Back to Journals » OncoTargets and Therapy » Volume 13
The Role of CCL20-CCR6 Axis in Ovarian Cancer Metastasis
Authors Liu W , Wang W, Zhang N, Di W
Received 3 September 2020
Accepted for publication 25 November 2020
Published 11 December 2020 Volume 2020:13 Pages 12739—12750
DOI https://doi.org/10.2147/OTT.S280309
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Wan Liu,1,2,* Wenjing Wang,1,2,* Ning Zhang,1,2 Wen Di1– 3
1Department of Obstetrics and Gynecology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, People’s Republic of China; 2Shanghai Key Laboratory of Gynecologic Oncology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, People’s Republic of China; 3State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wen Di; Ning Zhang
Department of Obstetrics and Gynecology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, 160 Pujian Road, Pudong New District, Shanghai 200127, People’s Republic of China
Email [email protected]; [email protected]
Background: Chemokine networks play a key and complex role in tumor progression. CCL20 and its unique receptor CCR6 have been reported to mediate malignant biological activities in various cancers, but their role in ovarian cancer metastasis remains unclear.
Purpose: Our study aims to explore the effect of CCL20-CCR6 axis on ovarian cancer metastasis and its potential mechanism.
Methods: The transwell assay was used to detect the cell migration and invasion after CCL20 treatment. The CCK-8 assay was used to detect the cell viability after CCL20 treatment and CCR6 depletion. The mRNA and protein expression were assayed through qRT-PCR and Western blotting. The siRNAs and CRISPR-Cas9 system were adopted to suppress CCR6 expression. Intraperitoneal xenograft mouse model was constructed to test the pro-metastasis effect of CCL20-CCR6 axis in vivo. The differentially expressed genes induced by CCL20 were identified through RNA-sequencing, and immunohistochemistry staining was used to detect their protein expression in tumor tissues.
Results: Our results revealed that CCL20 treatment selectively promoted the migration and invasion of CCR6high ovarian cancer cells, but had no effect on CCR6low cells. Blockade of CCR6 expression effectively reversed the cell migration and invasion induced by CCL20 stimulation. Animal experiment proved that CCL20-CCR6 axis mediated ovarian cancer metastasis in vivo. The differentially expressed genes after CCL20 stimulation were associated with metastasis, and CCL20 induced an increased expression of CDH2 and VCAN and decreased CDH1 expression in cancer cells. Moreover, CCL20 stimulated the expression of N-cadherin and versican in tumor tissues and inhibited the expression of E-cadherin, while CCR6 knockout successfully blocked the expression changes.
Conclusion: Our findings revealed that CCL20-CCR6 axis promotes ovarian cancer metastasis both in vivo and in vitro, probably through increasing cancer cell adhesion and epithelial–mesenchymal transition. Blockade of CCL20-CCR6 axis might become a novel anti-tumor therapeutic target for ovarian cancer.
Keywords: CCL20, CCR6, ovarian cancer, metastasis, transcriptional regulation
Introduction
In 2020, the mortality rate of ovarian cancer ranks fifth among American women.1 Due to the lack of effective screening and early clinical diagnosis methods, more than 70% of patients are diagnosed as advanced stage and often accompanied by extensive cancer metastasis.2,3 The current standard treatment for ovarian cancer is cytoreductive surgery followed by platinum and taxane chemotherapy.4 Although the initial response rate of chemotherapy is up to 80%, more than 70% of patients will still experience cancer recurrence.5 In the past decade, targeted drugs have been widely tested in clinical trials for the treatment of recurrent ovarian cancer, and some targeted drugs have been approved by the United States Food and Drug Administration (FDA) and/or European Medicine Agency for clinical use.6 Such targeted drugs include angiogenesis inhibitors (bevacizumab), poly (ADP-ribose) polymerase (PARP) inhibitors (olaparib, rucaparib and niraparib), neurotrophic tropomyosin receptor kinase (NTRK) inhibitors (entrectinib and larotrectinib) and immune checkpoint inhibitors (pembrolizumab). Several targeted drugs have improved the prognosis of patients to a certain extent and showed less side effects than chemotherapy,7,8 but there are some limitations in application. For example, olaparib is only recommended for the treatment of ovarian cancer patients with a BRCA1/2 mutation and entrectinib only for patients with NTRK fusion-positive solid tumors,9–11 and the efficacy of pembrolizumab as a single-agent therapy is very limited in treatment of ovarian cancer.12 Therefore, it is urgent to further investigate the mechanisms underlying the metastasis and aggressiveness of ovarian cancer and develop novel antitumor strategies for ovarian cancer.
