Back to Journals » Cancer Management and Research » Volume 10
The role of external beam radiotherapy for hepatocellular carcinoma patients with lymph node metastasis: a meta-analysis of observational studies
Authors Rim CH , Kim CY, Yang DS, Yoon WS
Received 29 May 2018
Accepted for publication 30 June 2018
Published 6 September 2018 Volume 2018:10 Pages 3305—3315
DOI https://doi.org/10.2147/CMAR.S175703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Chai Hong Rim,1 Chul Yong Kim,2 Dae Sik Yang,3 Won Sup Yoon1
1Department of Radiation Oncology, Ansan Hospital, Korea University Medical College, Ansan, Gyeonggi-do, Republic of Korea; 2Department of Radiation Oncology, Anam Hospital, Korea University Medical College, Seoul, Republic of Korea; 3Department of Radiation Oncology, Guro Hospital, Korea University Medical College, Seoul, Republic of Korea
Purpose: Lymph node metastasis of hepatocellular carcinoma is categorized as advanced in Barcelona Clinic of Liver Cancer staging, and sorafenib is a sole treatment recommended. However, appliance of local treatment including external beam radiotherapy (EBRT) has not been uncommon. We performed a meta-analysis and systemically reviewed current literature to evaluate the efficacy and safety of EBRT.
Methods: PubMed, Medline, Cochrane library, and Embase were systemically searched until December 17, 2017. The primary endpoint of analyses was response rate (RR), and 1-year overall survival and complication rates of grade ≥3 were secondary endpoints. Complications were primarily assessed descriptively.
Results: A total of 8 studies comprising 521 patients were included. The pooled RR was 73.1% (95% confidence interval [CI]: 63.6–80.9), and high-dose EBRT groups had better RR than the low-dose group (82.2% [95% CI: 74.4–88.1] vs 51.1% [95% CI: 40.3–61.7]; P=0.001]. The pooled 1-year overall survival rate was 41.0% (95% CI: 32.9–49.6). Six studies assessed the survival benefit according to RR, and 5 (83.3%) of these 6 studies reported statistically significant survival benefit. The most common grade ≥3 toxicities were thrombocytopenia and gastrointestinal complication, with pooled rates of 3.4% (95% CI: 1.2–9.5) and 3.5% (95% CI:1.7–7.2), respectively.
Conclusion: EBRT showed a pooled RR of 73.1% and was safely performed. EBRT might palliate symptoms through tumor reductions and improve survival. Use of sorafenib combined or sequentially with EBRT can be recommended rather than monotherapy.
Keywords: hepatocellular carcinoma, lymph node metastasis, meta-analysis, radiation therapy
Introduction
Lymph node metastasis (LNM) of hepatocellular carcinoma (HCC) is a rare condition of the disease, with an incidence of 5.1%–7.5% in large surgical series.1,2 LNM of HCC has poor prognosis, and no effective standard therapeutic modality has been established. Although surgery, including lymphadenectomy, might be performed in selective patients,1,3,4 the majority of patients are not suitable surgical candidates owing to uncontrolled primary cancer, poor liver function, and concurrent distant metastasis.5 Barcelona Clinic of Liver Cancer (BCLC) guidelines categorized LNM into advanced stage with portal invasion and distant metastasis, recommending sorafenib.6 However, in its landmark randomized trials, the tumor response rate (RR) was only 3%–5%, and the survival benefit was modest and insignificant in the subgroup of extrahepatic metastasis.7,8
Although external beam radiation therapy (EBRT) was known to be ineffective for HCC due to poor tolerance of whole liver, the application has been emerged with availability of local tumor irradiation while sparing non-tumorous liver.9 EBRT is also an effective local treatment option for HCC with LNM, where EBRT provides a noninvasive methodology that achieves high tumor RRs. Several researchers have reported their clinical experiences in the case series.10–17 The purpose of this meta-analysis and systemic review was to evaluate the efficacy and safety of EBRT for LNM of HCC, and discuss the optimal treatment strategy in consideration of other treatment modalities.
