Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
The Role of Gut Microbiota in the Progression of Parkinson’s Disease and the Mechanism of Intervention by Traditional Chinese Medicine
Authors Huan P, Wang L, He Z, He J
Received 18 March 2022
Accepted for publication 7 July 2022
Published 26 July 2022 Volume 2022:18 Pages 1507—1520
DOI https://doi.org/10.2147/NDT.S367016
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Pengfei Huan,1,2 Li Wang,1,2 Zhuqing He,1,2 Jiancheng He1,2
1School of Basic Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China; 2Shanghai Key Laboratory of Health Identification and Assessment, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China
Correspondence: Jiancheng He, Email [email protected]
Abstract: Parkinson’s disease (PD) is a common degenerative disease of the nervous system that seriously affects the quality of life of the patients. The pathogenesis of PD is not yet fully clear. Previous studies have confirmed that patients with PD exhibit obvious gut microbiota imbalance, while intervention of PD by regulating the gut microbiota has become an important approach to the prevention and treatment of this disease. Traditional Chinese medicine (TCM) has been shown to be safe and effective in treating PD. It has the advantages of affecting multiple targets. Studies have shown TCM can regulate gut microbiota. However, the specific mechanism of action is still unclear. Therefore, this article will mainly discuss the association of the alteration of the gut microbiota and the incidence of PD, the advantages of TCM in treating PD, and the mechanism of regulating gut microbiota by TCM to treat PD. It will clarify the target and mechanism of TCM treating PD by acting gut microbiota and provided a novel methodology for the prevention and treatment of PD.
Keywords: Parkinson’s disease, gut microbiota, traditional Chinese medicine, pathogenesis
Parkinson’s disease (PD) is a common neurological degenerative disease in the elderly, characterized by lesions of the substantia nigra and striatum. The main symptoms of this disease are tremor, muscle rigidity, bradykinesia, and unstable posture.1 An annual increase in the incidence of PD has been noted in the elderly population. The prevalence rate of the elderly over 65 years old with this disease is as high as 1–2%, and the incidence rate of the group over 85 years old reaches 4%.2 PD has caused a serious burden on both patients and their families. Current treatments for PD either increase/replace DA, or prevent the breakdown of DA, or prolong the action of levodopa to help control tremors.3 Medications and surgery have been used to treat PD, but both have moderate side effects and often produce disappointing results.4,5 Therefore, it is particularly important to focus on addressing different treatment strategies for this disease. The clinical application of Traditional Chinese Medicine (TCM) is gradually supported by clinical evidence. However, the specific mechanism of action is still unclear. TCM is usually taken orally and is absorbed in the intestinal tract. A variety of studies have shown TCM regulate gut microbiota.6–8 Gut microbiota refer to the assembly of microorganisms, such as bacteria, archaea, eukaryotes, and viruses, which colonize the intestine.9 Gut microbiota have been shown to be closely associated with PD.10 However, it remains unclear whether Chinese herbal medicine can be used to treat PD by acting on the gut microbiota. The present study addressed this hypothesis.
The Association of the Alteration of the Gut Microbiota and the Incidence of PD
Alterations of Gut Microbiota Composition in PD Patients
The human intestine has been reported to contain a high number of microorganisms (microbiota). Microbiota comprise a higher number of genomes than those noted in human cells.10 Therefore, retaining the stability of the gut microbiota is the key to maintain normal physiological activities of the human body. Previous studies have shown that the imbalance of the gut microbiota is closely related to the occurrence of type 2 diabetes, obesity, and atherosclerosis.11 As a common neurological disease, PD has been studied for nearly two centuries. However, the mechanism of PD remains unclear. A large number of studies have found a close relationship between disorders of the gut microbiota and the incidence of PD, the gut microbiota of PD patients is disordered [Table 1]. Several groups of differences are evident between patients and control groups. Previous studies have shown that the number of Lactobacillus in the feces of PD patients is higher, while the numbers of Prevotella, Clostridium and Bacteroides fragilis are lower. In addition, the decline in the number of Prerevotella may result in decreased mucin synthesis and increased intestinal permeability.12 In the feces samples of PD patients, the numbers of anti-inflammatory-related bacteria, such as Coprococcus, Blautia, and Roseburia were significantly reduced. This bacterial species may promote the occurrence of inflammation in the colon.13 In addition, a previous study indicated that the numbers of Butyricicoccus and Clostridium XlVb of PD patients were significantly increased compared with the corresponding numbers noted in the healthy control group, which may be related to the cognitive impairment of PD.14 In PD patients, Christensenellaceae and Desulfovibrionaceae, Bifidobacterium, Collinsella, Bilophila, and Akkermansia are relatively abundant. By contrast, Lachnospiraceae, Roseburia, Lachnospiraceae, and Faecalibacterium exhibited lower abundance.15 Nishiwaki et al used a meta-analysis to compare 223 PD patients with 137 healthy controls and demonstrated that the numbers of Akkermansia, Catabacter, and of the bacterial species Akkermansiaceae were increased, while those of Roseburia, Faecalibacterium, and Lachnospiraceae ND3007 were decreased.16 A previous study utilized two large datasets from a Microbiome/Metagenome-wide association study to specify changes in the gut microbiota of patients with PD. Specific pathogenic bacteria were identified in PD patients, such as Porphyromonas, Prevotella Corynebacterium, Bifidobacterium, and Lactobacillus, their abundance was decreased, whereas the abundance of short-chain fatty acid (SCFA)-producing bacteria, such as the Rumenococcus and Lacetospiraceae was also decreased in PD patients, moreover, PD exhibited an increase in the abundance of Bifidobacterium and Lactobacillus, which are probiotics for sugar metabolism that may become conditional pathogens in PD.17 In general, the data indicated that the composition of the gut microbiota of PD patients had changed, which was manifested as a decrease in the relative abundance of SCFA-producing bacteria and an increase in the relative abundance of Akkermansia. These changes may reflect the changes in some PD-specific gut microbiota.
 |
Table 1 Bacterial Composition Alterations in PD Patients Compared to Healthy Controls |
The Imbalance in the Gut Microbiota Can Promote the Occurrence of PD Through the ENS-Vagus Nerve Pathway
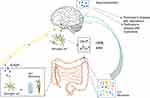
At present, a variety of studies have shown a close relationship between the occurrence and development of PD and the disorders associated with dysfunction of the gut microbiota (Figure 1). The main pathological features of PD are accumulation of α-syn in Lewy bodies in cells and accumulation of Lewy neurites in axons and dendrites. α-syn was initially discovered in 1980 in the gastrointestinal tract, suggesting that this protein of the nervous system may originate from the gastrointestinal tract.18 On this basis, Break proposed that this pathogen of PD initially caused intestinal lesions and destroyed the enteric nervous system (ENS), this situation caused the intestinal nerves to produce Lewy bodies, which act on the vagus nerve reaching the substantia nigra and striatum and finally cause the development of PD.19–21 Svensson et al demonstrated by the removal of the abdominal vagus nerve that this operation could significantly reduce the risk of PD.22 These studies provide strong support for the Break hypothesis. The latter was confirmed in animal experiments. By using rotenone to induce PD-like neuropathy in mice by intragastric administration, it was shown that neuropathy initially appeared in the intestinal ENS of mice, whereas, this change was finally present in the substantia nigra of experimental mice.23 Previous studies have shown that following injection of α-syn into the intestinal wall of rats, this protein is transported from the intestine to the brain.24 These results indicate that ENS is one of the starting sites of α-syn accumulation and that it is closely related to the occurrence of PD. Qin et al25 demonstrated that the disturbance of gut microbiota could activate intestinal glial cells, leading to the accumulation of α-syn, which can enter the substantia nigra, translocate to the vagus nerve, and cause PD. Sampson et al26 transplanted the feces of PD patients and normal subjects into sterile mice. As a result, the mice developed dyskinesia and abnormal intestinal function. When antibiotics were provided to PD mice, which were pre-induced by bacterial endotoxins, it was found that the inflammation of the substantia nigra was improved. The antibiotics could reduce the activation of microglia and increase the number of dopaminergic neurons in the compact substantia nigra. This evidence indicated that the change of the gut microbiota was closely related to the occurrence of PD. The aforementioned evidence indicated that the dysbiosis of gut microbiota was closely related to the occurrence of PD.
