced a significantly shorter PFS (HR 2.01 [95% CI 1.09–3.72], P = 0.025 and 1.90 [95% CI 1.02–3.51], P = 0.042, respectively).
 |
Table 2 Univariate Analysis of Patients’ Characteristics and Survival Time |
In the case of OS, patients with better ECOG PS (0–1) experienced a significantly longer OS (47.4 months [95% CI NR-NR] vs 26.2 months [95% CI 20.6–31.8], Log rank P = 0.005; HR 0.24 [95% CI 0.08–0.69], P = 0.008). By contrast, a high baseline NLR predicted a shorter OS (HR 3.85 [95% CI 1.39–10.66], P = 0.009). Of note, baseline NLR was the only factor associated with both PFS (26.2 months [95% CI 21.0–31.3] vs 18.0 months [95% CI 15.3–20.6], Log rank P = 0.038) and OS (47.4 months [95% CI 42.2–52.9] vs 35.6 months [95% CI 20.9–50.3], Log rank P = 0.005) (Figure 1A and B); a high baseline NLR predicted a worse outcome.
 |
Figure 1 Influence of the baseline neutrophil-to-lymphocyte ratio on progression-free survival (A) and overall survival (B). |
Association Between Baseline NLR and Patients’ Characteristics
As the baseline NLR correlated with survival time significantly in the univariate analysis, we further analyzed the association between NLR and patients’ characteristics to clarify its significance in outcome prediction. The results are summarized in Table 3. Of note, only tumor stage was significantly associated with baseline NLR. Patients with stage IVB disease were more likely to have a high baseline NLR than those with stage IIIB-IVA (33.9% vs 15.1%, P = 0.029). Age, gender, smoking status, ECOG PS, and EGFR mutation types did not correlate with baseline NLR.
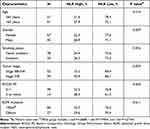 |
Table 3 Association Between the Baseline Neutrophil-to-Lymphocyte Ratio and Patients’ Characteristics |
In the multivariate analysis, adjusted for tumor stage, EGFR mutation types, and ECOG PS, baseline NLR only predicted OS significantly (aHR 3.45 [95% CI 1.18–10.27], P = 0.023) but not PFS (aHR 1.46 [95% CI 0.77–2.77], P = 0.253). With respect to PFS, EGFR 19Del (aHR 0.41 [95% CI 0.21–0.77], P = 0.006) was the only significant predictor of longer PFS. There was a trend toward shorter PFS in patients with stage IVB disease (aHR 1.87 [95% CI 0.99–3.54], P = 0.056). Regarding OS, ECOG PS 0–1 also predicted a significantly longer survival time (aHR 0.12 [95% CI 0.04–0.42], P = 0.001).
Baseline NLR Was Associated with Higher Metastatic Burden Leading to Worse Outcome
Since NLR correlated with tumor stage, we further analyzed its association with tumor burden and metastatic sites. Interestingly, patients with a high baseline NLR had significantly more metastatic organs than those with a low NLR (2.5 ± 1.3 vs 1.8 ± 0.9, P = 0.012). As shown in Figure 2, a high baseline NLR was associated with more brain and liver metastases (P = 0.022 and 0.042, respectively) and there was also a trend toward more bone metastases (P = 0.075). By contrast, we did not observe any significant association between lung and pleural metastases with baseline NLR (P = 0.507 and 1.000, respectively). These results suggest that a high NLR was associated with higher metastatic burden, particularly extrathoracic metastasis.
 |
Figure 2 Association between the baseline neutrophil-to-lymphocyte ratio and metastatic organs. |
PFS was significantly shorter in patients with stage IVB disease than in those with non-stage IVB disease (18.2 months [95% CI 15.9–20.4] vs NR [95% CI NR-NR]) (Table 2). The OS curve also disclosed a trend toward a shorter survival time among patients with stage IVB disease despite the lack of statistical significance (Supplementary Figure 1). We analyzed the influence of baseline NLR on the outcome of patients with stage IVB and non-stage IVB disease separately. As shown in Figure 3, a high baseline NLR was associated with both a shorter PFS (15.9 vs 19.0 months, P = 0.070) and OS (35.6 vs 47.4 months, P = 0.005). By contrast, for patients with non-stage IVB disease, neither PFS nor OS correlated with baseline NLR (P = 0.889 and 0.679, respectively; data not shown). Taken together, patients with a high baseline NLR had higher metastatic burden, more extrathoracic metastases, and a worse outcome, particularly for those with stage IVB disease.