Materials and methods
Study protocol
Our study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. We systemically searched the studies from PubMed, Embase, Medline, and Cochrane library published until December 24, 2017. The search term was designed to find all researches related to EBRT for HCC with LNM: (HCC or “hepatocellular carcinoma”) and (“lymph node” or LN) and (metastasis or metastases) and (radiotherapy or EBRT or RT or “radiation therapy”). We included only the articles written in English and excluded the unpublished studies. The reference list of relevant articles was also searched.
Selection criteria
Our inclusion criteria for the meta-analysis were as follows: 1) clinical trials of HCC patients with LNM treated with EBRT, 2) inclusion of ≥5 HCC patients with LNM, and 3) provision of at least 1 main outcome (LN RR or 1-year overall survival [OS] rate). Reviews, conference abstracts, editorials, letters, case reports, and in vitro or animal studies were excluded.
Duplicated studies among the databases or researches other than clinical trials were filtered at the first screening using titles and citations. At the second screening, abstracts, studies with irrelevant subjects and <5 HCC patients with LNM, and some remaining reviews and case reports that were not filtered in the first screening were excluded. The final screening with full-text review was performed to include only the studies that fully met the inclusion criteria. For multiple studies published in a single institution, the following criteria were used for selection prioritized in numerical order: 1) included HCC patients with LNM only rather than all HCC patients, 2) the study with the largest number of patients, and 3) the most recently published study. All the screening processes were performed by 2 independent researchers, and disagreements during the process and decision of final inclusion were agreed upon discussion. The study inclusion process is described in Figure 1.
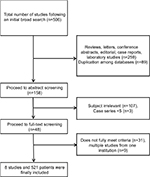  | Figure 1 The study inclusion process. |
Data extraction
Data extraction was performed by 2 independent researchers using a standardized form. Disagreement was resolved through discussion and mutual consent. The data obtained included 1) general information such as authors, country, and year of publication; 2) clinical information such as the number of patients, sex, rate of Child–Pugh Classification A, age, control of primary HCC, chemotherapy, tumor response, and toxicity criteria; 3) EBRT profiles such as modality, radiation dose, clinical target of EBRT; 4) and clinical outcomes such as median OS (overall and tumor responders vs nonresponders), 1-year OS rate, 2-year OS rate, tumor RR (overall and low-dose vs high-dose EBRT), and grade ≥3 toxicity. RR should be evaluated with tumor size criteria, such as the World Health Organization criteria or Response Evaluation Criteria in Solid Tumors,18 not with symptomatic responses. If the study included both the HCC patients with and without LNM, we only used the clinical information of HCC patients with LNM.
Quality assessment
Because most of the studies were retrospective in nature, we used the Newcastle–Ottawa Scale (NOS)19 to assess the quality of included studies. The studies rated from 7 to 9 points on the NOS were regarded as high quality, and those rated from 4 to 6 points were defined as moderate quality.
Statistical analysis
The primary endpoint was tumor RR and was defined as the sum of complete remission and partial RRs. The OS and grade ≥3 toxicities were the secondary endpoints. Toxicities were primarily assessed descriptively. We calculated the pooled rates of RR, OS, and grade ≥3 toxicities using random-effects model20 because the included studies were conducted in different institutions and the patient groups were heterogeneous. The Cochran Q21 test and I2 statistics were used to evaluate the heterogeneity among included studies. If the P-value was <0.1 and the I2 value was ≥50%, the heterogeneity was considered as significant. The visual inspection of funnel plot and quantitative analysis of Egger’s test of intercept22 were used to evaluate publication bias. Publication bias was considered to be present if the 2-tailed P-value at Egger’s test was <0.1. The trimmed result using Duval and Tweedie’s method23 was presented at the results. To compare the RRs according to the EBRT dose (high vs low), Q-test based on an analysis of the variance and random-effects model was used. All statistical analyses were performed using Comprehensive Meta-Analysis software version 3 (Biostat Inc., Englewood, NJ, USA).
