Back to Journals » Drug Design, Development and Therapy » Volume 16
The Role of Dexmedetomidine in Tumor-Progressive Factors in the Perioperative Period and Cancer Recurrence: A Narrative Review
Authors Cai Q, Liu G, Huang L, Guan Y, Wei H, Dou Z, Liu D, Hu Y, Gao M
Received 12 January 2022
Accepted for publication 28 May 2022
Published 6 July 2022 Volume 2022:16 Pages 2161—2175
DOI https://doi.org/10.2147/DDDT.S358042
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Qiang Cai,1,* Guoqing Liu,2,* Linsheng Huang,3,* Yuting Guan,2,* Huixia Wei,4 Zhiqian Dou,5 Dexi Liu,6 Yang Hu,1 Meiling Gao4
1Department of Orthopedics, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, People’s Republic of China; 2Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 3Department of Hepatobiliary Surgery, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, People’s Republic of China; 4Department of Anesthesiology, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, People’s Republic of China; 5Department of Obstetrics, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, People’s Republic of China; 6Department of Stomatology, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Meiling Gao, Department of Anesthesiology, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, People’s Republic of China, Tel +86-15971849819, Email [email protected] Yang Hu, Department of Orthopedics, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, 441000, People’s Republic of China, Tel +86-13995744850, Email [email protected]
Abstract: Dexmedetomidine, a specific α 2 adrenergic receptor agonist, is highly frequently used in the perioperatively for its favorable pharmacology, such as mitigating postoperative cognitive dysfunction. Increasing attention has been recently focused on the effect of whether dexmedetomidine influences cancer recurrence, which urges the discussion of the role of dexmedetomidine in tumor-progressive factors. The pharmacologic characteristics of dexmedetomidine, the tumor-progressive factors in the perioperative period, and the relationships between dexmedetomidine and tumor-progressive factors were described in this review. Available evidence suggests that dexmedetomidine could reduce the degree of immune function suppression, such as keeping the number of CD3+ cells, NK cells, CD4+/CD8+ ratio, and Th1/Th2 ratio stable and decreasing the level of proinflammatory cytokine (interleukin 6 and tumor necrosis factor-alpha) during cancer operations. However, dexmedetomidine exhibits different roles in cell biological behavior depending on cancer cell types. The conclusions on whether dexmedetomidine would influence cancer recurrence could not be currently drawn for the lack of strong clinical evidence. Therefore, this is still a new area that needs further exploration.
Keywords: dexmedetomidine, cancer recurrence, surgery, immune, inflammation
Introduction
The leading cause of mortality currently in patients <85 years is cancer, which adds a tremendous economic and medical burden worldwide.1 Surgery under anesthesia remains the first choice for cancer patients and plays a crucial role in cancer diagnosis, stage confirmation, and reconstruction. Metastatic recurrence is still reasonably frequent although surgical resection should be curative in local tumor lesions. An increasing number of studies have recently indicated that anesthetic drugs, as one of the essential perioperative components, may be involved in cancer recurrence by influencing the factors of tumor progression,2 such as propofol and locoregional anesthesia leading less immunosuppression,3 opioids stimulating cytological behavior of several tumor cells.4 Dexmedetomidine is a frequently increasingly used anaesthetic in the department of anesthesiology and intensive care unit (ICU) for its favorable pharmacology of suitable sedation, pain alleviation, and reduced odds of postoperative cognitive dysfunction.5 However, there still lacks of reviews on the theme of whether it plays a role in cancer recurrence as the amount of related researches grows sharply. Some studies showed that dexmedetomidine could protect immunity function, reduce inflammation reaction in patients who underwent cancer surgeries, and inhibit tumor cell growth, which may be favorable for outcomes of cancer patients.6–8 However, some studies claimed dexmedetomidine decreased overall survival after lung cancer surgery9 and stimulated the growth of some kinds of cancer cells.10,11 Hence, this review makes a comprehensive description, exploring the role of dexmedetomidine in tumor-progressive factors in the perioperative period that may affect cancer recurrence.
Methods
Two authors (QC and MLG) comprehensively searched PUBMED, EMBASE, and SCOPUS using the terms ((Dexmedetomidine or DEX or Dexmedetomide) and (Tumor or Tumour or Oncology or Cancer or Neoplasm)) from inception to July 2021 to gather fundamental and clinical studies exploring the relationship between dexmedetomidine and cancer. Moreover, references related to this topic were also searched. Clinical Registration Websites (https://clinicaltrials.gov/ and http://www.chictr.org.cn) were searched for ongoing clinical trials observing whether dexmedetomidine is associated with cancer recurrence.
Chemistry and Clinical Pharmacology of Dexmedetomidine
Dexmedetomidine is a usual anesthetic agent approved in the United States by the Food and Drug Administration in 1999 for the sedation of critical patients in the ICU. Furthermore, the applied range of dexmedetomidine in the clinical setting expanded to patients being operated on in 2008.12 The chemical structure of dexmedetomidine is 5-[(1S)-1-(2,3-dimethyl phenyl) ethyl]-1H-imidazole with molecular formula C13H16N2 (Figure 1).12 As a particular agonist to α2 adrenergic receptor, the selectivity ratio of α2 adrenoceptor to α1 adrenoceptor is 1600:1, which was more potent than clonidine with a selectivity ratio of 200:1.5 The protein binding of dexmedetomidine was 94%, and the half-life distribution was approximately 6 min with a clearance half-life of approximately 2–3 h. Dexmedetomidine could be metabolized by direct glycosylation and cytochrome P450 enzymes. Moreover, 95% and 4% of its metabolites are excreted in the urine and feces, respectively, and are not affected by fat mass.13 The α2 adrenergic receptors were distributed in the brain and other peripheral organs (eg, the spine, spleen, kidney, aorta, lung, skeletal muscle, heart, and liver; Table 1). Dexmedetomidine exerts diverse pharmacologic actions by specific binding to α2 adrenergic receptors in different tissues and cells. The most remarkable feature of dexmedetomidine is that patients remain easily rousable under dexmedetomidine-based sedation,14 which is primarily mediated by the activation of pre- and postsynaptic α2 adrenergic receptors in locus coeruleus15 where the brain is responsible for mediating wakefulness and sleep. Furthermore, multiple studies showed that dexmedetomidine-based sedation could reduce the risk of postoperative delirium in surgery patients, especially in the elderly.16–19 The analgesic effect of dexmedetomidine is mediated by activating α2 adrenergic receptors in locus coeruleus and the spine20 through interneuron hyperpolarization and reduction of neurotransmitter release (eg, substance P and glutamate).12 In addition, dexmedetomidine exerts a protective role in ischemia–reperfusion injury in cerebral,21 spinal cord,22 kidney,23 lung,24 heart,25 liver,26 and intestine,27 which has promising application and benefit for patients.