Dysbiosis of Gut Microbiota Further Exacerbates the Severity of PD
The imbalance of the gut microbiota is not only closely related to the occurrence of PD, but it is also inseparable from the development of the disease. The accumulation of α-syn in the brain can induce microglia to differentiate into M1 type microglia that secrete pro-inflammatory factors, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and interleukin-12 (IL-12).27 Previous studies have shown that IL-6, IL-1β, and other inflammatory factors can stimulate the secretion of corticosteroid releasing hormone and adrenocorticotropic hormone causing abnormal activation of the Hypothalamic–pituitary–adrenal (HPA) axis.28,29 It has also been shown that overactivation of the HPA axis can lead to disorders of the gut microbiota.30 This exacerbates the imbalance of the gut microbiota of patients with PD, leading to the development of PD.
Depression is a common complication of PD. The risk of depression in PD patients is 30% higher compared with patients who do not suffer from PD.31 As a neuroprotective factor, Brain-derived neurotrophic factor (BDNF) can regulate the growth and remodeling of nerve cells. Previous studies have shown that the decrease in the content of BDNF in the brain of PD patients will affect the function of neurons in the amygdala, leading to depression, the increase in the levels of BDNF can improve depression-like behavior in PD patients.32 The change in the BDNF-mediated signal transduction is the common basis for accelerating dopaminergic damage and can alter different brain structures to cause depression-like behavior in PD.33 Therefore, the lack of BDNF levels is one of the important mechanisms leading to the development of depression in PD. Gut microbiota can affect the levels of BDNF in the brain. Previous studies have shown that the ability of gut microbiota to fight depression and improve spatial memory may be achieved by restoring BDNF concentration in the brain.34 Feng et al35 demonstrated that compared with the control group, the levels of BDNF in the brain of the rats in the antibiotic group were significantly decreased, which demonstrated that the gut microbiota could affect the expression of BDNF in the brain.
During the treatment of PD, Levodopa (L-DOPA) is mainly used to replace the deficiency of DOPA. However, the long-term intake of L-DOPA can cause dyskinesia, which is manifested as involuntary, aimless, irregular and repetitive movements of the limbs, trunk and face, these effects are the main causes of disability in PD patients.36 In PD, dyskinesia significantly reduces the quality of life of patients and increases the cost of treatment.37 5-hydroxytryptamine (5-HT) is derived from the raphe nucleus of the brainstem, it is an important neurotransmitter and vasoactive substance, which acts extensively throughout the body. Previous studies have shown that the number of dopamine cells in the patient’s body is constantly reduced with the continuous development of the disease.38 Concomitantly, exogenous L-DOPA is absorbed by 5-HT cells and competes with 5-HT for entry into the vesicles at the end of the 5-HT neurons, this leads to decreased concentration of 5-HT.39 In the PD monkey model experiment, long-term L-DOPA treatment caused a reduction in the 5-HT levels in the striatum, hippocampus or amygdala. However, the 5-HT levels of the healthy control group did not significantly change.40 All these data indicate that the abnormality of the serotoninergic system is an important cause of PD-associated dyskinesia. It has also been shown that in sterile mice, the expression of tryptophan hydroxylase 1, which is the rate-limiting enzyme for the synthesis of 5-HT is reduced.41 The intestinal 5-HT levels are reduced, leading to weakening of the intestinal motility. Following administration of probiotics, the intestinal motility of sterile mice was enhanced, and the activity levels of the 5-HT4 receptors were enhanced in vivo.42 Previous studies have shown that probiotics can increase the levels of 5-HT in the brain of rats.43 Human-derived bacteria and intestinal bacteria, which are found in mice, were implanted into sterile mouse intestines. This led to an increase in gene transcription of the 5-HT synthesis rate-limiting enzyme and to a concomitant increase in its protein expression in mice, which in turn resulted in increased levels of the 5-HT positive markers in mouse tissues.44
In summary, it has been shown that various reasons can cause gut microbiota disorders, leading to the activation of intestinal microglia. This leads to accumulation of α-syn in the enteric nerve. As the disease progresses, α-syn can move along the vagus nerve into the substantia nigra, resulting in the occurrence of PD. The accumulation of α-syn in the brain can cause abnormal activation of microglia in the brain and hyperfunction of the HPA axis, further aggravating the intestinal dysbiosis and leading to neurotransmitter disorders in the brain, which exacerbate PD. The management of PD by regulating the gut microbiota is considered to be a new treatment method. At present, various types of drugs have been developed that can regulate the gut microbiota. TCM plays an important role among them.
The Advantages of TCM in Treating PD
Traditionally, L-DOPA, which is a dopamine receptor agonist, monoamine oxidase B inhibitors, and other drugs have been used for the treatment of PD. Previous studies have shown that long-term use of these chemicals can cause significant side effects, including nausea, constipation, headaches, and sleep disturbances.45 At present, the conventional treatment targets of the westernized methods are relatively simple and can cause various adverse events. These treatment methods do not follow a personalized treatment plan. Considering the disadvantages of the long-term use of westernized medicine, an urgent need is required to identify other, safer, and more effective ways to treat PD.
TCM has been used for centuries to treat tremors with optimal results. Until now, the treatment of TCM is still popular in various Asian countries, including China, Japan, and South Korea. In the past few decades, several active substances and potential molecular targets of Chinese herbal medicine have been discovered. Chinese medicine is rich in various substances, such as protein, amino acids, trace elements, vitamins, and other nutritional active substances. The advantage of the application of TCM is its ability to target multiple proteins, which can be used to directly treat PD. In addition, it can also synergize with western medicine to reduce the adverse effects caused by the latter and increase its therapeutic effects.46 Various TCM include compounds, such as flavonoids, Madecassoside, baicalein, resveratrol, and forsythiae, which can protect neurons and potentially prevent the development of PD.47–51
In addition to showing optimal laboratory efficacy in preventing PD, TCM has also achieved satisfactory clinical results. A randomized study indicated that the Chinese herbal compound Bu Shen Huo Xue Yin could improve the motor symptoms of PD patients.52 Besides, Chinese medicine can also improve the motor and non-motor symptoms of PD. Sleep disorders are common non-motor symptoms of PD, a randomized study indicated that Yang Xue Qing Nao granules could significantly improve sleep dysfunction.53 In addition, TCM treatment exhibits certain advantages compared with the corresponding western-based approaches. Firstly, it can improve clinical symptoms and the quality of life of the patients,54 while prolonging the therapeutic effect of L-DOPA by reducing its dose usage and decreasing the occurrence and severity of L-DOPA-induced dyskinesia55,56 [Table 2].