Results
Study characteristics
An initial broad search found 506 studies. After excluding reviews, letters, conference abstracts, editorial, case reports, laboratory studies, and duplicated studies, 158 studies were screened for their abstracts. Of these, studies with irrelevant subject or those that included HCC patients with LNM of <5 were excluded. The full text of the remaining 48 studies was reviewed, and we excluded those that did not fully meet the inclusion criteria. Multiple studies conducted in a single institution were selected according to the criteria detailed in the selection criteria section. Finally, 8 studies comprising 521 HCC patients with LNM were included in the meta-analysis.10–17 The inclusion process is described in Figure 1.
All 8 studies were retrospectively designed and had scores ranging from 5 to 6, corresponding with moderate quality according to the NOS scale. Six of the 8 studies provided the rates of Child–Pugh Classification A patients, which ranged from 57.1% to 94.2%. The rate of distant metastasis, controlled primary lesion, and intra-abdominal LN were available in 5, 6, and 7 of the 8 studies, respectively. Since LNM of HCC is a rare disease and its prognosis are known to be as poor as systemic metastasis,24 most studies did not exclude the patients with distant metastases or extra-abdominal LNM. The data about use of concomitant or post-EBRT chemotherapy were available in 2 studies. In one study, sorafenib and capecitabine plus cisplatin were used. In another study, transarterial infusion of epirubicin and cisplatin and systemic infusion of 5-FU were used. The characteristics of the included studies are summarized in Table 1.
Treatment profiles and clinical outcomes
Regarding EBRT modalities, 4 of the 8 studies (50%) used 3-dimensional conformal radiotherapy (3DCRT), 2 studies used 3DCRT and intensity-modulated radiotherapy (IMRT), 1 study used IMRT, and 1 study used 3DCRT and 2-dimensional radiotherapy. The information of EBRT target was available in 7 of 8 studies. The clinical target of EBRT was LNM in 6 of the 8 studies (75%), while 1 study targeted LNM and the elective nodal station. The median prescribed radiation dose, which was calculated to biologically equivalent dose (BED) using an α/β ratio of 10, ranged from 50 to 75 Gy10 with the median value of 60 Gy10. One study, which had the highest BED, used a hypofractionated scheme of 10 fractions of 5 Gy, while the other 7 studies used the conventional scheme of >10 fractions with 1.8–4 Gy per fraction.
RR was available in all 8 studies, and the median was 77.6% (range: 56.7–86.7). The pooled RR was 73.1% (95% confidence interval [CI]: 63.6–80.9). Significant heterogeneity was found among the studies (P<0.001, I2=75.2%). Six of the 8 studies reported the RR according to high-dose vs low-dose groups. The borderline dose between low- and high- dose groups ranged from 50 to 60 Gy10 in BED. The pooled RR of the high-dose groups was 82.2% (95% CI: 74.4–88.1), while that of the low-dose groups was 51.1% (95% CI: 40.3–61.7). The pooled RR of the high-dose and low-dose groups was significantly different (P<0.001). Significant heterogeneities were observed in studies in terms of overall RR (P<0.001, I2=75.2) and in the high-dose group (P=0.035, I2=58.2%), but not in the low-dose group (P=0.362, I2=8.5%). As dose comparisons were used as variables for survival or tumor control rates assessment from included studies (rather than as subgroups), further analysis was not performed although heterogeneity was observed in the high-dose group.
The 1-year OS rate was available in 5 studies, and the pooled rate was 41.0% (95% CI: 32.9–49.6). The 2-year OS rate was available in 5 studies, and the pooled rate was 19.9% (95% CI: 16.1–24.3). The heterogeneities among studies were not significant in 1-year OS (P=0.129, I2=43.9%) and 2-year OS rates (P=0.566, I2=0%). The median OS was reported in 7 of the 8 studies, with median value of 8 months (range: 5.8–19). Six studies compared the survival rates between tumor responders and nonresponders, and tumor responders had favorable survival in 5 of these 6 (83.3%) studies.