Among various factors that affect tumor progression, one of the important factors is the chemokine superfamily. Members of this family include a large number of low-molecular-weight (8~14 kDa) chemotactic cytokines, which exert various biological functions by activating seven transmembrane domain G protein-coupled receptors on target cells.13–15 To date, approximately 50 chemokine ligands and 20 receptors have been identified. In malignant tumors, chemokine receptors are not only expressed in immune cells in the tumor microenvironment, but also in tumor cells and endothelial cells.16,17 Therefore, in addition to recruit anti- or pro-tumor immune cells to the tumor site, chemokines can also directly act on tumor cells and endothelial cells to regulate tumor cell growth, angiogenesis, invasiveness and metastasis.18–20 Different cytokines can play different roles in different types of cancers. For instance, CCL5 derived from ovarian cancer stem cells could recruit Treg cells to the tumor site and thereby facilitating tumor metastasis,20,21 whereas CCL14 could promote hepatocellular carcinoma (HCC) cell apoptosis by inhibiting cell cycle progression via Wnt/β-catenin signaling pathway, making it a favorable prognostic indicator in HCC patients.22,23 Despite increasing studies exploring the functions of chemokines in tumors, knowledge about the complex roles of chemokines in different types of tumors remains limited, and further studies should be focused on deciphering the roles and mechanisms of specific chemokines in specific types of tumors.
Recent studies have shown that the CCL20-CCR6 axis plays a key role in tumor progression. CCL20 is a 9 kDa chemokine and the only known ligand for CCR6 receptor.24 Non-tumor cells in the tumor microenvironment, such as stromal cells in giant cell tumor of bone, astrocytes in glioblastoma, and tumor-associated macrophages in melanoma are important sources of CCL20, which can recruit suppressive immune cells to the tumor site to maintain an immunosuppressive microenvironment, or act on endothelial cells to promote angiogenesis.25–30 In addition, CCL20 can also directly mediate the migration and/or invasion process of various cancer cells, including lung adenocarcinoma, cholangiocarcinoma, colon cancer and HCC,31–34 but its role in ovarian cancer metastasis remains unclear. Our previous studies have demonstrated that classically activated macrophages after cisplatin stimulation promoted the migration of ovarian cancer cells through the CCL20-CCR6 axis.35 In this study, we further verified the role of the CCL20-CCR6 axis in the metastasis of ovarian cancer and explored its related molecular mechanisms.
Materials and Methods
Cell Culture
The human ovarian epithelial cell line IOSE and human ovarian cancer cell lines A2780, SKOV3, HEY and ES2 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in DMEM/high glucose medium (HyClone, South Logan, UT, USA) containing 10% fetal bovine serum (FBS) (Gibco, Gaithersburg, MD, USA) and 1% penicillin-streptomycin (Sigma Aldrich, St Louis, MO, USA) in a humidified atmosphere with 5% CO2 at 37°C.
Transwell Migration and Invasion Assays
For migration assay, ovarian cancer cells were harvested and resuspended in FBS-free medium with recombinant human CCL20 protein (rhCCL20, 20 ng/mL) (Peprotech, Cranbury, NJ, USA). Cell suspensions (A2780: 1.5 × 105 cells/200 μL, SKOV3, HEY and ES2: 1.5 × 104 cells/200 μL) were then placed into the upper chamber of transwell plates (8 μm; Corning, Corning, NY, USA). For invasion assays, 40 μL matrigel matrix (pre-diluted 1:5 with serum-free medium) (BD Biosciences, Franklin Lakes, NJ, USA) was placed into the upper chamber of transwell plates, and cell suspensions (A2780: 3 × 105 cells/200 μL, SKOV3, HEY and ES2: 3 × 104 cells/200 μL) were added 2 hours later. Subsequently, 700 μL medium containing 20 ng/mL rhCCL20 and 10% FBS was added into the lower chamber. After incubation for 24 hours (migration) or 72 hours (invasion) at 37°C, cells in the upper chamber were carefully removed. Migrated or invaded cells were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet (Sigma Aldrich), and quantified using an IX71 inverted microscope (Olympus Corp, Shinjuku-ku, Japan) at × 200 magnification.