 |
Table 2 The Advantages of TCM in Treating PD |
The Mechanism of TCM in Regulating PD is Mediated via the Gut Microbiota
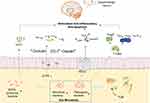
Gut microbiota play an important role in the occurrence and development of PD. TCM protects the intestinal mucosal barrier, and restores the diversity of intestinal microbes by regulating the amount and proportion of gut microbiota and metabolites, which not only can decrease release of pro-inflammatory cytokines from the gut into the bloodstream, but also increase the level of SCFAs and certain neuroactive factors, that exerts anti-oxidative, anti-inflammatory, anti-apoptotic via neuronal mechanism, endocrine mechanism, and immunological mechanism.57,58 This in turn can protect dopamine neurons in the brain and prevent the development of PD. [Figure 2]
Regulation of the Structure of the Gut Microbiota and Their Corresponding Metabolites
As mentioned in the aforementioned sections, a high number of studies have shown that patients with PD present with dysbiosis of gut microbiota, which can be summarized as a decrease in the number of beneficial bacteria and an increase in the abundance of pathogenic bacteria. TCM is a type of multi-target medicine. Previous studies have shown that Chinese herbal medicine can regulate the structure of gut microbiota and their metabolites.
Prevotellaceae can participate in the biosynthesis and degradation of mucin in the intestine. The levels of mucin are positively related to the permeability of the intestine.59 When the levels of mucin decrease, external pathogens are more likely to enter the intestinal submucosal nerve plexus.60 Prevotellaceae bacteria can affect the level of auxin in the human body and the change in auxin content can further affect the function of the substantia nigra striatum, which alters the patient’s nerve function.61,62 Lyciumbarbarum can increase the relative abundance of Prevotellaceae.63 Hu et al demonstrated that following Hua Feng Dan and 70W (Rannasangpei) treatment of PD mice for 35 days, the abundance of Prevotellaceae in the intestine was significantly increased.64
SCFA are important metabolites of the gut microbiota, mainly produced by Bacteroides, Bifidobacterium, Lactobacillus, Clostridium, Rosella, and Prevotella.65 SCFA reaches the central nervous system via the blood circulation and can strengthen the blood-brain barrier by increasing the expression levels of tight junction proteins. It also nourishes the nerves and exhibits anti-inflammatory effects.66 Moreover, SCFA exhibits extensive effects on nerve regeneration genes; it inhibits histone deacetylase activity, upregulates the expression levels of brain-derived nerve growth factor and glial cell-derived neurotrophic factor, and protects dopaminergic neurons from further injury.67 Unger et al demonstrated that the composition of the gut microbiota of PD patients was significantly changed and that the concentration of the SCFA mixture in the feces was significantly reduced, which caused enteric nervous dysfunction and gut microbiota imbalance.68 Astragaloside IV is an effective ingredient of TCM that can increase the levels of SCFAs produced and their generation in vivo.69 Previous studies have shown that berberine can improve the structure of the gut microbiota, repair the damaged microflora enzyme system, and convert the polysaccharides that are not fully absorbed in the intestine into SCFAs.70 Hydroxysafflor yellow A is an extract of Safflower. Administration of this extract to mice can significantly increase the concentrations of acetic, butyric, and propionic acids in their intestines. This process is related to the increase in the level of SCFA production.71 Xiexin Tang can increase the production capacity of intestinal microbe-derived SCFA by increasing the mRNA and activity levels of the key SCFA synthesis enzyme acetate kinase.72
Regulation of Intestinal Immunity
Previous studies have shown that the intestine is the largest immune organ of the human body, which is mainly composed of intestinal epithelial cells, intestinal intraepithelial lymphocytes, laminal lymphocytes, mesentery-associated lymph nodes, and other tissues.73 Gut microbiota can promote the development of the intestinal mucosal immune system and maintain the homeostasis of the intestinal environment.74
The intestinal mucosal immune system is mainly composed of three parts. The first involves the antibacterial peptides secreted by intestinal epithelial cells; the second contains the immunoglobulin A secreted by the B lymphocytes, which can prevent bacterial translocation; the third part involves immune cells, such as dendritic cells, B lymphocytes, T lymphocytes, and innate lymphocytes.75 Autopsy analysis of brain tissues indicated that cluster of differentiation 4+ (CD4+) and cluster of differentiation 8+ (CD8+) T cells in the substantia nigra of PD patients exhibited significant lymphocyte infiltration and microglia activation.76 The administration of the T cell signal transduction inhibitor FK506 in the α-syn overexpression rat model can significantly reduce the activation of substantia nigra microglia and the number of CD4+ T cells, thereby increasing the survival of dopaminergic neurons. CD4+ T cells can differentiate into helper T cells (Th1, Th2, and Th17) and regulatory T cell (Treg) subsets. They interact and inhibit each other by secreting cytokines, forming a network that maintains a stable internal environment. Tregs induce apoptosis of effector T cells through cell-to-cell contact and the action of soluble factors. Treg cells can reduce the activation of microglia and increase the survival rate of neurons.77 This function may be achieved by inhibiting Th17. Treg cells can upregulate the expression of certain neurotrophic factors, such as BDNF, while they downregulate the expression of inflammatory factors, which can induce apoptosis of activated microglia.78 This study demonstrated that compared with the healthy control group, the Treg cells of PD patients exhibited a weaker effect in inhibiting the proliferation of effector T cells and the release of cytokines.79 The inhibitory effect of Tregs, which were isolated from PD patients, on effector T cells from healthy allogeneic donors was decreased, indicating that Treg cell dysfunction was involved in the progression of PD. In contrast to these observations, Th17 cells promoted the deterioration of neuroinflammation mediated by IL-1, IL-6, IL-17, TNF-α, and IFN-γ.80 All these data indicate a close association between immune system imbalance and PD. Gut microbiota play a key role in regulating the dynamic balance of intestinal immune cells. Garidou et al provided mice with a high-fat diet, which caused disturbances in the gut microbiota of the ileum, resulting in a decrease in Th1 cells in the lamina propria.81 Previous studies have shown that Treg cells in the intestinal mucosa can be induced by intestinal symbiotic bacteria that differentiate and proliferate. For example, Bacteroides fragilis in the intestine can induce Treg development through polysaccharide A, while Clostridium can promote the proliferation of Treg cells.82,83 Chinese medicine exerts multiple effects on the regulation of the intestinal mucosal immunity. Polysaccharides and Ginsenosides from American ginseng restored the composition of the gut microbiota, and increased the abundance of various beneficial mucosal-related flora, such as Clostridium, Bifidobacterium, while decreasing the abundance of several harmful bacteria, such as Escherichia coli, Shigella, and Ruminococcus to stimulate small intestinal CD4+ T cells and immunoglobulin A secreting cells, and regulate the intestinal immune barrier.84 Grape seed procyanidin extract significantly increased the proportion of Tregs in the mouse intestinal mucosa and decreased the expression levels of TNF-α, IL-1β, and IL-6. Grape seed procyanidin extract treatment increased the abundance of slime mold, suggesting that it may have a regulatory effect on the intestinal immune balance.85 Clematichinenoside AR is a saponin extracted from TCM. It can inhibit the function of IL-10-/- mouse Th17 cells and promote the Treg cell response. The specific mechanism may be related to the inhibition of the PI3K/Akt signaling pathway.86 Activated PI3K and Akt play an important role in the differentiation of Th17 cells87 and the inactivation of the PI3K/Akt signaling pathway is involved in the regulation of the immune balance by inducing T cell differentiation to counteract the activity of Th17 cells.88 Dendrobium polysaccharide stimulates intraepithelial lymphocyte and lamina propria lymphocyte to secrete cytokines. It also increases the content of IL-10 and regulatory T cells, which is important for maintaining intestinal immune homeostasis and the integrity of the mucosal barrier.89 TCM exhibits a significant therapeutic effect on intestinal immune barrier damage. By regulating the gut microbiota and enhancing the defense function of the intestinal immune barrier, TCM can be used for the treatment of PD.