The treatment profiles and clinical outcomes are summarized in Table 2, and the pooled rates with relevant statistics are shown in Table 3. Forest plots of the 1-year OS, RR, and RR of the high- and low-dose groups are shown in Figure 2.
  | Table 3 Pooled rates of main clinical outcomes Abbreviations: CI, confidence interval; OS, overall survival; RR, response rate. |
  | Figure 2 (A) Forest plots of the RRs and 1-year OS rates. The P-values from the Cochran Q test21 and I2 statistics are described below the figures. Significant heterogeneity among the studies was found in regard to RRs, but not in 1-year OS rates. (B) Forest plots of RRs comparing high- and low-dose groups. The P-value was derived from a Q-test, based on an analysis of the variance and a random-effects model, where P (total between) <0.001 suggests a significant difference of RRs between high-dose and low-dose subgroups. Abbreviations: OS, overall survival; RR, response rate. |
Toxicities
Treatment-related toxicities were available in all included studies, except in 1 study in which the toxicity of both patients with and without LNM was reported. Treatment toxicities were evaluated using the Radiation Therapy Oncology Group criteria and the Common Terminology Criteria for Adverse Event in 4 and 3 studies, respectively. The most commonly reported grade ≥3 toxicities were thrombocytopenia and gastrointestinal (GI) bleeding or ulcer. Two of the 7 studies (28.6%) reported grade 3 thrombocytopenia at a rate of 9.9% and 14.3%. Grade ≥3 GI bleeding or ulcer was reported in 4 studies, at a rate of 1.5%, 2.9%, 8.9%, and 5.9%. One study reported that grade ≥3 toxicity did not occur. Overall, the number of grade ≥3 toxicities was 23 cases (4.5%) of thrombocytopenia and 11 cases (2.2%) of GI toxicity. The toxicities of the included studies are summarized in Table 2.
Pooled analyses were performed for the 2 most common grade ≥3 complications. The pooled rate of grade ≥3 thrombocytopenia was 3.4% (95% CI: 1.2%–9.5%), and grade ≥3 GI toxicity was 3.5% (95% CI: 1.7%–7.2%). Significant heterogeneities were not shown for GI toxicity (P=0.196, I2=30.5%) but shown for thrombocytopenia (P=0.023, I2=59.0%). Further analysis was not performed despite the heterogeneity because it might be explained with that thrombocytopenia of grade ≥3 was reported in only 2 of 7 available studies (9.9% and 14.3%) but not in other studies. Forest plots of pooled analyses are shown in Figure 3.
  | Figure 3 (A) Forest plot of GI toxicities of grade 3 or higher, (B) forest plot of thrombocytopenia of grade 3 or higher. Abbreviations: CI, confidence interval; GI, gastrointestinal. |
Publication bias
Pooled analyses of overall RR, high- and low-dose group RR, 1-year OS rate, and 2-year OS rate did not have publication bias according to Egger’s test and visual inspection of the funnel plot. Funnel plots assessing publication biases are demonstrated in Figure 4. Publication bias assessments were not performed for complications, as too few studies (4 for GI toxicities and 2 for thrombocytopenia) reported relevant rates and most of the rates were lower than 10%; biases might not be detected via funnel plot-based analyses.25
  | Figure 4 Funnel plots assessing RRs and OS rates. Abbreviations: OS, overall survival; RR, response rate. |
Discussion
LNM of HCC is a rare clinical condition with poor prognosis, and its standard treatment has yet to be established. Surgical treatment including lymphadenectomy can be an option for local treatment,1,3,4,26 and systemic treatment with sorafenib is a sole recommended option in the BCLC guidelines.6
Surgery including lymphadenectomy in HCC patients with LNM has been controversial. Of the series that reported surgical experience, Sun et al1 performed surgery on 49 HCC patients with LNM, and lymphadenectomy was performed in 26 patients. The lymphadenectomy group showed similar survival rates (P=0.944) as the patients who did not undergo lymphadenectomy, and operation time was prolonged for approximately 1 hour (P=0.033). Considering these findings, the authors did not recommend routine lymphadenectomy and endorsed the use of radiotherapy, in reference to a study by Zeng et al27 that showed effective treatment response (the study by Chen et al11 was included in our meta-analysis, and the 2 studies were both performed at the same institution.)