 |
Table 1 Classification of α2 Adrenergic Receptors |
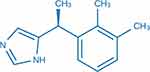 |
Figure 1 The chemical structure of dexmedetomidine. |
Direct and Indirect Tumor-Progressive Factors in the Perioperative Period
The perioperative period is a critical time because the physiological status of patients dramatically changes. The equilibrium between the immune system and neoplasm growth was considered steady before surgery trauma.28 Surgery alters the interplay of neuroendocrine, inflammatory, immune, and metabolic pathways of patients,29 which initiates a cascade of stress responses by activating the sympathetic nervous system (SNS) and hypothalamic–pituitary–adrenal (HPA) axis.30 SNS and HPA axis activation is closely related to immune dysfunction.31 Adrenoreceptors are also distributed on lymphoid organs and immune cells32 that could be activated by catecholamines and glucocorticoids secreted from adrenal glands, leading to an imbalance between Th1 and Th2 cells, shifting in favor of the Th2 cells, decreasing NK cell cytotoxicity, resulting in immune function suppression.33,34
Circulating tumor cells (CTCs), which are the cause of distant metastases,35 are shed from the solid tumor into the blood in many cancer patients, which are also significantly increased intraoperatively during tumor resection, especially in open approach than minimally invasive surgery, in the central vein than peripheral venous blood.36,37 Moreover, the detection rate of CTCs is much higher during surgical manipulation, particularly in cancer with lymphatic invasion.38
Moreover, tissue injury, stress, and infection caused by surgery trauma could lead to the inflammatory response involved in the coordinated delivery of blood components to the site of infection and injury.39 Tissue-resident macrophages and mast cells will recognize the initial signal subsequently, leading to the production of various inflammatory mediators, including chemokines, cytokines, vasoactive amines, eicosanoids, and products of proteolytic cascades and inducing neutrophils to move to the position of the inflammation.40 However, the inflammatory process persists and acquires new characteristics if the acute inflammatory response fails to eliminate the pathogen. Thus, it would transform into the persistent inflammation state involving the formation of granulomas and tertiary lymphoid tissues,41 providing favorite sites by disrupting endothelial surfaces and liberating growth factors for the seedings from CTCs released by surgery manipulation,42,43 which was called inflammatory oncotaxis.44 Moreover, neutrophil extracellular traps (NETs), relative to the inflammatory reaction induced by surgical trauma,45 could attract cancer cells to form distant metastases.46
Hence, the depressed immunologic function, the inflammation state, may be indirect tumor-progressive factors aroused by surgery stress or injury, which acted as helpers in cancer recurrence. Moreover, the CTCs and distant microscopic metastases could be the direct tumor-progressive factors, whose vitality is essential to cancer recurrence. The form of distant metastases facilitated by tumor-progressive factors by surgery stress or injury in the perioperative period is presented below (Figure 2).
The Role of Dexmedetomidine in Indirect Tumor-Progressive Factors (Immunologic Function and Inflammation State) During Cancer Operations
Dexmedetomidine is widely used to maintain anesthesia in operations,17 including cancer surgeries.7,47 Moreover, dexmedetomidine infusion may influence the immunologic function and inflammation state of cancer patients. Sixty-two patients undergoing radical mastectomy were pumped with 1 μg/kg dexmedetomidine for 10 min before anesthesia induction showed increased CD4+ and NK cell levels compared with the control.7 This high level was maintained for 48 h and only returned to normal in about 72 h. Patients pumped with 1 μg/kg dexmedetomidine intravenously at 0.2 μg/kg·h during radical gastric cancer resection showed elevated CD3+ and CD4+ levels and CD4+/CD8+ ratio and reduced interleukin (IL-6) and tumor necrosis factor-alpha (TNF-α) levels compared with the control.47 Dexmedetomidine could also maintain Th1/Th2 balance and decrease IL-6 and TNF-α levels in patients undergoing radical gastrectomy.48,49 Patients pumped intravenously with 1 μg/kg dexmedetomidine for 10 min as a loading dose and maintained at 0.3 μg/(kg·h) until the end of hepatectomy had reduced IL-6 and TNF-α levels compared with the control.27 Patients pumped with 1 μg/kg dexmedetomidine intravenously for 10–15 min as a loading dose and maintained at 1 μg/(kg·h) before colon cancer operation showed increased CD3+ and CD4+ levels, CD4+/CD8+ ratio, and Th1/Th2 ratio compared with the control.50 Furthermore, Guo et al found that 1 μg/kg dexmedetomidine pumped intravenously for 10 min as a loading dose and maintained at 0.4 μg/(kg·h) until 30 min before the end of lung cancer operation reduced the TNF-α level of patients,51 which was also reported by other studies with different dexmedetomidine infusion rates.52 Moreover, dexmedetomidine infusion could also promote CD3+ and CD4+ levels and CD4+/CD8+ ratio and decrease IL-6 levels in lung cancer operation6,53–56 compared with the control. Patients pumped with 0.5 μg/kg dexmedetomidine intravenously for 15 min as a loading dose and maintained at 0.4 μg/(kg·h) until the end of oral cancer operation had elevated CD3+ and CD4+ levels and CD4+/CD8+ ratio.57 In addition, patients pumped with 1 μg/kg dexmedetomidine intravenously for 15 min and maintained at 0.5 μg/(kg·h) until brain cancer operation ended had elevated CD3+ and CD4+ levels, CD4+/CD8+ ratio, and NK cell numbers.58 In patients pumped with 0.3 μg/(kg·h) dexmedetomidine intravenously until the end of esophagus cancer operation had significantly decreased IL-6 levels compared with the control.59 Other studies also demonstrated that intravenous infusion of dexmedetomidine could decrease IL-6 and TNF-α levels in esophagus cancer operation.60 Moreover, dexmedetomidine infusion could decrease IL-6 and TNF-α levels in colorectum operation61,62 (the studies involved are listed in Table 2, and the details of the changes are shown in Supplementary Table 1).