Cell Counting Kit-8 (CCK-8) Cell Viability Assay
Ovarian cancer cells (4000/well) were cultured in 96-well plates with increasing concentrations of rhCCL20. At indicated time-points, 10 μL CCK-8 reagent (Dojindo, Kumamoto, Japan) was added for 2-hour incubation, and then the absorbance was measured using a microplate reader (Thermo Scientific, Waltham, MA, USA) at 450 nm. Concentration-response curves were created using GraphPad Prism 6.0 (La Jolla, CA, USA).
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) and reversely transcribed into cDNA using a PrimeScript RT Reagent Kit (Takara, Otsu, Japan). qRT-PCR was performed using QuantStudio™ Test Development Software (Thermo Scientific) with SYBR Green qPCR Master Mix (EZBioscience, Roseville, MN, USA). The data were analyzed using the 2−ΔΔCTmethod. Glyceraldehyde 3-phosphate dehydrogenase was used for data normalization. The sequences of primers were as follows: CCR6, forward: GCT TGA GCT ACT CTT TGG TTT C, reverse: CAA GCA CCA CAG CTA TGA TTA C; VCAN, forward: ACT GAA ACT TCC TAC GTA TGC A, reverse: CTC ACA AAG TGC ACC AAC ATA A; CDH1, forward: AGT CAC TGA CAC CAA CGA TAA T, reverse: ATC GTT GTT CAC TGG ATT TGT G; CDH2, forward: CGA TAA GGA TCA ACC CCA TAC A, reverse: TTC AAA GTC GAT TGG TTT GAC C. All primers were purchased from Sangon Biotech (Shanghai, China).
Western Blotting
Total protein was extracted using RIPA lysis buffer (Beyotime, Shanghai, China) containing 1% phenylmethanesulfonyl fluoride (Beyotime). Equal quantities of protein were electrophoresed in polyacrylamide gel (Beyotime), transferred onto PVDF membranes (Millipore, Billerica, MA, USA), and blocked in 5% bovine serum albumin for 1 hour. Antibodies used in Western blotting were CCR6 (Abcam, Cambridge, UK) and β-actin (Sigma-Aldrich). Species-specific secondary anti-mouse and anti-rabbit antibodies (LI-COR, Lincoln, NE, USA) were used to visualize the blots under the Odyssey imaging system (LI-COR).
Cell Transfection
CCR6-specific siRNAs (siCCR6-1: 5ʹ-GCUCCGAUCCAGAACACUATT-3ʹ, siCCR-2: 5ʹ-GGGCAGAAGUUCAGAAACUTT-3ʹ) and negative control siRNA (siRNA-NC: 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ) were constructed by Genephama Biotechnologies (Shanghai, China). A2780 and SKOV3 cells were transiently transfected with siRNAs using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocols and were collected for further transwell migration and invasion experiments.
CRISPR-Cas9 System-Mediated Knockout of CCR6
The construction of transfer plasmids (lentiCRISPRv2) and packaging plasmids (delta8.9 and VSVG) was entrusted to Obio technology corporation (Shanghai, China). Briefly, a pair of annealed oligonucleotides was cloned into lentiCRISPRv2 plasmids to obtain lentiviral single-guide RNA (sgRNA) plasmids. The forward sequence of sgCCR6 was: ACC GTT ACT GTG CTC CTT GCA GG, and the reverse sequence was: AAA CCC TGC AAG GAG CAC AGT AA. The lentiviral sgRNA plasmids and packaging plasmids were co-transfected into 293T cells using lipofectamine 3000 (Invitrogen). After purification and ultracentrifugal concentration, the supernatant was collected for titer detection and ovarian cancer cell infection. To generate CCR6 knock-out cells, A2780 cells were cultured in supernatant containing lentiviral particles together with 5 μg/mL polybrene (Sigma-Aldrich). 72 hours after infection, 5 µg/mL puromycin was added for selection, and the surviving cells were collected for future monoclonal cell selection.