On the other hand, recently published surgical studies have investigated the long-term survival of patients after surgery and claimed the need for lymphadenectomy. Awazu et al3 performed surgery with lymphadenectomy on 15 patients and achieved a 1-year survival rate of 76.9% and a 2-year survival rate of 52.7%. Kobayashi et al4 also reported favorable results, with a 1-year survival rate of 85%, and a 2-year survival rate of 42% for 18 patients. Kobayashi et al4 also stated that the median survival time of EBRT studies has only been 7–9 months and that the rate of GI bleeding was fairly high, at 9%–22%, which mitigated the utility of EBRT.
However, it should be noted that the inclusion criteria of the patients in the above 2 studies were very strict. Both studies included cases with controlled primary liver cancer, adequate liver function and performance status for surgery, no extrahepatic metastasis other than LNM, and isolated LNM. For the EBRT studies that were included in our meta-analysis, most studies included significant proportions of patients with uncontrolled primary HCCs, extrahepatic LNM, and distant metastases other than LNM.10,11,13–15,17 Furthermore, patients with portal vein thrombosis were not excluded in most of the studies,10,12,13 and the rate of grade ≥3 or higher GI bleeding was <10% in all of the studies that reported adverse events. The GI bleeding rate of 9%–22% might be the number encompassed grade ≤2 GI bleeding, which could be adequately controlled. Taken together, it might be unreasonable to compare the surgical and EBRT series directly regarding survival.
Sorafenib is the only systemic agent that has demonstrated survival benefit in well-designed randomized controlled trials. However, in its landmark trials the survival benefits of subgroups with extrahepatic metastases were not significant.7,8 In addition, the tumor RRs of patients who were treated with sorafenib was as low as 2%–3.5%. LNM of HCC can present with severe symptoms, such as obstructive jaundice, pyloric obstruction, and inferior vena cava obstruction.27 The symptom improvement is difficult to be expected with use of sorafenib because tumor response rate is less than moderate. In addition, sorafenib treatment has complications such as hand–foot syndrome, which can be distressing for patients with HCCs. Treatment with sorafenib alone, as the BCLC guidelines recommended, should be carefully determined for patients with extrahepatic metastases, where improved survival rates have not been apparent. We recommend sorafenib in combination with local treatments, such as lymphadenectomy or EBRT, which might palliate the local LNM symptoms and improve survival.
In our meta-analysis, the overall pooled RR was 73.1% (95% CI: 63.6–80.9), and the high-dose group showed an excellent pooled RR of 82.2% (95% CI: 74.4%–88.1%). Because 5 of the 6 (83.3%) available studies11,13–15,17 reported a survival benefit of EBRT for responders, EBRT with higher dose than 55-60Gy2 in BED (equivalent to 46–50 Gy in conventional 2 Gy per fraction) is recommended when possible. Wee et al15 reported that the symptoms related with LNM nearly triples the risk of death; the high RR of LNM after EBRT might be expected to relieve the symptom through tumor reductions and prolong survival. Radiosensitive organs, such as the duodenum, can be included in the radiation field when performing EBRT for LNMs. Therefore, in order to perform high-dose EBRT, techniques that increase treatment precision, such as respiratory gating, cone-beam or megavoltage CT, and real-time tumor tracking, are essential.28–30 The presence of GI ulcer history increases the likelihood of severe GI toxicity and should be endoscopically evaluated prior to treatment.17,31 In our meta-analysis, the overall grade ≥3 complication rates were as low as 3.4% and 3.5% for thrombocytopenia and GI toxicity, respectively, but this result should be interpreted with caution because all of the included studies were retrospective.