 |  |  |
Table 2 Studies Investigating the Effect of Dexmedetomidine on Immune Cells and Inflammatory Cytokines in Patients Undergoing Cancer Surgery |
These studies suggested that dexmedetomidine could regulate immunologic function and decrease the proinflammatory cytokine release in the perioperative period of cancer operations. The leading mechanism is that SNS and HPA activity stimulated by surgery stress could induce a redistribution of immune cells (eg, neutrophils, monocytes, and T cells) by secretion of catecholamines and cortisol.63 Catecholamines induce the T and NK cells to move from the marginated pool (eg, bone marrow and lymph nodes) to the bloodstream temporarily.64 Consequently, the T cells and monocytes were induced out of the bloodstream to the surgical site or the marginated pool. Thus, the number of NK and T cells decreased postoperatively.65 However, α2 adrenergic receptors are highly expressed in the pineal gland;66 dexmedetomidine could reduce the adrenocorticotropin (ACTH) secretion and cortisol levels by binding to it.63
IL-6 and TNF-α are mainly secreted from monocytes and macrophages. The inhibiting effect of dexmedetomidine on TNF-a and IL-6 secretions depend on two possible mechanisms. First, the attenuation of surgery stress by ACTH and cortisol reduction via dexmedetomidine could indirectly reduce the inflammation reaction given the close relationship between stress and inflammation.67 Second, dexmedetomidine could directly influence monocytes and macrophages. Li et al reported that dexmedetomidine could attenuate NFκB-p65 phosphorylation to decrease TNF-α production from LPS-stimulated murine BV-2 microglial cells and RAW264.7 macrophage cells.68 A similar study also proved that dexmedetomidine could reduce TNF-a and IL-6 levels and enhanced IL-10 secretion from bone marrow-derived macrophages.26
The current study shows that dexmedetomidine infusion could mediate the immunologic function and inflammation state of cancer patients. Similarly, a meta-analysis including 4842 patients suffering from different diseases showed that dexmedetomidine infusion significantly inhibited the release of epinephrine, norepinephrine, and cortisol,30 leading to (1) increased number of NK cells; (2) increased ratio of CD4+/CD8+ and Th1/Th2 cells; and (3) decreased TNF-a and IL-6 levels. Moreover, a study from Shin et al69 demonstrated that BALB/c nude mice with tumor pumped with Dexmedetomidine exhibited faster NK cell activity recovery and lower cortisol levels and TNF-α levels at 4 weeks after surgery when compared with the control that BALB/c nude mice with tumor pumped with the saline. The underlying mechanisms accounting for the phenomenon might be dexmedetomidine relieves the stress responses by regulating the sympathetic nervous system (SNS) and hypothalamic–pituitary–adrenal (HPA) axis reaction, which were highly correlated to the equilibrium of immunity function and the inflammatory state, as presented in the introduction. As an evidence, Li et al70 established a unique (lipopolysaccharide) LPS-induced acute lung injury (ALI) rate model with the bilateral cervical vagus nerve cut off (vagotomy), they found that dexmedetomidine could reduce LPS-induced IL-1β, TNF-α, and catecholamine but increased acetylcholine in blood serum in the rate without vagotomy, but partially abolished by vagotomy, which suggested dexmedetomidine could play the role by high vagal nerve tone and α2-adrenoceptor activation.
Although Clinical trials have shown the effects of dexmedetomidine on immunomodulatory and anti-inflammatory. There are still several limitations. Firstly, the number of studies is relatively small, which requires multicenter studies with large samples to confirm the conclusion further. Secondly, long-term role of dexmedetomidine regulating immune function and inflammation in cancer patients, such as 5 year survival period, has not been investigated. Therefore, this is still a new area worthy of further research.
The Role of Dexmedetomidine in Direct Tumor-Progressive Factors (Proliferation, Migration, and Invasion of Cancer Cells)
Dexmedetomidine and Lung Cancer Cell
The latest fundamental study11 from Wang et al found that dexmedetomidine could promote human lung cancer cell A549 proliferation and migration at the <0.001 nM level, which was far less than the blood concentration used in clinical settings. A549 cell quantity could be increased 1.2- and 1.7-folds at the 0.001- and 10-nM levels, respectively, and enhance cell migration by 2.2-fold vs vehicle at the 1-nM level. Moreover, Ki67 is one kind of nucleoprotein engaged in ribosomal RNA transcription.72 As one of the cell proliferation markers, it is expressed in the G1, S, G2, and M phases of the cell cycle, but not in the silent G0 stage. Ki67 expression was 2.9-fold over the control when the A549 cell was treated with 1 nM dexmedetomidine, meaning the A549 cell was in the active growth period.
Dexmedetomidine and Breast Cancer Cell
Some articles73,74 recently indicated that dexmedetomidine could promote the growth of human breast cancer cells. Xia et al found that dexmedetomidine could promote proliferation, migration, and invasion of human MDA-MB-231 breast cancer cells via the activation of α2-adrenoceptor/ERK1/2 signaling.73 The ERK1/2 signaling pathway is one of the classical pathways involved in many essential cell functions and regulates tumor cell progression.75 The protein level of the phosphorylated ERK, α2 adrenoceptor increased when the MDA-MB-231 cell was treated with different dexmedetomidine levels (>0.1 µM) for 48 h.73 This promotion role confirmed in vivo that the volumes and weight of the tumor in dexmedetomidine-treated mice were more massive than in the control group. Similarly, the migration capacity of cells was significantly improved when human MCF-7 and MDA-MB-231 cancer cells were treated with dexmedetomidine (1 µM) for 16 h.76
Prolactin and relevant receptors (PrlR) had been found in several breast cancer cells,77 and the role of PrlR stimulating breast cell proliferation78 and the association of cancer risk and PrlR level before diagnosis <10 years had been confirmed.79 Moreover, PrlR levels were an independent prognostic marker for breast cancer.80 An interesting study by Castillo74 revealed that the prolactin secretions of human T47D and MCF-7 breast cancer cells were promoted by dexmedetomidine at the 1-nM level and could be reversed by rauwolscine, an α2 adrenergic antagonist. The increasing PrlR could cause rapid STAT5 and ERK1/2 phosphorylation in MCF-7 and T47D cells and activate relevant cancer pathways.