Regulation of Intestinal Endocrine Cells and Products
Enteroendocrine cell (EECs) are derived from intestinal stem cells. Although EECs account for less than 1% of the entire gastrointestinal lumen epithelial cells, their number exceeds the sum of all endocrine cells in other organs of the body.90 EECs secrete more than 20 different types of hormones, and are considered to be the largest and most complex endocrine organ in the body.91 Studies have shown that EECs also have electrical excitability and chemical sensitivity, allowing the production of gastrointestinal hormones or peptides under various stimuli, and releasing them into the blood to play a systemic role.92 Previous studies have shown that a variety of hormones secreted by intestinal endocrine cells are closely related to PD. Glucagon-like peptide-1 (GLP-1) is a polypeptide hormone secreted by small intestinal L cells, which can efficiently pass through the blood-brain barrier and influence neuronal pathways associated with neuroinflammation, thus having a certain neuroprotective effect.93 GLP-1 receptor agonists or analogs activate GLP-1 to stimulate cell proliferation, which can improve dopaminergic neurotransmission, neuron and synaptic function, disease motor symptoms, and exert an optimal neuroprotective effect.94 Ghrelin is mainly produced by enteroendocrine cells and promotes the release of the growth hormone. It also participates in a variety of endocrine regulation processes and exerts anti-apoptotic and anti-oxidant effects. Finally, it exerts neuroprotective effects by antagonizing 1-methyl-4-phenyl-1,2,3,6-tetrahydropyran-induced neurotoxicity.95,96 Leptin (LEP) is mainly secreted by fat cells, whereas a previous study has found that endocrine cells can also secrete it, LEP can significantly improve the neurological function of patients with PD.97,98 Taken together, this evidence indicates that EECs play an important role in the occurrence and development of PD. Previous studies have found that the gut microbiota can regulate the secretion of gastrointestinal hormones, such as GLP-1, Ghrelin, and LEP.99,100 TCM plays an important role in the regulation of ENSs. Ginseng soluble dietary fiber increases the abundance of beneficial bacteria, such as Firmicutes and Bacteroides and regulates the levels of hormones, such as GLP-1, Ghrelin, and cholecystokinin.101 Banana resistant starch can improve the diversity of the gut microbiota and adjust the overall structure of intestinal microbes, by increasing the ratio of Bacteroides/Firmicutes and the relative abundance of Cyanobacterium. It also downregulates the relative abundance of Deferribacteres and Tenericutes. Banana resistant starch can inhibit the proliferation of Turicibacter, Romboutsia, and Oligella. Their abundance and the expression levels of Ghrelin are negatively correlated.102 The Sangzhi alkaloids of the Chinese medicine can regulate the total amount of SCFA-producing bacteria in the intestine, which causes upregulation in the expression of the GLP-1 syngenesis-related gene proglucagon, and promotes the synthesis of GLP-1.103 The Shenqi compound can improve the imbalance of the gut microbiota, increase the proportion of Bacteroides, butyrate-producing bacteria, and other beneficial bacteria, as well as promote the secretion of GLP-1 by L cells.104 In addition, Sorghum resistant starch extracted from sorghum has also been shown to regulate the structure of the gut microbiota and promote the synthesis of LEP.105 Panax notoginseng saponins increase the abundance of Akkermansia muciniphila and Parabacteroides distasonis, which shape the intestinal microbiota of mice and increase the levels of LEP.106 TCM can regulate ENSs, increase the synthesis of gastrointestinal hormones, such as GLP-1, Ghrelin, and LEP, and exert a therapeutic effect on PD by affecting the gut microbiota.
Regulation of the Intestinal Mucosal Barrier
The intestinal mucosal barrier is mainly composed of mechanical, biological, immune, and chemical barriers. The most important interaction in the mechanical barriers involves the tight junctions,107 which are composed of tight junction proteins, including cytoplasmic proteins, such as claudin-1, occludin, zonula occludens-1 (ZO-1), the junction adhesion molecules, and the cytoskeleton. The intestinal mucosal barrier has a barrier and fence function.108 When the expression levels of claudin-1, occludin, and ZO-1 in the intestinal mucosa are reduced, the tight junctions become unstable and the “gap” between cells increases.109 In parallel, the permeabilization of the cells increases, leading to intestinal mucosal barrier damage.110 The increase in intestinal permeability and the translocation of bacteria and inflammatory bacterial products, such as lipopolysaccharide (LPS), may cause inflammation and oxidative stress in the gastrointestinal tract, which may trigger the accumulation of α-synuclein in ENS, the latter promotes neuroinflammation by enhancing the activation of astrocytes and microglia.111 Concomitantly, the destruction of the gastrointestinal barrier will further change the structure of the gut microbiota, which is conducive to the colonization of inflammatory bacteria.112 In Keshavarzian’s study, PD patients indicated increased intestinal mucosal permeability and systemic poisoning of colon endotoxin.17 In patients with PD, the intestinal permeability or “intestinal leakage” increases and the deposition of α-syn in the ENS increases.113 In addition, the accumulation of α-syn in the colonic mucosa of PD patients also leads to impairment of the intestinal barrier function.114 Scutellaria-Coptis can upregulate the expression levels of the tight junction proteins claudin-1, occludin and ZO-1, which significantly improves intestinal epithelial damage and inhibits the excessive transport of lipopolysaccharide to the blood circulation.115 Fucoidan is an effective ingredient in seaweed. It has been shown that following fucoidan treatment, the levels of D-lactic acid, diamine oxidase, and endotoxin are decreased, whereas the expression levels of occludin, claudin-1, and ZO-1 are increased. The main functions of these proteins involve the repair of the intestinal barrier.116 Treatment of Dextran sodium sulfate-induced mice with Huang Lian Jie Du Decoction (HLJDD) significantly increased the expression levels of ZO-1 and occludin in the colon tissues. In addition, HLJDD intervention significantly inhibited the decrease in mucin secretion induced by dextran sodium sulfate. The aforementioned results indicated that HLJDD could protect the intestinal mucosa by increasing the expression of tight junction proteins and the secretion of mucus proteins.117 Salvia miltiorrhiza stems and leaves can also enhance the intestinal barrier function by upregulating the expression levels of the ileum and colon tight junction protein ZO-1, as well as those of occlusion and tight junction donor protein-5.118
Study has shown that continuous inflammation can lead to imbalance in the expression levels of adhesion molecules and can cause damage to the intestinal mucosal barrier.119 LPS is considered to be the trigger of the low-grade inflammatory response, it is the main component of the cell wall of intestinal gram-negative bacteria.120 The increase in the abundance of Enterobacteriaceae in PD can increase the serum LPS concentration.121 LPS can induce the synthesis of TNF-α, IL-1, IL-6, IL-8, and other inflammatory factors and destroy the intestinal mucosal barrier.122 The expression levels of pro-inflammatory cytokines in PD patients were significantly increased.123 Berberine is the main ingredient of Coptis, which can significantly reduce the levels of serum LPS, IL-1β, TNF-α, and IL-6 in mice. This effect may be related to the increase in the expression levels of ZO-1 and occludin in the colonic mucosa and to the increase in the thickness of the mucosa.115 Gastrodia can reduce the levels of LPS in the serum by remodeling of the gut microbiota; they concomitantly reduce the expression levels of serum TNF-α, IL-1β, IL-6, IL-8, and other pro-inflammatory factors.124 Curcumin is an extract of turmeric that exhibits significant inhibitory activity against Enterococcus and Clostridium, by downregulating the expression levels of Toll-like receptor 4 mRNA and protein in the intestine, and inhibiting the release of key inflammatory molecules (IL-1β, TNF-α). Concomitantly, it increases the secretion of immunoglobulins and alleviates intestinal inflammation.125 The protection of the intestinal mucosa by TCM can prevent bacteria and their harmful metabolites from entering the blood, which is of important significance for the prevention and treatment of PD.