Recently, our team and the Korean Liver Cancer Study Group analyzed the Korean nationwide cohort from 2008–2012 including 1,015 BCLC C patients, to find the subpopulation who can be benefited with local treatment including EBRT. Regarding patients with LNM, initial treatment including EBRT showed a trend to favorable survival than sorafenib (1-year OS rates: 16.3% vs 6.4%, P=0.079).32 The statistical analysis might be underpowered due to the small numbers of patients with LNM, and additional data from 2013 to 2014 are being added and analyzed. Upon completion of this analysis, the results will be helpful in identifying the role of EBRT for the HCC patients with LNM, as well as with the result of the present study.
In summary, EBRT is a local treatment option with a high RR, which provides possible symptom relief through tumor reduction and prolongs survival for treating HCC patients with LNM. EBRT has the additional benefit of being noninvasive and can be performed in an outpatient setting. Surgery, including lymphadenectomy, might provide long-term survival in selective cases. Hence, surgical treatment should be attempted after a multidisciplinary discussion about the general condition and disease status of the patient, when therapeutic benefit from resection can be expected. Local treatment such as EBRT and surgery might be considered if the patients’ condition and disease status allow, and sorafenib should be used as a final systemic option if the disease progresses.
The following limitations should be considered when interpreting this study. The use of meta-analysis on observational studies is controversial.33 The heterogeneity among study designs and patient populations might affect the estimation of pooled analysis.34 Although randomized controlled studies provide the most robust evidence, not all clinical cases can be supported by the best evidence in oncology, and treatment decisions can be made based on small studies, clinical observations, and clinical experiences.35 In particular, designing randomized controlled trials for rare diseases with poor expected prognosis, such as LNM of HCC, is difficult. Therefore, a meta-analysis of observational studies may be one of the best ways to assess the efficacy and safety of a particular treatment modality.34 Additionally, the relatively small numbers of studies and patients included in the present meta-analysis can also be a drawback. Owing to rarity of incidence, most of the included studies encompassed every HCC patient with LNM, regardless of whether the patient was having distant metastases or extrahepatic LNMs. Although LNM of HCC is a rare disease and its prognosis is known to be as poor as systemic metastasis,24 clinical heterogeneity might be present; that is why we set the RR as a primary endpoint rather than survival rate.
Conclusion
EBRT confers a favorable tumor response, might relieve the symptoms of LNM, and is expected to prolong survival. High-dose EBRT is associated with higher tumor RRs, and technical considerations are necessary to reduce possible complications. Surgery can be considered for selective candidates. We recommend, through the current study, to use sorafenib combined or sequentially with local treatments rather than monotherapy.
Acknowledgment
This study was supported by a Korea University Ansan Hospital grant (O1801171).
Disclosure
The authors report no conflicts of interest in this work.
References
Sun HC, Zhuang PY, Qin LX, et al. Incidence and prognostic values of lymph node metastasis in operable hepatocellular carcinoma and evaluation of routine complete lymphadenectomy. J Surg Oncol. 2007;96(1):37–45. | ||
Ercolani G, Grazi GL, Ravaioli M, et al. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg. 2004;239(2):202–209. | ||
Awazu M, Fukumoto T, Takebe A, et al. Lymphadenectomy combined with locoregional treatment for multiple advanced hepatocellular carcinoma with lymph node metastases. Kobe J Med Sci. 2013;59(1):E17–E27. | ||
Kobayashi S, Takahashi S, Kato Y, et al. Surgical treatment of lymph node metastases from hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2011;18(4):559–566. | ||
Katyal S, Oliver JH, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216(3):698–703. | ||
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. | ||