Another study81 found that dexmedetomidine could alter the collagen structure of 4T1 mice breast cancer cells to promote growth. Second-harmonic generation (SHG) is a particular optical signal generated when laser contacts with nonlinear materials are more sensitive to microstructure change than the best fluorescence signal.82 Fibrillar collagen with detectable SHG signal was confirmed to promote tumor cell locomotion in breast tumor models83,84 and was associated with tumor cell proliferation, invasion, and metastasis.85 The researchers treated the tumor mice with dexmedetomidine at a concentration of 10 or 25 mg/kg for 19 days and showed the increased tumor growth rate and the notable change in the number of SHG image pixels in the tumor removed from mice treated with dexmedetomidine.81
Moreover, [3H]thymidine is a raw material of compounding DNA, which has a radioactive character that could be detected to assess the reactivity of cells to drugs to promote proliferation. However, dexmedetomidine could enhance the incorporation rate of [3H]thymidine into the cell and enhance mouse breast tumor volume of C4-HD at a follow-up of 25 days of the experimental period without losing sensitivity to the α2 adrenoceptor after continuous treatment.86 Also, another study from the same team showed that the stromal fibroblasts from breast tumors could also express α2 adrenergic receptors, and dexmedetomidine could promote fibroblast proliferation. Furthermore, the effect of proliferation could be reversed by α2 adrenergic antagonist, rauwolscine.87
Dexmedetomidine and Colon Cancer Cell
Lavon et al explored the role of dexmedetomidine in the progression of mouse CT26 colon adenocarcinoma cells. They found that dexmedetomidine administered at the hypnotic dose of 3 or 12.5 µg·kg−1 h−1 could promote CT26 tumor metastasis numbers in the livers of female mice with CT26 tumor cells injected into the spleen 3 weeks previously.10
Dexmedetomidine and Ovarian Cancer Cell
Cai et al8 [6] found that dexmedetomidine could inhibit the growth rate of the NUTU-19 rat ovarian cancer cell by inhibiting the p38MAPK/NF-κB signaling pathway. The researchers injected NUTU-19 ovarian carcinoma cells into the right armpit of rats to form a solid tumor, then distributed the rats into different groups treated by different doses of dexmedetomidine or saline. Moreover, they set the rat group without tumors planted as the healthy group. However, the rats with the tumors in the dexmedetomidine group displayed more energy and better appetite than the saline group but not the healthy group. The same situation was presented when measuring the weight of the tumor. When comparing the pathological changes of ovarian cancer tissues from the saline group, the ovarian cancer tissues from the dexmedetomidine groups exhibited shrinkage of tumor cell and chromatin migration and patchy necrosis at different degrees. The p38 MAPK-dependent NF-κB signaling pathway is seen as playing the primary role in chemoresistance and cell damage and having a crucial influence on the proliferation of malignant tumor cells, including ovarian cancer cells.88 Moreover, Cai et al8 [6] discovered that the dexmedetomidine group presented significantly fewer expression signals of that pathway than the saline group.
Dexmedetomidine and Osteosarcoma Cell
A study by Wang et al89 cultivated the human osteosarcoma cell MG63 combined with dexmedetomidine at different doses and found that 100 ng/mL of dexmedetomidine could significantly suppress cell viability after 12 h of treatment. Furthermore, 100 ng/mL of dexmedetomidine could significantly decrease the number of migrated MG63 cells and elevate the percentage of apoptotic MG63 cells after 24 h of treatment. MiR-520-3p is one of the noncoding RNAs that could suppress various human cancers.90–92 Moreover, AKT/mTOR pathway is essential in the disease process, especially in tumor progression,93,94 and could regulate human osteosarcoma cell proliferation and apoptosis.95 They89 found that miR-520-3p induced by dexmedetomidine could specifically bind to the 3′-UTR of AKT1 to inhibit MG63 osteosarcoma cell.
In summary, dexmedetomidine could directly promote proliferation, migration, and metastasis in some cancer cells (eg, lung, breast, and colon cancer cells) and restrain some ovarian cancer cells and osteosarcoma cancer cells by different mechanisms (Table 3).
 |
Table 3 The Mechanism of the Role of Dexmedetomidine in Cell Biological Behaviors |
Future Perspectives
Accumulating clinical studies have shown that dexmedetomidine is inclined to protect immunologic function and reduce inflammatory cytokine in the perioperative period of cancer surgeries, which may inhibit cancer recurrence factors. However, a few fundamental studies indicate that dexmedetomidine could facilitate the vitality of some human cancer cell lines (eg, MDA-MB-231, MCF-7, T47D, 4T1, and A549), which originate from breast or lung tissue. Thus, this arouses the interesting question: could the clinical application of dexmedetomidine be deleterious to the survival of cancer patients or accelerative to cancer recurrence? However, a lack of robust clinical evidence (ie, RCTs or meta-analysis of high-quality) exists on this theme. A study from MD Anderson Cancer Center9 investigating the relationship between the intraoperative use of dexmedetomidine and lung cancer recurrence presented a decreased overall survival in patients using dexmedetomidine. However, the author reminded us of the significant limitation that dexmedetomidine may be used in patients with more severe comorbidities that were not captured in the database. Dexmedetomidine could improve the outcomes of critical patients (eg, heart disease patients). Perioperative dexmedetomidine used decreases the postoperative mortality of patients who underwent heart surgeries and decreased the delirium rate.98,99
Moreover, some essential variables (eg, consumption of opioids or intraoperative volatile anesthetics) were not included in the final analysis, which was considered as potential factors influencing cancer recurrence.100,101 In addition, no difference in recurrence-free survival time with or without dexmedetomidine was noted. Another retrospective study from the same research group indicated that dexmedetomidine administration could not influence the survival of children and adolescents who had undergone major oncologic surgery.102
Hence, it is still common to apply dexmedetomidine to cancer patients for its excellent pharmacological effect at this stage, especially in reducing postoperative delirium. Nevertheless, exploring the effect of the administration of dexmedetomidine on outcomes of cancer patients is still meaningful. A certain number of prospective randomized controlled trials are currently ongoing (NCT03109990: Impact of Dexmedetomidine on Breast Cancer Recurrence After Surgery; NCT03012971: Dexmedetomidine Supplemented Analgesia and Long-term Survival After Cancer Surgery. NCT04111926: Intraoperative Dexmedetomidine and long-term outcomes in the elderly after major surgery), aiming to assess whether dexmedetomidine would influence cancer recurrence and long-term survival in different cancer operations. Thus, this will bring more specific information to clinicians.