 |
Figure 3 Influence of the baseline neutrophil-to-lymphocyte ratio on progression-free survival (A) and overall survival (B) among stage IVB patients. |
Discussion
The treatment of advanced NSCLC has been directed towards personalized therapy, typically involving histological classification and biomarker assessment, which play a crucial role in the decision of front-line therapy.24,25 Since osimertinib showed consistent benefits of varying degrees across all subgroups in the FLAURA study and no biomarkers other than EGFR mutations have been identified, relevant prognostic factors that could help in clinical decision-making are still lacking.5,26,27 In the present study, we found real-world evidence showing comparable efficacy of first-line osimertinib and more importantly, we attempted to determine whether baseline serum NRL could serve as a useful biomarker in outcome prediction. As compared with previous reports, our study consisted of a relatively homogeneous population, which included advanced NSCLC patients harboring sensitizing EGFR mutations and all of them received osimertinib as the first-line treatment. Our results suggest that baseline serum NLR is an important prognostic factor for these patients, particularly those with stage IVB disease.
There have been many studies evaluating NLR as a predictive marker for EGFR-mutant NSCLC patients receiving EGFR-TKI treatment.13,15,16 Consistently, a high NLR was associated with a worse outcome. However, most data came from first-generation EGFR-TKI, gefitinib and erlotinib,13 and many of the study populations were heterogeneous in histological types, EGFR mutation subtypes, and race. In studies by Xu et al and Yun et al, 3 and 36 patients with osimertinib treatment were enrolled, respectively.15,16 Given the small case numbers, subgroup analysis focusing on the third-generation EGFR-TKI was not possible. Park et al investigated the role of pretreatment NLR in advanced NSCLC patients treated with osimertinib. Sixty-one eligible patients were included retrospectively.17 Thirty-one of the patients received second-line treatment with osimertinib, while the remaining 30 patients received third line or later treatment with osimertinib. The T790M mutation status of these patients came from either tissue rebiopsy or liquid biopsy. The results suggested that never-smoking status (HR 0.54 [95% CI 0.30–0.98], P = 0.041) and a baseline NLR ≤ 3.5 (HR 0.23 [95% CI 0.12–0.45], P < 0.001) were independently associated with a longer PFS following osimertinib treatment. Currently, data regarding first-line osimertinib treatment remain limited. Herein, our study consisted of a larger cohort and our data support the prognostic role of baseline NLR among EGFR-mutant NSCLC patients receiving first-line osimertinib treatment.