In vivo Experiment
All animal experiments followed the guidelines of the Institutional Animal Care and Use Committee of Ren Ji Hospital, and all experimental procedures were approved by the Institutional Animal Care and Use Committee of Ren Ji Hospital. For in vivo metastasis mouse model, 2 ×106 of wild type (WT) A2780 cells or CCR6 knock-out (KO) A2780 cells were injected into the abdominal cavity of 5-week-old female nude mice. One week after intraperitoneal inoculation of tumor cells, each group was further randomly divided into two groups and treated with PBS or 1.5 mg/kg rhCCL20 (dissolved in PBS) intraperitoneally, and twice a week afterwards. Thus experiment mice were divided into 4 groups (six mice per group) based on the types of inoculated cells and treatments: WT-PBS group (WT-A2780 cells inoculation and PBS treatment), WT-CCL20 group (WT-A2780 cells inoculation and rhCCL20 treatment), KO-PBS group (CCR6-KO-A2780 cells inoculation and PBS treatment) and KO-CCL20 group (CCR6-KO-A2780 cells inoculation and rhCCL20 treatment). Mice were euthanized 1 month after inoculation and the metastatic tumors were surgically excised and weighted.
Immunohistochemical (IHC) Staining and Hematoxylin and Eosin (H&E) Staining
Tumor tissues were fixed in 10% formalin for 24h, embedded in paraffin, and sectioned into 5 μm thick sections. After deparaffinization and rehydration, the sections were stained with hematoxylin and eosin. For immunohistochemistry, after neutralizing the endogenous peroxidase with 1% H2O2 and a specific protein block, the slices were incubated with antibodies against N-cadherin (Servicebio, Wuhan, China), E-cadherin (Servicebio) or versican (Boster, Wuhan, China) at 4°C overnight followed by incubating with HRP-conjugated anti-rabbit or anti-mouse antibody for 45 minutes. The sections were visualized with diaminobenzidine and counterstained with hematoxylin. Images were scanned at 40× magnification using a P-MIDI (3D HISTECH, Budapest, Hungary).
RNA-Sequencing
The RNA-sequencing was entrusted to OE biotechnology corporation (Shanghai, China). Briefly, total RNA was extracted using a mirVana miRNA Isolation Kit (Invitrogen), and the integrity of RNA was assessed using the Agilent Bio analyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). The RNA integrity number of all samples was ≥7, suggesting good RNA quality. The cDNA libraries were constructed using the TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, San Diego, CA, USA) in accordance with the manufacturer’s instructions, and then were sequenced on an Illumina Hiseq X Ten platform. P value <0.05 and fold change >1.5 or <0.67 was set as the threshold for significantly differential expression. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of differentially expressed genes was performed using an R program.
Statistical Analysis
All the experiments were performed in triplicate. Data were presented as mean ± standard deviation (SD) and analyzed by SPSS 21.0 software. The differences between two groups were analyzed by two-tailed Student’s t-test. One-way analysis of variance (ANOVA) was used for comparisons of more than two groups. P < 0.05 was considered statistically significant.
Results
Effects of CCL20 on Migration and Invasion of Ovarian Cancer Cell Lines
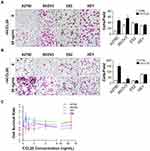
Our previous study revealed that cisplatin-stimulated classically activated macrophages promote ovarian cancer cell migration through CCL20-CCR6 axis.35 To further study the role of CCL20-CCR6 axis in ovarian cancer metastasis and its underlying mechanisms, we first examined the effects of rhCCL20 protein on the migration and invasion capacity as well as proliferation of ovarian cancer cell lines. The results showed that rhCCL20 protein significantly promoted the migration and invasion of A2780 and SKOV3 cells, but had no such pro-effect on ES2 and HEY cells (Figure 1A and B). In addition, rhCCL20 protein had no significant effect on the proliferation of A2780, SKOV3, ES2 and HEY cells (Figure 1C).