Conclusion
Dexmedetomidine could protect immunologic function, reduce inflammatory cytokine in the perioperative period of cancer surgeries, and have diverse roles in cancer cell biology. That means the roles of dexmedetomidine on the tumor-progressive factors were still complex and non-uniform. It is still cautious to make a conclusion concerning whether dexmedetomidine is harmful for some kinds of cancer patients. More future clinical trials should be held to provide more specific information. This may lead to optimization in the strategy of anesthesia in cancer patients in the future.
Data Sharing Statement
The data presented in the article could be requested by consulting the corresponding author.
Ethical Approval
No ethical approval was required for the review.
Author Contributions
Yang Hu, Meiling Gao performed the conception of this review; Yuting Guan, Huixia Wei, Zhiqian Dou, Dexi Liu contributed to the related article collection; Qiang Cai, Meiling Gao, Linsheng Huang, Guoqing Liu performed reviewing the related articles and wrote the review. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Doctoral Start-up Fund of Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science.
Disclosure
The authors declared no competing interest.
References
1. Dubowitz JA, Sloan EK, Riedel BJ. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin Exp Metastasis. 2018;35(4):347–358. doi:10.1007/s10585-017-9862-x
2. Eden C, Esses G, Katz D, DeMaria S. Effects of anesthetic interventions on breast cancer behavior, cancer-related patient outcomes, and postoperative recovery. Surg Oncol. 2018;27(2):266–274. doi:10.1016/j.suronc.2018.05.001
3. Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 2018;16(1):8. doi:10.1186/s12967-018-1389-7
4. Song Z, Tan J. Effects of anesthesia and anesthetic techniques on metastasis of lung cancers: a narrative review. Cancer Manag Res. 2022;14:189–204. doi:10.2147/CMAR.S343772
5. Bischoff P, Kochs E. Alpha 2-agonists in anesthesia and intensive medicine. AINS. 1993;28(1):2–12. doi:10.1055/s-2007-998867
6. Kong L, Lu XH. [Effect of dexmedetomidine on perioperative inflammatory response and cellular immune in patients undergoing radical operation of thoracoscopic lung cancer]. Zhonghua Yi Xue Za Zhi. 2018;98(36):2929–2932. Chinese. doi:10.3760/cma.j.issn.0376-2491.2018.36.011
7. Yang XH, Bai Q, Lv MM, Fu HG, Dong TL, Zhou Z. Effect of dexmedetomidine on immune function of patients undergoing radical mastectomy: a double blind and placebo control study. Eur Rev Med Pharmacol Sci. 2017;21(5):1112–1116.
8. Cai QH, Tang Y, Fan SH, et al. In vivo effects of dexmedetomidine on immune function and tumor growth in rats with ovarian cancer through inhibiting the p38MAPK/NF-κB signaling pathway. Biomed Pharmacother. 2017;95:1830–1837. doi:10.1016/j.biopha.2017.09.086
9. Cata JP, Singh V, Lee BM, et al. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol. 2017;33(3):317–323. doi:10.4103/joacp.JOACP_299_16
10. Lavon H, Matzner P, Benbenishty A, et al. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth. 2018;120(1):188–196. doi:10.1016/j.bja.2017.11.004
11. Wang C, Datoo T, Zhao H, et al. Midazolam and dexmedetomidine affect neuroglioma and lung carcinoma cell biology in vitro and in vivo. Anesthesiology. 2018;129(5):1000–1014. doi:10.1097/ALN.0000000000002401
12. Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi:10.1007/s40262-017-0507-7
13. Rolle A, Paredes S, Cortínez LI, et al. Dexmedetomidine metabolic clearance is not affected by fat mass in obese patients. Br J Anaesth. 2018;120(5):969–977. doi:10.1016/j.bja.2018.01.040
14. Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90(3):699–705. doi:10.1097/00000539-200003000-00035
15. Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76(6):948–952. doi:10.1097/00000542-199206000-00013
16. Djaiani G, Silverton N, Fedorko L, et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. 2016;124(2):362–368. doi:10.1097/ALN.0000000000000951
17. Duan X, Coburn M, Rossaint R, Sanders RD, Waesberghe JV, Kowark A. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth. 2018;121(2):384–397. doi:10.1016/j.bja.2018.04.046
18. Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–1902. doi:10.1016/S0140-6736(16)30580-3
19. Subramaniam B, Shankar P, Shaefi S, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA. 2019;321(7):686–696. doi:10.1001/jama.2019.0234
20. Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84(4):873–881. doi:10.1097/00000542-199604000-00015
21. Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology. 1991;75(2):328–332. doi:10.1097/00000542-199108000-00022
22. Sun Z, Zhao T, Lv S, Gao Y, Masters J, Weng H. Dexmedetomidine attenuates spinal cord ischemia-reperfusion injury through both anti-inflammation and anti-apoptosis mechanisms in rabbits. J Transl Med. 2018;16(1):209. doi:10.1186/s12967-018-1583-7
23. Si Y, Bao H, Han L, et al. Dexmedetomidine attenuation of renal ischaemia-reperfusion injury requires sirtuin 3 activation. Br J Anaesth. 2018;121(6):1260–1271. doi:10.1016/j.bja.2018.07.007
24. Liang S, Wang Y, Liu Y. Dexmedetomidine alleviates lung ischemia-reperfusion injury in rats by activating PI3K/Akt pathway. Eur Rev Med Pharmacol Sci. 2019;23(1):370–377. doi:10.26355/eurrev_201901_16785