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. | ||
Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. | ||
Rim CH, Seong J. Application of radiotherapy for hepatocellular carcinoma in current clinical practice guidelines. Radiat Oncol J. 2016;34(3):160–167. | ||
Toya R, Murakami R, Yasunaga T, et al. Radiation therapy for lymph node metastases from hepatocellular carcinoma. Hepatogastroenterology. 2009;56(90):476–480. | ||
Chen YX, Zeng ZC, Fan J, et al. Defining prognostic factors of survival after external beam radiotherapy treatment of hepatocellular carcinoma with lymph node metastases. Clin Transl Oncol. 2013;15(9):732–740. | ||
Jang JW, Kay CS, You CR, et al. Simultaneous multitarget irradiation using helical tomotherapy for advanced hepatocellular carcinoma with multiple extrahepatic metastases. Int J Radiat Oncol Biol Phys. 2009;74(2):412–418. | ||
Lee DY, Park JW, Kim TH, et al. Prognostic indicators for radiotherapy of abdominal lymph node metastases from hepatocellular carcinoma. Strahlenther Onkol. 2015;191(11):835–844. | ||
Park YJ, Lim DH, Paik SW, et al. Radiation therapy for abdominal lymph node metastasis from hepatocellular carcinoma. J Gastroenterol. 2006;41(11):1099–1106. | ||
Wee CW, Kim K, Chie EK, Yu SJ, Kim YJ, Yoon JH. Prognostic stratification and nomogram for survival prediction in hepatocellular carcinoma patients treated with radiotherapy for lymph node metastasis. Br J Radiol. 2016;89(1065):20160383. | ||
Yamashita H, Nakagawa K, Shiraishi K, et al. Radiotherapy for lymph node metastases in patients with hepatocellular carcinoma: retrospective study. J Gastroenterol Hepatol. 2007;22(4):523–527. | ||
Yoon SM, Kim JH, Choi EK, et al. Radioresponse of hepatocellular carcinoma-treatment of lymph node metastasis. Cancer Res Treat. 2004;36(1):79. | ||
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. | ||
Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute; 2011. | ||
DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114. | ||
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89–98. | ||
Hasegawa K, Makuuchi M, Kokudo N, et al. Impact of histologically confirmed lymph node metastases on patient survival after surgical resection for hepatocellular carcinoma. Ann Surg. 2014;259(1):166–170. | ||
Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. | ||
Amini N, Ejaz A, Spolverato G, Maithel SK, Kim Y, Pawlik TM. Management of lymph nodes during resection of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a systematic review. J Gastrointest Surg. 2014;18(12):2136–2148. | ||
Zeng ZC, Tang ZY, Fan J, et al. Consideration of role of radiotherapy for lymph node metastases in patients with HCC: retrospective analysis for prognostic factors from 125 patients. Int J Radiat Oncol Biol Phys. 2005;63(4):1067–1076. | ||
Guckenberger M, Sweeney RA, Wilbert J, et al. Image-guided radiotherapy for liver cancer using respiratory-correlated computed tomography and cone-beam computed tomography. Int J Radiat Oncol Biol Phys. 2008;71(1):297–304. | ||
Guckenberger M, Meyer J, Wilbert J, et al. Intra-fractional uncertainties in cone-beam CT based image-guided radiotherapy (IGRT) of pulmonary tumors. Radiother Oncol. 2007;83(1):57–64. | ||
Zhao JD, Xu ZY, Zhu J, et al. Application of active breathing control in 3-dimensional conformal radiation therapy for hepatocellular carcinoma: the feasibility and benefit. Radiother Oncol. 2008;87(3):439–444. | ||
Chon YE, Seong J, Kim BK, et al. Gastroduodenal complications after concurrent chemoradiation therapy in patients with hepatocellular carcinoma: endoscopic findings and risk factors. Int J Radiat Oncol Biol Phys. 2011;81(5):1343–1351. | ||
Rim CH, KLCS Group, Registry KCC. Selection of patients with the benefit of local treatment including radiotherapy in Barcelona clinic of liver cancer c patients: the preliminary result. In: The 12th Annual Conference of the Korean Liver Cancer Association; February 9, 2018; Seoul, Korea. | ||
Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol. 1999;28(1):1–9. | ||
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. | ||
Poonacha TK, Go RS. Level of scientific evidence underlying recommendations arising from the National Comprehensive Cancer Network clinical practice guidelines. J Clin Oncol. 2011;29(2):186–191. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