CCR6 Expression in Ovarian Cancer Cell Lines
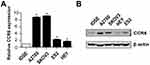
CCR6 is currently the only known receptor for CCL20 ligand. Our previous studies have shown that ovarian cancer patients with higher CCR6 expression had a worse clinical prognosis, and their gene expression profiles were highly enriched in gene sets associated with metastasis.35 Considering that rhCCL20 protein had inconsistent effects on the promotion of migration and invasion in different ovarian cancer cells, we tested the expression of its receptor CCR6 in ovarian cancer cell lines. The result (Figure 2A) showed that compared to human ovarian epithelial cell line IOSE, CCR6 mRNA was generally highly expressed in ovarian cancer cell lines (SKOV3, A2780, ES2 and HEY cells), and mRNA expression of CCR6 in SKOV3 and A2780 cells was significantly higher than that in ES2 and HEY cells. We further verified the protein expression of CCR6 in IOSE and ovarian cancer cells by Western blotting, and the result was consistent with CCR6 expression at the mRNA level (Figure 2B). Taken all together, we can conclude that rhCCL20 protein selectively promotes the migration and invasion of CCR6high ovarian cancer cells (A2780 and SKOV3 cells), but has no such effect on CCR6low ovarian cancer cells (ES2 and HEY cells).
Blockade of CCR6 Reverses CCL20-Mediated Migration and Invasion of Ovarian Cancer Cells
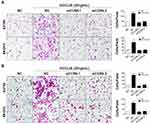
To further confirm the role of CCR6 activation in ovarian cancer cell migration and invasion, two CCR6-specific siRNAs (siCCR6-1 and siCCR6-2) were transfected into A2780 and SKOV3 cells to inhibit CCR6 expression. Our previous study proved that these two siRNAs were efficient to reduce CCR6 expression by at least 50% and did not affect the viability of ovarian cancer cells.35 Here the result showed that rhCCL20 protein significantly promoted migration and invasion of ovarian cancer cells transfected with negative siRNA (siRNA-NC), whereas knockdown of CCR6 by siCCR6 significantly decreased ovarian cancer cell migration (Figure 3A) and invasion (Figure 3B) induced by rhCCL20 protein, indicating that CCR6 activation by CCL20 mediated the migration and invasion of ovarian cancer cells.
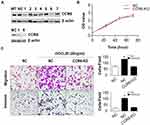
In addition, we used the CRISPR-Cas9 system to construct sgCCR6 lentivirus and transfected it into A2780 cells to construct CCR6 knock-out (CCR6-KO) A2780 cells. After lentivirus transfection and puromycin selection, we collected 7 monoclonal cell lines. Western blotting was used to verify the knockout effect. The result showed that the expression of CCR6 in No.6 monoclonal cells was significantly suppressed (Figure 4A); therefore, we chose No.6 monoclonal cells as CCR6-KO cells for subsequent experiments. Consistent with the above results, knockout of CCR6 by sgCCR6 lentivirus did not affect viability of A2780 cells (Figure 4B), but did inhibit ovarian cancer cell migration and invasion promoted by rhCCL20 protein (Figure 4C), further confirming the conclusion that CCL20-CCR6 axis mediates the migration and invasion of ovarian cancer cells.
CCL20-CCR6 Axis Mediates Ovarian Cancer Metastasis in vivo
To further investigate the pro-metastasis effect of CCL20-CCR6 axis on ovarian cancer in vivo, we randomly divided nude mice into 4 groups based on the inoculated cells and treatments: WT-PBS group (WT-A2780 cells inoculation + PBS treatment), WT-CCL20 group (WT-A2780 cells inoculation + rhCCL20 treatment), KO-PBS group (CCR6-KO-A2780 cells inoculation + PBS treatment) and KO-CCL20 group (CCR6-KO-A2780 cells inoculation + rhCCL20 treatment). Mice were euthanized 1 month after inoculation and the tumors were surgically excised (n = 6/group). There were no significant differences in body weight among the four groups throughout the experiment (Figure 5A). Strikingly, the mice in WT-CCL20 group exhibited obvious tumor metastasis compared with that in WT-PBS group, whereas no significant differences were observed between KO-PBS and KO-CCL20 groups (Figure 5B). Next, nude mice were dissected out of abdominal metastatic tumors (Figure 5C), and the number (Figure 5D) and weight (Figure 5E) of the tumors were counted and compared. Results showed that rhCCL20 protein could significantly promote the number and weight of WT-A2780 tumors in the abdominal cavity of nude mice, but it had no such effect on CCR6-KO-A2780 tumors. Collectively, these data suggest that CCL20-CCR6 axis facilitates ovarian cancer metastasis in vivo.