25. Riquelme JA, Westermeier F, Hall AR, et al. Dexmedetomidine protects the heart against ischemia-reperfusion injury by an endothelial eNOS/NO dependent mechanism. Pharmacol Res. 2016;103:318–327. doi:10.1016/j.phrs.2015.11.004
26. Zhou H, Sun J, Zhong W, et al. Dexmedetomidine preconditioning alleviated murine liver ischemia and reperfusion injury by promoting macrophage M2 activation via PPARγ/STAT3 signaling. Int Immunopharmacol. 2020;82:106363. doi:10.1016/j.intimp.2020.106363
27. Wang ZX, Huang CY, Hua YP, Huang WQ, Deng LH, Liu KX. Dexmedetomidine reduces intestinal and hepatic injury after hepatectomy with inflow occlusion under general anaesthesia: a randomized controlled trial. Br J Anaesth. 2014;112(6):1055–1064. doi:10.1093/bja/aeu132
28. Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130(6):1237–1250. doi:10.1002/ijc.26448
29. Kehlet H. Manipulation of the metabolic response in clinical practice. World J Surg. 2000;24(6):690–695. doi:10.1007/s002689910111
30. Wang K, Wu M, Xu J, et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br J Anaesth. 2019;123(6):777–794. doi:10.1016/j.bja.2019.07.027
31. Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun. 2007;21(6):736–745. doi:10.1016/j.bbi.2007.03.008
32. Scanzano A, Cosentino M. Adrenergic regulation of innate immunity: a review. Front Pharmacol. 2015;6:171. doi:10.3389/fphar.2015.00171
33. Irwin M. Stress-induced immune suppression: role of brain corticotropin releasing hormone and autonomic nervous system mechanisms. Adv Neuroimmunol. 1994;4(1):29–47. doi:10.1016/S0960-5428(06)80188-9
34. Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora’s box? Mol Med. 2008;14(3–4):195–204. doi:10.2119/2007-00105.Flierl
35. García SA, Weitz J, Schölch S. Circulating tumor cells. Methods Mol Biol. 2018;1692:213–219.
36. Wind J, Tuynman JB, Tibbe AG, et al. Circulating tumour cells during laparoscopic and open surgery for primary colonic cancer in portal and peripheral blood. Eur J Surg Oncol. 2009;35(9):942–950. doi:10.1016/j.ejso.2008.12.003
37. Huang HB, Ge MJ. The effects of different surgical approaches on the perioperative level of circulating tumor cells in patients with non-small cell lung cancer. Thorac Cardiovasc Surg. 2016;64(6):515–519. doi:10.1055/s-0035-1552925
38. Hashimoto M, Tanaka F, Yoneda K, et al. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg. 2014;18(6):775–783. doi:10.1093/icvts/ivu048
39. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi:10.1038/nature07201
40. Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–815. doi:10.1038/nri2171
41. Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–353. doi:10.1038/ni1330
42. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. doi:10.1186/s12199-018-0740-1
43. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
44. DerHagopian RP, Sugarbaker EV, Ketcham A. Inflammatory oncotaxis. JAMA. 1978;240(4):374–375. doi:10.1001/jama.1978.03290040052023
45. Huang H, Tohme S, Al-Khafaji AB, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62(2):600–614. doi:10.1002/hep.27841
46. Willigers HM, Prinzen FW, Roekaerts PM, de Lange S, Durieux ME. Dexmedetomidine decreases perioperative myocardial lactate release in dogs. Anesth Analg. 2003;96(3):657–664, table of contents. doi:10.1213/01.ANE.0000048708.75957.FF
47. Dong W, Chen MH, Yang YH, et al. The effect of dexmedetomidine on expressions of inflammatory factors in patients with radical resection of gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(15):3510–3515.
48. Wang Y, Xu X, Liu H, Ji F. Effects of dexmedetomidine on patients undergoing radical gastrectomy. J Surg Res. 2015;194(1):147–153. doi:10.1016/j.jss.2014.10.008
49. Zheng L, Zhao J, Zheng L, Jing S, Wang X. Effect of dexmedetomidine on perioperative stress response and immune function in patients with tumors. Technol Cancer Res Treat. 2020;19:1533033820977542. doi:10.1177/1533033820977542
50. Wang K, Li C. Effects of dexmedetomidine on inflammatory factors, T lymphocyte subsets and expression of NF-κB in peripheral blood mononuclear cells in patients receiving radical surgery of colon carcinoma. Oncol Lett. 2018;15(5):7153–7157. doi:10.3892/ol.2018.8205
51. Guo YB, Xu JD, Ji XX, Zhang JX, Liang JX, Zhou GB. Protective effect of dexmedetomidine against perioperative inflammation and on pulmonary function in patients undergoing radical resection of lung cancer. J South Med Univ. 2017;37(12):1673–1677.
52. Gao S, Wang Y, Zhao J, Su A. Effects of dexmedetomidine pretreatment on heme oxygenase-1 expression and oxidative stress during one-lung ventilation. Int J Clin Exp Pathol. 2015;8(3):3144–3149.
53. Wen QP, Miao Z, Wu P, et al. Whole-course application of dexmedetomidine combined with ketorolac in nonnarcotic postoperative analgesia for patients with lung cancer undergoing thoracoscopic surgery: a randomized control trial. Pain Physician. 2020;23(2):E185–e193. doi:10.36076/ppj.2020/23/E185
54. Liu GC, Sun K, Fu HG, Dong TL, Yuan F. [Effects of dexmedetomidine on injury of lungs and CHOP protein expression in elderly patients with lung cancer during one-lung ventilation]. Zhonghua Yi Xue Za Zhi. 2020;100(1):37–41. Chinese. doi:10.3760/cma.j.issn.0376-2491.2020.01.009
55. Xie Y, Jiang W, Zhao L, Wu Y, Xie H. Effect of dexmedetomidine on perioperative inflammation and lung protection in elderly patients undergoing radical resection of lung cancer. Int J Clin Exp Pathol. 2020;13(10):2544–2553.