Although NLR can be widely applied for outcome prediction in cancer management, the underlying mechanism remains uncertain. Most studies have linked NLR with inflammation. In the tumor microenvironment, cancer cells may secrete cytokines that reprogram neutrophils to a cancer-promoting polarity,28,29 and lymphopenia was reported to be associated with an inadequate cell-mediated immune response to cancer.30 Additionally, NRL is a reliable and valid biomarker of systemic inflammation in both cancer and non-cancer diseases.10,31 Given that there is a strong link between inflammation and lung tumorigenesis and cancer progression,32 it would not be surprising that these inflammatory markers are associated with the outcomes of cancer patients. Clinically, it is not easy to define whether an elevated NLR is a “cause” or an “effect” since the causal relationship between cancer and inflammation may be reciprocal.33
In the case of hepatocellular carcinoma, preoperative NLR is associated with more aggressive phenotypes, including the presence of vascular invasion, satellite tumor numbers, and high levels of alpha-fetoprotein.34 Among patients with head and neck cancer, subjects with higher T and N staging were more likely to have a high NLR.35 A similar situation can also be observed in patients with breast cancer and colorectal cancer.36,37 In the present study, tumor stage was the only factor among the different patients’ characteristics that was significantly associated with baseline NLR. Indeed, patients with stage IVB disease were more likely to have a high baseline NLR. It is not surprising that a high NLR corresponded to higher tumor stage and therefore a worse outcome.19
In addition to tumor stage, we further clarified the association between the NLR and tumor metastatic burden. Of note, patients with a high NLR had significantly more metastatic organs, particularly extrathoracic metastases, and these organs are important metastatic sites for determining the M1c status and stage IVB stage. By contrast, neither lung nor pleural metastases, which belong to M1a status and stage IVA disease, were significantly correlated with baseline NLR. In the stratified analysis, NLR could only predict the outcome of patients with stage IVB disease but not those with stage III or IVA disease. In the study by Yun et al, there was a trend towards more metastatic sites and more brain metastasis among those with NLR ≥ 5.16 Xu et al also showed that baseline NLR was significantly higher in stage IV than in stage III NSCLC patients.15 Along with the wide application of NLR in various cancer types and treatments, we suggest that NLR can serve as an important prognostic factor, representing the tumor burden and extension of metastasis, rather than a predictive factor specific to a certain disease or regimen.
The major limitation of this investigation was the retrospective nature of the study design, and the study was done at a single cancer center. Although the data were collected retrospectively, we analyzed a relatively homogeneous patient population to maximize the validity of the patients’ characteristics, treatment response, and survival follow-up. More importantly, considerable efforts were undertaken to clarify and qualify the extension of tumor metastasis. Our results suggested the associations between NLR and metastatic burden and its value to predict the outcome of first-line osimertinib treatment.
Conclusion
Baseline serum NLR could act as an important prognostic marker. A high NLR was associated with higher metastatic burden, more extrathoracic metastases, and therefore, a worse outcome.
Acknowledgments
Kuan-Chih Chen and Yen-Hsiang Huang contributed equally to the work as co-first authors. We would like to thank the Cancer Prevention and Control Center of Taichung Veterans General Hospital for assisting with the data collection and management and we also thank the NRPB Pharmacogenomics Lab and the NCFPB Integrated Core Facility for Functional Genomics for their technical support.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
2. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–162. doi:10.1097/jto.0000000000000033
3. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl4):iv192–iv237. doi:10.1093/annonc/mdy275
4. National Comprehensive Cancer Network. Non-small cell lung cancer (Version: 1.2023). Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
5. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi:10.1056/NEJMoa1713137
6. Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. 2022;23(7):3636. doi:10.3390/ijms23073636
7. Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L. Update on neutrophil function in severe inflammation. Front Immunol. 2018;9:2171. doi:10.3389/fimmu.2018.02171
8. Lee MJ, Park SD, Kwon SW, et al. Relation between neutrophil-to-lymphocyte ratio and index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2016;118(9):1323–1328. doi:10.1016/j.amjcard.2016.07.072
9. Lowsby R, Gomes C, Jarman I, et al. Neutrophil to lymphocyte count ratio as an early indicator of blood stream infection in the emergency department. Emerg Med J. 2015;32(7):531–534. doi:10.1136/emermed-2014-204071
10. Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14.
11. Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi:10.1093/jnci/dju124
12. Bryant AK, Sankar K, Strohbehn GW, et al. Prognostic and predictive value of neutrophil-to-lymphocyte ratio with adjuvant immunotherapy in stage III non-small-cell lung cancer. Lung Cancer. 2022;163:35–41. doi:10.1016/j.lungcan.2021.11.021
13. Tang M, Gao X, Sun H, et al. Neutrophil-lymphocyte ratio as a prognostic parameter in NSCLC patients receiving EGFR-TKIs: a systematic review and meta-analysis. J Oncol. 2021;2021:6688346. doi:10.1155/2021/6688346
14. Wang Z, Zhan P, Lv Y, et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis. Transl Lung Cancer Res. 2019;8(3):214–226. doi:10.21037/tlcr.2019.06.10
15. Xu C, Yao X, Li T, et al. Pretreatment neutrophil-to-lymphocyte ratio is a predictive biomarker for EGFR TKI-treated patients with advanced EGFR-mutant non-small cell lung cancer. Transl Cancer Res. 2020;9(4):2875–2883. doi:10.21037/tcr.2020.02.28
16. Yun NK, Rouhani SJ, Bestvina CM, et al. Neutrophil-to-lymphocyte ratio is a predictive biomarker in patients with epidermal growth factor receptor (EGFR) mutated advanced non-small cell lung cancer (NSCLC) treated with tyrosine kinase inhibitor (TKI) therapy. Cancers. 2021;13(6):1426. doi:10.3390/cancers13061426
17. Park JY, Jang SH, Lee CY, et al. Pretreatment neutrophil-to-lymphocyte ratio and smoking history as prognostic factors in advanced non-small cell lung cancer patients treated with osimertinib. Tuberc Respir Dis. 2022;85(2):155–164. doi:10.4046/trd.2021.0139
18. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi:10.1056/NEJMoa1612674
19. Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual.
20. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
21. Hsu KH, Ho CC, Hsia TC, et al. Identification of five driver gene mutations in patients with treatment-naïve lung adenocarcinoma in Taiwan. PLoS One. 2015;10(3):e0120852. doi:10.1371/journal.pone.0120852
22. Malapelle U, Sirera R, Jantus-Lewintre E, et al. Profile of the Roche cobas(R) EGFR mutation test v2 for non-small cell lung cancer. Expert Rev Mol Diagn. 2017;17(3):209–215. doi:10.1080/14737159.2017.1288568
23. Bowen RC, Little NAB, Harmer JR, et al. Neutrophil-to-lymphocyte ratio as prognostic indicator in gastrointestinal cancers: a systematic review and meta-analysis. Oncotarget. 2017;8(19):32171–32189. doi:10.18632/oncotarget.16291
24. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322(8):764–774. doi:10.1001/jama.2019.11058
25. Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27(8):1345–1356. doi:10.1038/s41591-021-01450-2
26. Gray JE, Okamoto I, Sriuranpong V, et al. Tissue and plasma EGFR mutation analysis in the FLAURA trial: osimertinib versus comparator EGFR tyrosine kinase inhibitor as first-line treatment in patients with EGFR-mutated advanced non-small cell lung cancer. Clin Cancer Res. 2019;25(22):6644–6652. doi:10.1158/1078-0432.Ccr-19-1126
27. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi:10.1056/NEJMoa1913662
28. Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133(20):2159–2167. doi:10.1182/blood-2018-11-844548
29. Grieshaber-Bouyer R, Radtke FA, Cunin P, et al. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat Commun. 2021;12(1):2856. doi:10.1038/s41467-021-22973-9
30. Beyer M, Schultze JL. Regulatory T cells: major players in the tumor microenvironment. Curr Pharm Des. 2009;15(16):1879–1892. doi:10.2174/138161209788453211
31. Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122(7):474–488. doi:10.4149/bll_2021_078
32. O’Callaghan DS, O’Donnell D, O’Connell F, O’Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5(12):2024–2036. doi:10.1097/jto.0b013e3181f387e4
33. Wang Q, Shi Q, Lu J, Wang Z, Hou J. Causal relationships between inflammatory factors and multiple myeloma: a bidirectional Mendelian randomization study. Int J Cancer. 2022;151(10):1750–1759. doi:10.1002/ijc.34214
34. Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:117. doi:10.1186/1471-2407-14-117
35. Ma SJ, Yu H, Khan M, et al. Evaluation of optimal threshold of neutrophil-lymphocyte ratio and its association with survival outcomes among patients with head and neck cancer. JAMA Netw Open. 2022;5(4):e227567. doi:10.1001/jamanetworkopen.2022.7567
36. Chen Y, Chen K, Xiao X, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. doi:10.1186/s12885-016-2352-8
37. Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181–184. doi:10.1002/jso.20329
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