Conclusion and Prospects
Gut microbiota have received considerable attention as a new potential target for the treatment of PD. TCM has been reported as a safe and effective strategy for regulating gut microbiota and improving PD in clinical trials. Chinese herbal compounds or single Chinese medicines have been used to treat PD for thousands of years and have achieved optimal results. In recent years, a large number of studies have found that TCM has a regulatory effect on intestinal microbes. This article summarized the relationship between the gut microbiota and the occurrence and development of PD, and the underlying regulatory mechanism. It also explained that TCM could alter the structure of the gut microbiota and their metabolites to regulate intestinal mucosal barrier, inflammation, immunity, and intestinal endocrine activity. By regulating these processes TCM can be used to treat PD. The present study provided a novel methodology for the prevention and treatment of PD by using TCM. At present, a limited number of studies have been reported on the treatment of PD with TCM via the regulation of the gut microbiota. The majority of these research studies focused on the effect of TCM on the gut microbiota and their metabolites. However, the molecular biological mechanism underlying the treatment of PD has been rarely examined. Therefore, additional research can be carried out in future studies on this topic. TCM affects multiple pathways and targets multiple proteins. Its mechanism of action is complex. Therefore, a systems-biology approach can be used in future studies in order to provide additional insight into the complex mechanism of action of TCM with regard to the treatment of PD.
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (No.81573899), The Youth Science Fund Project of National Natural Science Fund of China (No.82104965), Shanghai Biomedical Science Technology Support Project (No.20S21901700) and Shanghai Science and Technology Project (NO.21DZ2271000).
Disclosure
The authors of this study declare that they have no conflict of interest.
References
1. Damier P, Hirsch EC, Agid Y, et al. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122:1437–1448. doi:10.1093/brain/122.8.1437
2. de Rijk MC, Launer LJ, Berger K, et al. Prevalence of Parkinson’s disease in Europe: a collaborative study of population-based cohorts. Neurologic diseases in the elderly research group. Neurology. 2000;54:21–23.
3. Isaacson SH, Hauser RA. Improving symptom control in early Parkinson’s disease. Ther Adv Neurol Disord. 2009;2:393–400. doi:10.1177/1756285609339383
4. Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351:2498–2508.
5. Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362:2077–2091. doi:10.1056/NEJMoa0907083
6. Liu B, Piao X, Niu W, et al. Kuijieyuan decoction improved intestinal barrier injury of ulcerative colitis by affecting TLR4-dependent PI3K/AKT/NF-κB oxidative and inflammatory signaling and gut microbiota. Front Pharmacol. 2020;11:1036. doi:10.3389/fphar.2020.01036
7. Ma ZJ, Wang HJ, Ma XJ, et al. Modulation of gut microbiota and intestinal barrier function during alleviation of antibiotic-associated diarrhea with Rhizoma Zingiber officinale (Ginger) extract. Food Funct. 2020;11(12):10839–10851. doi:10.1039/D0FO01536A
8. Sheng YA, Chao S, Yi J, et al. Herbal medicine WangShiBaoChiWan improves gastrointestinal health in mice via modulation of intestinal tight junctions and gut microbiota and inhibition of inflammation. Biomed Pharmacother. 2021;138:11426.
9. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi:10.1038/nature08821
10. Heinzel S, Aho VTE, Suenkel U, et al. Gut microbiome signatures of risk and prodromal markers of Parkinson disease. Ann Neurol. 2020;88(2):320–331. doi:10.1002/ana.25788
11. Heianza Y, Ma W, Manson JE, et al. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. 2017;6(7):e004947. doi:10.1161/JAHA.116.004947
12. Hasegawa S, Goto S, Tsuji H, et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS One. 2015;11:1–15.
13. Keshavarzian A, Green J, Engen PA, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30:1351–1360. doi:10.1002/mds.26307
14. Qian YW, Yang XD, Xu SQ, et al. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun. 2018;5:194–202. doi:10.1016/j.bbi.2018.02.016
15. Cirstea MS, Yu AC, Golz E, et al. Microbiota composition and metabolism are associated with gut function in Parkinson’s disease. Mov Disorders. 2020;35:1695–1697. doi:10.1002/mds.28208
16. Nishiwaki H, Ito M, Ishida T, et al. Meta-analysis of gut dysbiosis in Parkinson’s disease. Mov Disord. 2020;35:1626–1635. doi:10.1002/mds.28119
17. Wallen ZD, Appah M, Dean MN, et al. Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. NPJ Parkinsons Dis. 2020;6:11. doi:10.1038/s41531-020-0112-6
18. Li X, Yang W, Li X, et al. Age-dependent elevations of oligomeric and phosphorylated alpha-synuclein synchronously occurs in the brain and gastrointestinal tract of cynomolgus monkeys. Neurosci Lett. 2018;662:276–282. doi:10.1016/j.neulet.2017.10.047
19. Braak H, Rüb U, Gai P, et al. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–536. doi:10.1007/s00702-002-0808-2
20. Ahn EH, Kang SS, Liu X, et al. Initiation of Parkinson’s disease from gut to brain by δ-secretase. Cell Res. 2020;30(1):70–87. doi:10.1038/s41422-019-0241-9
21. Phillips RJ, Walter GC, Wilder SL, et al. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson’s disease? Neuroscience. 2008;153:733–750. doi:10.1016/j.neuroscience.2008.02.074
22. Svensson E, Horváth-Puhó E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78:522–529. doi:10.1002/ana.24448
23. Francisco PM, Oleg A, Yanina D, et al. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One. 2010;5:e8762. doi:10.1371/journal.pone.0008762
24. Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi:10.1007/s00401-014-1343-6