56. Yin H, Cao L, Zhao H, Yang Y. Effects of dexmedetomide, propofol and remifentanil on perioperative inflammatory response and lung function during lung cancer surgery. Am J Transl Res. 2021;13(4):2537–2545.
57. Huang L, Qin C, Wang L, Zhang T, Li J. Effects of dexmedetomidine on immune response in patients undergoing radical and reconstructive surgery for oral cancer. Oncol Lett. 2021;21(2):106. doi:10.3892/ol.2020.12367
58. Wu L, Lv H, Luo W, Jin S, Hang Y. Effects of dexmedetomidine on cellular immunity of perioperative period in children with brain neoplasms. Int J Clin Exp Med. 2015;8(2):2748–2753.
59. Gong Z, Long X, Wei H, et al. Dexmedetomidine combined with protective lung ventilation strategy provides lung protection in patients undergoing radical resection of esophageal cancer with one-lung ventilation. J South Med Univ. 2020;40(7):1013–1017. doi:10.12122/j.issn.1673-4254.2020.07.15
60. Tang C, Hu Y, Zhang Z, et al. Dexmedetomidine with sufentanil in intravenous patient-controlled analgesia for relief from postoperative pain, inflammation and delirium after esophageal cancer surgery. Biosci Rep. 2020;40(5). doi:10.1042/BSR20193410
61. Zhang J, Liu G, Zhang F, et al. Analysis of postoperative cognitive dysfunction and influencing factors of dexmedetomidine anesthesia in elderly patients with colorectal cancer. Oncol Lett. 2019;18(3):3058–3064. doi:10.3892/ol.2019.10611
62. Yi XL, Wang JT, Chu CQ, Li YX, Yin JH, Liu SL. Cardiocerebral protective effects of dexmedetomidine as anesthetic in colorectal cancer surgery. Eur Rev Med Pharmacol Sci. 2018;22(11):3570–3576. doi:10.26355/eurrev_201806_15183
63. Yuki K. The immunomodulatory mechanism of dexmedetomidine. Int Immunopharmacol. 2021;97:107709. doi:10.1016/j.intimp.2021.107709
64. Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells–from barracks to boulevards to battlefields: a tale of three hormones–Curt Richter Award winner. Psychoneuroendocrinology. 2012;37(9):1345–1368. doi:10.1016/j.psyneuen.2012.05.008
65. Bartal I, Melamed R, Greenfeld K, et al. Immune perturbations in patients along the perioperative period: alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav Immun. 2010;24(3):376–386. doi:10.1016/j.bbi.2009.02.010
66. Muñóz-Hoyos A, Fernández-García JM, Molina-Carballo A, et al. Effect of clonidine on plasma ACTH, cortisol and melatonin in children. J Pineal Res. 2000;29(1):48–53. doi:10.1034/j.1600-079X.2000.290107.x
67. Umamaheswaran S, Dasari SK, Yang P, Lutgendorf SK, Sood AK. Stress, inflammation, and eicosanoids: an emerging perspective. Cancer Metastasis Rev. 2018;37(2–3):203–211. doi:10.1007/s10555-018-9741-1
68. Li R, Lai IK, Pan JZ, Zhang P, Maze M. Dexmedetomidine exerts an anti-inflammatory effect via α2 adrenoceptors to prevent lipopolysaccharide-induced cognitive decline in mice. Anesthesiology. 2020;133(2):393–407. doi:10.1097/ALN.0000000000003390
69. Shin S, Kim KJ, Hwang HJ, Noh S, Oh JE, Yoo YC. Immunomodulatory effects of perioperative dexmedetomidine in ovarian cancer: an in vitro and xenograft mouse model study. Front Oncol. 2021;11:722743. doi:10.3389/fonc.2021.722743
70. Li Y, Wu B, Hu C, et al. The role of the vagus nerve on dexmedetomidine promoting survival and lung protection in a sepsis model in rats. Eur J Pharmacol. 2022;914:174668. doi:10.1016/j.ejphar.2021.174668
71. Guo Y, Sun L, Zhang J, Li Q, Jiang H, Jiang W. Preventive effects of low-dose dexmedetomidine on postoperative cognitive function and recovery quality in elderly oral cancer patients. Int J Clin Exp Med. 2015;8(9):16183–16190.
72. Schonk DM, Kuijpers HJ, van Drunen E, et al. Assignment of the gene(s) involved in the expression of the proliferation-related Ki-67 antigen to human chromosome 10. Hum Genet. 1989;83(3):297–299. doi:10.1007/BF00285178
73. Xia M, Ji NN, Duan ML, et al. Dexmedetomidine regulate the malignancy of breast cancer cells by activating α2-adrenoceptor/ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20(16):3500–3506.
74. Castillo LF, Rivero EM, Goffin V, Lüthy IA. Alpha-adrenoceptor agonists trigger prolactin signaling in breast cancer cells. Cell Signal. 2017;34:76–85. doi:10.1016/j.cellsig.2017.03.003
75. Huntington JT, Shields JM, Der CJ, et al. Overexpression of collagenase 1 (MMP-1) is mediated by the ERK pathway in invasive melanoma cells: role of BRAF mutation and fibroblast growth factor signaling. J Biol Chem. 2004;279(32):33168–33176. doi:10.1074/jbc.M405102200
76. Gargiulo L, Copsel S, Rivero EM, et al. Differential beta(2)-adrenergic receptor expression defines the phenotype of non-tumorigenic and malignant human breast cell lines. Oncotarget. 2014;5(20):10058–10069. doi:10.18632/oncotarget.2460
77. Clevenger CV, Chang WP, Ngo W, Pasha TL, Montone KT, Tomaszewski JE. Expression of prolactin and prolactin receptor in human breast carcinoma. Evidence for an autocrine/paracrine loop. Am J Pathol. 1995;146(3):695–705.
78. Goffin V, Touraine P. The prolactin receptor as a therapeutic target in human diseases: browsing new potential indications. Expert Opin Ther Targets. 2015;19(9):1229–1244. doi:10.1517/14728222.2015.1053209
79. Tworoger SS, Eliassen AH, Zhang X, et al. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res. 2013;73(15):4810–4819. doi:10.1158/0008-5472.CAN-13-0665
80. Hachim IY, Hachim MY, Lopez VM, Lebrun JJ, Ali S. Prolactin receptor expression is an independent favorable prognostic marker in human breast cancer. AIMM. 2016;24(4):238–245. doi:10.1097/PAI.0000000000000178
81. Szpunar MJ, Burke KA, Dawes RP, Brown EB, Madden KS. The antidepressant desipramine and alpha2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev Res. 2013;6(12):1262–1272. doi:10.1158/1940-6207.CAPR-13-0079
82. Moreaux L, Sandre O, Charpak S, Blanchard-Desce M, Mertz J. Coherent scattering in multi-harmonic light microscopy. Biophys J. 2001;80(3):1568–1574. doi:10.1016/S0006-3495(01)76129-2
83. Wang W, Wyckoff JB, Frohlich VC, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62(21):6278–6288.
84. Sidani M, Wyckoff J, Xue C, Segall JE, Condeelis J. Probing the microenvironment of mammary tumors using multiphoton microscopy. J Mammary Gland Biol Neoplasia. 2006;11(2):151–163. doi:10.1007/s10911-006-9021-5
85. Conklin MW, Eickhoff JC, Riching KM, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178(3):1221–1232. doi:10.1016/j.ajpath.2010.11.076
86. Bruzzone A, Piñero CP, Castillo LF, et al. Alpha2-adrenoceptor action on cell proliferation and mammary tumour growth in mice. Br J Pharmacol. 2008;155(4):494–504. doi:10.1038/bjp.2008.278
87. Bruzzone A, Pinero CP, Rojas P, et al. alpha(2)-Adrenoceptors enhance cell proliferation and mammary tumor growth acting through both the stroma and the tumor cells. Curr Cancer Drug Targets. 2011;11(6):763–774. doi:10.2174/156800911796191051
88. Yan H, Xin S, Wang H, Ma J, Zhang H, Wei H. Baicalein inhibits MMP-2 expression in human ovarian cancer cells by suppressing the p38 MAPK-dependent NF-kappaB signaling pathway. Anticancer Drugs. 2015;26(6):649–656. doi:10.1097/CAD.0000000000000230
89. Wang X, Xu Y, Chen X, Xiao J. Dexmedetomidine inhibits osteosarcoma cell proliferation and migration, and promotes apoptosis by regulating miR-520a-3p. Oncol Res. 2018;26(3):495–502. doi:10.3727/096504017X14982578608217
90. Yu J, Tan Q, Deng B, Fang C, Qi D, Wang R. The microRNA-520a-3p inhibits proliferation, apoptosis and metastasis by targeting MAP3K2 in non-small cell lung cancer. Am J Cancer Res. 2015;5(2):802–811.
91. Su H, Ren F, Jiang H, Chen Y, Fan X. Upregulation of microRNA-520a-3p inhibits the proliferation, migration and invasion via spindle and kinetochore associated 2 in gastric cancer. Oncol Lett. 2019;18(3):3323–3330. doi:10.3892/ol.2019.10663
92. Ren Z, Yang T, Ding J, et al. MiR-520d-3p antitumor activity in human breast cancer via post-transcriptional regulation of spindle and kinetochore associated 2 expression. Am J Transl Res. 2018;10(4):1097–1108.
93. Xu Z, Xu M, Liu P, et al. The mTORC2-Akt1 cascade is crucial for c-Myc to promote hepatocarcinogenesis in mice and humans. Hepatology. 2019;70(5):1600–1613. doi:10.1002/hep.30697
94. Citro S, Miccolo C, Meloni L, Chiocca S. PI3K/mTOR mediate mitogen-dependent HDAC1 phosphorylation in breast cancer: a novel regulation of estrogen receptor expression. J Mol Cell Biol. 2015;7(2):132–142. doi:10.1093/jmcb/mjv021
95. Liu Y, Bi T, Dai W, et al. Lupeol induces apoptosis and cell cycle arrest of human osteosarcoma cells through PI3K/AKT/mTOR pathway. Technol Cancer Res Treat. 2016;15(6):Np16–np24. doi:10.1177/1533034615609014
96. Chi M, Shi X, Huo X, Wu X, Zhang P, Wang G. Dexmedetomidine promotes breast cancer cell migration through Rab11-mediated secretion of exosomal TMPRSS2. Ann Transl Med. 2020;8(8):531. doi:10.21037/atm.2020.04.28
97. Zheng L, Jia R, Zhao J. Dexmedetomidine regulates proliferation, apoptosis, migration, and invasion in ovarian cancer cells via MiR-155-HIF-1α axis. Med Sci Monit. 2019;25:10164–10172. doi:10.12659/MSM.919112
98. Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127(15):1576–1584. doi:10.1161/CIRCULATIONAHA.112.000936
99. Peng K, Shen YP, Ying YY, et al. Perioperative dexmedetomidine and 5-year survival in patients undergoing cardiac surgery. Br J Anaesth. 2021;127(2):215–223. doi:10.1016/j.bja.2021.03.040
100. Forget P, Aguirre JA, Bencic I, Borgeat A, Cama A. How anesthetic, analgesic and other non-surgical techniques during cancer surgery might affect postoperative oncologic outcomes: a summary of current state of evidence. Cancers. 2019;11(5). doi:10.3390/cancers11050592
101. Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev. 2017;36(1):159–177. doi:10.1007/s10555-016-9647-8
102. Owusu-Agyemang P, Cata JP, Kapoor R, Zavala AM, Williams UU, Van Meter A. An analysis of the survival impact of dexmedetomidine in children undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia. 2018;35(1):435–440. doi:10.1080/02656736.2018.1506167
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.