25. Qin XY, Zhang SP, Cao C, et al. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 2016;7311:1–9.
26. Sampson TR, Debeliusm JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinsons’s disease. Cell. 2016;167:1469–1480. doi:10.1016/j.cell.2016.11.018
27. Daniele SG, Béraud D, Davenport C, et al. Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders. Sci Signal. 2015;8(376):ra45. doi:10.1126/scisignal.2005965
28. Girotti M, Donegan JJ, Morilak DA. Influence of hypothalamic IL-6/gp130 receptor signaling on the HPA axis response to chronic stress. Psychoneuroendocrinology. 2013;38:1158–1169. doi:10.1016/j.psyneuen.2012.11.004
29. Matsuwaki T, Eskilsson A, Kugelberg U, et al. Interleukin-1β induced activation of the hypothalamus-pituitary-adrenal axis is dependent on interleukin-1 receptors on non-hematopoietic cells. Brain Behav Immun. 2014;40:166–173. doi:10.1016/j.bbi.2014.03.015
30. Galley JD, Nelson MC, Yu ZT, et al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. doi:10.1186/1471-2180-14-189
31. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;4(174):307–311. doi:10.1192/bjp.174.4.307
32. Enomoto S, Shimizu K, Nibuya M, et al. Increased expression of endocytosis-Related proteins in rat hippocampus following 10-day electroconvulsive seizure treatment. Neurosci Lett. 2016;624:85–91. doi:10.1016/j.neulet.2016.05.015
33. Tuon T, Valvassori SS, Dal Pont GC, et al. Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res Bull. 2014;108:106–112. doi:10.1016/j.brainresbull.2014.09.006
34. Ait-Belgnaoui A, Colom A, Braniste V, et al. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol Motil. 2014;26:510–520. doi:10.1111/nmo.12295
35. Feng C, Wang LJ, Li ZZ. The changes of intestinal microbiota affect the expression of brain-derived neurotropic factor in rat hippocampus. Chin J Microecol. 2015;27:10–13.
36. Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi:10.1002/mds.1090
37. Dodel DRC, Berger K, Oertel WH. Health-related quality of life and healthcare utilisation in patients with Parkinson’s disease. Pharmaco Econ. 2001;19:1013–1038. doi:10.2165/00019053-200119100-00004
38. Tiklová K, Gillberg L, Volakakis N, et al. Disease duration influences gene expression in neuromelanin-positive cells from Parkinson’s disease patients. Front Mol Neurosci. 2021;14:763777. doi:10.3389/fnmol.2021.763777
39. Jaunarajs KE, George JA, Bishop C. L-DOPA-induced dysregulation of extrastriatal dopamine and serotonin and affective symptoms in a bilateral rat model of Parkinson’s disease. Neuroscience. 2012;218:243–256. doi:10.1016/j.neuroscience.2012.05.052
40. Michel E, Philippe DD, Li Q, et al. Widespread monoaminergic dysregulation of both motor and non-motor circuits in parkinsonism and dyskinesia. Cerebral Cortex. 2015;25:2783–2792. doi:10.1093/cercor/bhu076
41. Engevik MA, Luck B, Visuthranukul C, et al. Human-derived bifidobacterium dentium modulates the mammalian serotonergic system and gut-brain axis. Cell Mol Gastroenterol Hepatol. 2021;11(1):221–248. doi:10.1016/j.jcmgh.2020.08.002
42. Gough NR. Microbes message gut secretory cells. Sci Signal. 2015;8(373):ec101–ec101.
43. Luo J, Wang T, Shan L, et al. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci China Life Sci. 2014;57:327–335. doi:10.1007/s11427-014-4615-4
44. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;163:256–258. doi:10.1016/j.cell.2015.09.017
45. Boelens Keun JT, Arnoldussen IA, Vriend C, et al. Dietary approaches to improve efficacy and control side effects of levodopa therapy in Parkinson’s disease: a systematic review. Adv Nutr. 2021;12(6):2265–2287. doi:10.1093/advances/nmab060
46. Chen SY, Xiao SJ, Lin YN, et al. Clinical efficacy and transcriptomic analysis of Congrong Shujing granules in patients with Parkinson’s disease and syndrome of Shen (Kidney) essence deficiency. Chin J Integr Med. 2020;26(6):412–419. doi:10.1007/s11655-020-3080-0
47. Li YQ, Wun XJ. Flavonoids from medicinal plants in prevention and treatment of Parkinson disease: advances and prospection on pharmacology. Chin J Pharmacol Toxicol. 2016;30:1125–1135.
48. Blanchet J, Longpré F, Bureau G, et al. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1243–1250. doi:10.1016/j.pnpbp.2008.03.024
49. Mu X, He GR, Yuan X, et al. Baicalein protects the brain against neuron impairments induced by MPTP in C57BL/6 mice. Pharmacol Biochem Behav. 2011;98:286–291. doi:10.1016/j.pbb.2011.01.011
50. Xu CL, Qu R, Jin Z, et al. Neuroprotective effects of madecassoside in early stage of Parkinson’s disease induced by MPTP in rats. Fitoterapia. 2013;90:112–118. doi:10.1016/j.fitote.2013.07.009
51. Zhang S, Shao SY, Song XY, et al. Protective effects of Forsythia suspense extract with antioxidant and anti-inflammatory properties in a model of rotenone induced neurotoxicity. Neurotoxicology. 2016;52:72–83. doi:10.1016/j.neuro.2015.09.009
52. Yang MH. Effects of Bushen Huoxue Granule on motor function in patients with Parkinson’s disease: a multicenter, randomized, double-blind and placebo-controlled trial. J Chin Integr Med. 2010;8:231–237. doi:10.3736/jcim20100306
53. Pan W, Kwak S, Li G, et al. Therapeutic effect of Yang-Xue-Qing-Nao granules on sleep dysfunction in Parkinson’s disease. Chin Med. 2013;8:14. doi:10.1186/1749-8546-8-14
54. Gu C, Shen T, An H, et al. Combined therapy of Di-Huang-Yi-Zhi with Donepezil in patients with Parkinson’s disease dementia. Neurosci Lett. 2015;606:13–17. doi:10.1016/j.neulet.2015.08.019
55. Wang Y, Xie CL, Lu L, et al. Chinese herbal medicine paratherapy for Parkinson’s disease: a meta-analysis of 19 randomized controlled trials. Evid Based Complement Altern Med. 2012;2012:534861.
56. Zhang G, Xiong N, Zhang Z, et al. Effectiveness of traditional Chinese medicine as an adjunct therapy for Parkinson’s disease: a systematic review and meta-analysis. PLoS One. 2015;10:e0118498. doi:10.1371/journal.pone.0118498
57. Derkinderen P, Shannon KM, Brundin P. Gut feelings about smoking and coffee in Parkinson’s disease. Mov Disord. 2014;29:976–979. doi:10.1002/mds.25882
58. Liu JM, Wang FY, Liu SZ, et al. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci. 2017;381:176–181. doi:10.1016/j.jns.2017.08.3235
59. Gresse R, Chau cheyras-Durand F, Garrido JJ, et al. Pathogen challenge and dietary shift alter microbiota composition and activity in a mucin-associated in vitro model of the Piglet Colon (MPigut-IVM) simulating weaning transition. Front Microbiol. 2021;12:703421. doi:10.3389/fmicb.2021.703421
60. Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi:10.1038/nm1511
61. Scheperjans F, Aho V, Pereira PAB, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2014;30:350–358. doi:10.1002/mds.26069
62. Forsyth CB, Shannon KM, Kordower JH, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6:e28032. doi:10.1371/journal.pone.0028032
63. Ding Y, Yan YM, Peng YJ, et al. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int J Biol Macromol. 2018;125:751–760. doi:10.1016/j.ijbiomac.2018.12.081
64. Hu AL, Song S, Li Y, et al. Mercury sulfide-containing Hua-Feng-Dan and 70W (Rannasangpei) protect against LPS plus MPTP-induced neurotoxicity and disturbance of gut microbiota in mice. J Ethnopharmacol. 2020;254:112674. doi:10.1016/j.jep.2020.112674
65. Rey FE, Faith JJ, Bain J, et al. Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem. 2010;285(29):22082–22090. doi:10.1074/jbc.M110.117713
66. Bordin M, D’Atri F, Guillemot L, et al. Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol Cancer Res. 2004;2(12):692–701. doi:10.1158/1541-7786.692.2.12
67. Fu SP, Wang JF, Xue WJ, et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J Neuroinflammation. 2015;12:1–14. doi:10.1186/s12974-014-0230-3
68. Unger MM, Spiegel J, Dillmann KU, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi:10.1016/j.parkreldis.2016.08.019
69. He QL, Han CP, Huang L, et al. Astragaloside IV alleviates mouse slow transit constipation by modulating gut microbiota profile and promoting butyric acid generation. J Cell Mol Med. 2020;24:9349–9361. doi:10.1111/jcmm.15586
70. Wang LL, Guo HH, Huang S, et al. Comprehensive evaluation of SCFA production in the intestinal bacteria regulated by berberine using gas-chromatography combined with polymerase chain reaction. J Chromatogr B. 2017;1057:70–80. doi:10.1016/j.jchromb.2017.05.004
71. Liu J, Yue SJ, Yang ZR, et al. Oral hydroxysafflor yellow A reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharmacol Res. 2018;134:40–50. doi:10.1016/j.phrs.2018.05.012
72. Xiao SW, Zhang ZM, Chen MJ, et al. Xiexin Tang ameliorates dyslipidemia in high-fat diet-induced obese rats via elevating gut microbiota-derived short chain fatty acids production and adjusting energy metabolism. J Ethnopharmacol. 2019;241:112032. doi:10.1016/j.jep.2019.112032
73. Mu C, Yang Y, Luo Z, et al. The colonic microbiome and epithelial transcriptome are altered in rats fed a high-protein diet compared with a normal-protein diet. J Nutr. 2016;146(3):474–483. doi:10.3945/jn.115.223990
74. Nagashima K, Sawa S, Nitta T, et al. Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat Immunol. 2017;18:675–682. doi:10.1038/ni.3732
75. Burcelin R. Gut microbiota and immune crosstalk in metabolic disease. Mol Metabol. 2016;5:771–781. doi:10.1016/j.molmet.2016.05.016
76. Chen YH, Qi BQ, Xu WF, et al. Clinical correlation of peripheral CD4+‑cell sub‑sets, their imbalance and Parkinson’s disease. Mol Med Rep. 2015;12:6105–6111. doi:10.3892/mmr.2015.4136
77. Reynolds AD, Stone DK, Mosley RL, et al. Proteomic studies of nitrated alpha-synuclein microglia regulation by CD4+CD25+ T cells. J Proteome Res. 2009;8:3497–3511. doi:10.1021/pr9001614
78. Liu J, Gong N, Huang X, et al. Neuromodulatory activities of CD4 + CD25 + regulatory T cells in a murine model of HIV-1-associated neurodegeneration. J Immunol. 2009;182(6):3855–3865. doi:10.4049/jimmunol.0803330
79. Liu Z, Huang Y, Cao BB, et al. Th17 cells induce dopaminergic neuronal death via LFA-1/ICAM-1 interaction in a mouse model of Parkinson’s disease. Mol Neurobiol. 2017;54:7762–7776. doi:10.1007/s12035-016-0249-9
80. Saunders JAH, Estes KA, Kosloski LM, et al. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J Neuroimmune Pharmacol. 2012;7:927–938. doi:10.1007/s11481-012-9402-z
81. Garidou L, Pomié C, Klopp P, et al. The gut microbiota regulates intestinal CD4 T cells expressing RORγt and controls metabolic disease. Cell Metab. 2015;22:100–112. doi:10.1016/j.cmet.2015.06.001
82. Chudnovskiy A, Mortha A, Kana V, et al. Host-protozoan interactions protect from mucosal infections through activation of the inflammasome. Cell. 2016;167(2):444–456. doi:10.1016/j.cell.2016.08.076
83. Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi:10.1126/science.1198469
84. Zhou RR, He D, Xie J, et al. The synergistic effects of polysaccharides and ginsenosides from American Ginseng (L.) ameliorating cyclophosphamide-induced intestinal immune disorders and gut barrier dysfunctions based on microbiome-metabolomics analysis. Front Immunol. 2021;12:665901. doi:10.3389/fimmu.2021.665901
85. González-Quilen C, Grau-Bové C, Jorba-Martín R, et al. Protective properties of grape-seed proanthocyanidins in human ex vivo acute colonic dysfunction induced by dextran sodium sulfate. Eur J Nutr. 2021;60:79–88. doi:10.1007/s00394-020-02222-3
86. Song X, Li J, Wang Y, et al. Clematichinenoside AR ameliorated spontaneous colitis in Il-10 mice associated with improving the intestinal barrier function and abnormal immune responses. Life Sci. 2019;239:117021. doi:10.1016/j.lfs.2019.117021
87. Yin N, Wang Y, Lu X, et al. hPMSC transplantation restoring ovarian function in premature ovarian failure mice is associated with change of Th17/Tc17 and Th17/Treg cell ratios through the PI3K/Akt signal pathway. Stem Cell Res Ther. 2018;9:37. doi:10.1186/s13287-018-0772-x
88. Zheng Q, Diao S, Wang Q, et al. IL-17A promotes cell migration and invasion of glioblastoma cells via activation of PI3K/AKT signalling pathway. J Cell Mol Med. 2019;23:357–369. doi:10.1111/jcmm.13938
89. Xie SZ, Shang ZZ, Li QM, et al. Dendrobium huoshanense polysaccharide regulates intestinal lamina propria immune response by stimulation of intestinal epithelial cells via toll-like receptor 4. Carbohydr Polym. 2019;222:115028. doi:10.1016/j.carbpol.2019.115028
90. Sjolund K, Sanden G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. doi:10.1016/S0016-5085(83)80080-8
91. Ahlman H, Nilsson O. The gut as the largest endocrine organ in the body. Ann Oncol. 2001;12:S63–S68. doi:10.1093/annonc/12.suppl_2.S63
92. Bohórquez DV, Samsa LA, Andrew R, et al. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS One. 2014;9:e89881. doi:10.1371/journal.pone.0089881
93. Parthsarathy V, Hölscher C. The type 2 diabetes drug liraglutide reduces chronic inflammation induced by irradiation in the mouse brain. Eur J Pharmacol. 2013;700:42–50. doi:10.1016/j.ejphar.2012.12.012
94. Bułdak Ł, Machnik G, Skudrzyk E, Bołdys A, Okopień B. The impact of exenatide (a GLP‑1 agonist) on markers of inflammation and oxidative stress in normal human astrocytes subjected to various glycemic conditions. Exp Ther Med. 2019;17:2861–2869.
95. Andrews ZB, Erion D, Beiler R, et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci. 2009;29:14057. doi:10.1523/JNEUROSCI.3890-09.2009
96. Wang H, Dou S, Zhu J, et al. Ghrelin protects dopaminergic neurons against MPTP neurotoxicity through promoting autophagy and inhibiting endoplasmic reticulum mediated apoptosis. Brain Res. 2020;1746:147023. doi:10.1016/j.brainres.2020.147023
97. Nakamura T, Suzuki M, Okada A, et al. Association of leptin with orthostatic blood pressure changes in Parkinson’s disease. Mov Disorders. 2016;31(9):1–5. doi:10.1002/mds.26678
98. Cammisotto PG, Renaud C, Gingras D, Delvin E, Levy E, Bendayan M. Endocrine and exocrine secretion of leptin by the gastric mucosa. J Histochem Cytochem. 2005;53:851–860. doi:10.1369/jhc.5A6620.2005
99. Arora T, Akrami R, Pais R, et al. OPEN microbial regulation of the L cell transcriptome. Sci Rep. 2018;8. doi:10.1038/s41598-017-18079-2
100. Frye RE, Rose S, Slattery J, et al. Gastrointestinal dysfunction in autism spectrum disorder: the role of the mitochondria and the enteric microbiome. Microb Ecol Health Dis. 2015;26:27458. doi:10.3402/mehd.v26.27458
101. Hua M, Fan M, Li Z, et al. Ginseng soluble dietary fiber can regulate the intestinal flora structure, promote colon health, affect appetite and glucolipid metabolism in rats. J Funct Foods. 2021;83:104534. doi:10.1016/j.jff.2021.104534
102. Fu J, Wang Y, Tan S, et al. Effects of banana resistant starch on the biochemical indexes and intestinal flora of obese rats induced by a high-fat diet and their correlation analysis. Front Bioeng Biotechnol. 2021;9:575724. doi:10.3389/fbioe.2021.575724
103. Liu SN, Liu Q, Li CN, et al. Innovative hypoglycemic traditional Chinese medicine Sangzhi alkaloids regulating intestinal-pancreatic islet axis and its mechanism. Chin J Pharmacol Toxicol. 2019;33:36–37.
104. Zhang XY, Chao J, Wang HT, et al. Effect of Shenqi compound on intestinal microflora and blood glucose fluctuations in diabetic GK rats. Chin Archiv Trad Chin Med. 2019;33:36–37.
105. Shen RL, Zhang WL, Dong JL, et al. Sorghum resistant starch reduces adiposity in high-fat diet-induced overweight and obese rats via mechanisms involving adipokines and intestinal flora. Food Agric Immunol. 2015;26:120–130. doi:10.1080/09540105.2013.876976
106. Xu Y, Wang N, Tan HY, et al. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesit. Theranostics. 2020;10:11302–11323. doi:10.7150/thno.47746
107. Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659.
108. Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol. 2014;36:166–176. doi:10.1016/j.semcdb.2014.09.002
109. Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018;10(1):a029314–a029330. doi:10.1101/cshperspect.a029314
110. Matter K, Balda MS. SnapShot: epithelial Tight Junctions. Cell. 2014;157:992. doi:10.1016/j.cell.2014.04.027
111. Chelpin MVE, Thomas VJ. Targets and mechanisms in prevention of Parkinson’s disease through immunomodulatory treatments. Scand J Immunol. 2017;85:321–330. doi:10.1111/sji.12542
112. Stecher B, Robbiani R, Walker AW, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5(10):2177–2189. doi:10.1371/journal.pbio.0050244
113. Kelly LP, Carvey PM, Keshavarzian A, et al. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disorders. 2013;29:999–1009. doi:10.1002/mds.25736
114. Clairembault T, Leclair-Visonneau L, Coron E, et al. Structural alterations of the intestinal epithelial barrier in Park s disease. Inson Acta Neuropathol Commun. 2015;3:12. doi:10.1186/s40478-015-0196-0
115. Zhang BX, Yue RS, Chen Y, et al. The herbal medicine Scutellaria-coptis alleviates intestinal mucosal barrier damage in diabetic rats by inhibiting inflammation and modulating the gut microbiota. Evid Based Complement Altern Med. 2020;2020:4568629. doi:10.1155/2020/4568629
116. Sun T, Liang H, Xue M, et al. Protective effect and mechanism of fucoidan on intestinal mucosal barrier function in NOD mice. Food Agric Immunol. 2020;31:922–936. doi:10.1080/09540105.2020.1789071
117. Yuan ZW, Yang LH, Zhang XS, et al. Huang-Lian-Jie-Du decoction ameliorates acute ulcerative colitis in mice via regulating NF-κB and Nrf2 signaling pathways and enhancing intestinal barrier function. Front Pharmacol. 2019;10:1354. doi:10.3389/fphar.2019.01354
118. Gu JF, Su SL, Guo JM, et al. The aerial parts of Salvia miltiorrhiza Bge. strengthen intestinal barrier and modulate gut microbiota imbalance in streptozocin-induced diabetic mice. J Funct Foods. 2017;36:362–374. doi:10.1016/j.jff.2017.06.010
119. Tuganbaev T, Mor U, Bashiardes S, et al. Diet diurnally regulates small intestinal microbiome-epithelial-immune homeostasis and enteritis. Cell. 2020;182(6):1441–1459.e21. doi:10.1016/j.cell.2020.08.027
120. Wu H, Xie S, Miao J, et al. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes. 2020;11(4):997–1014. doi:10.1080/19490976.2020.1734423
121. Longhena F, Faustini G, Spillantini M, et al. Living in promiscuity: the multiple partners of alpha-synuclein at the synapse in physiology and pathology. Int J Mol Sci. 2019;20:141. doi:10.3390/ijms20010141
122. Zhang X, Wu D, Liang J, et al. Effects of Salvia miltiorrhizae on ICAM-1, TLR4, NF-kappa B and Bax proteins expression in multiple organs of rats with severe acute pancreatitis or obstructive jaundice. Inflammation. 2009;32:218–232. doi:10.1007/s10753-009-9124-4
123. Chen X, Hu Y, Cao Z, et al. Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Front Immunol. 2018;9:2122. doi:10.3389/fimmu.2018.02122
124. Fyl A, Jing WA, Jh A, et al. Gastrodia remodels intestinal microflora to suppress inflammation in mice with early atherosclerosis. Int Immunopharmacol. 2021;96:107758. doi:10.1016/j.intimp.2021.107758
125. Gan Z, Wei W, Li Y, et al. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Molecules. 2019;24:1220. doi:10.3390/molecules24071220
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.