Differentially Expressed Genes Elicited by CCL20 in Ovarian Cancer Cells
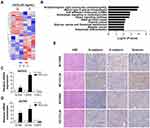
To further study the mechanism of CCL20-mediated migration and invasion of ovarian cancer cells, we used RNA sequencing to examine the effect of rhCCL20 treatment on gene expression profiles in ovarian cancer cells. The data have been deposited in NCBI’s gene expression omnibus (GEO) and is accessible through GEO series accession number GSE161799 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE161799). The differentially expressed genes are shown in Figure 6A and Table S1. KEGG analysis of these differential genes revealed a significant enrichment in cell adhesion molecules (CAMs) (Figure 6B). VCAN and CDH2, whose protein products play a crucial role in the regulation of cell motility, cell-cell adhesion and epithelial–mesenchymal transition (EMT), were greatly up-regulated after rhCCL20 treatment (Figure 6C and D). In addition, rhCCL20 exhibited a profound reduction in the transcription of CDH1, whose loss is considered the key process of EMT (Figure 6C and D). Moreover, IHC analysis of tumor tissues from in vivo experiment revealed that compared with WT-PBS group, N-cadherin and versican, the products of CDH2 and VCAN, were obviously increased in the tumors of WT-CCL20 group, and the product of CDH1 E-cadherin decreased dramatically. However, no significant differences were observed in the expression of N-cadherin, E-cadherin and versican between KO-PBS group and KO-CCL20 group (Figure 6E). Taken together, these data indicate that CCL20 promotes ovarian cancer cell migration and invasion through promoting the motility and epithelial–mesenchymal transition in ovarian cancer cells.
Discussion
Emerging evidence suggests that chemokines and their receptors are associated with the pathogenesis, progression and metastasis of various types of tumors, including ovarian cancer.36 Up to now, several achievements have been made on cancer targeted therapy targeting the chemokine system. For example, mogamulizumab, an anti-CCR4 antibody, is used to treat patients with relapsed and refractory adult T cell leukemia-lymphoma.37 Plerixafor, a CXCR4 antagonist, has been approved for the mobilization of hematopoietic stem cells for transplantation in non-Hodgkin’s lymphoma or multiple myeloma.38 Therefore, in-depth study about the roles and mechanisms of chemokine networks can help develop novel targeted drugs for cancer treatment. In this study, we studied the effect of CCL20-CCR6 axis on metastasis of ovarian cancer and explored its potential mechanisms.
Our previous study revealed that classically activated macrophages after cisplatin stimulation promoted ovarian cancer cell migration, and CCL20 played a vital role in the process.35 In this study, we firstly treated ovarian cancer cells with rhCCL20 protein and found that rhCCL20 selectively promoted the migration and invasion of A2780 and SKOV3 cells, but did not affect ES2 and HEY cells. Since CCR6 is currently the only receptor for CCL20, and our previous study revealed a potential correlation between CCR6 expression and ovarian cancer metastasis, therefore we tested CCR6 expression in ovarian cancer cell lines. The result showed that CCR6 was generally highly expressed in ovarian cancer cell lines compared to normal ovarian epithelial cells IOSE, and its expression in A2780 and SKOV3 cells was significantly higher than that in ES2 and HEY cells. Furthermore, blockade of CCR6 by CCR6-specific siRNAs and sgCCR6 lentivirus effectively reversed cell migration and invasion induced by CCL20 stimulation in A2780 and SKOV3 cells. Moreover, rhCCL20 strongly promoted the tumorigenesis of WT-A2780 metastatic tumors in the abdominal cavity of nude mice, but had no effect on CCR6-KO-A2780 metastatic tumors. Taken all above, we can conclude that CCL20-CCR6 axis mediates ovarian cancer metastasis both in vivo and in vitro.
Recent studies have shown that the CCL20-CCR6 axis plays an important role in cancer progression. The expression of CCR6 is significantly up-regulated in various tumor tissues or tumor cells, including laryngeal cancer, thyroid cancer, colon cancer, gastric cancer and liver cancer.33,34,39–41 In colon cancer, CCR6 expression is closely related to lymph node status and distant metastasis: CCR6 expression is higher in lymph node positive tumor tissues and the highest in metastatic cases;33 In gastric cancer, high expression of CCR6 is significantly correlated with cancer relapse and poor overall survival.41 CCL20, the ligand of CCR6, can directly regulate the malignant biological activities of tumor cells. Studies have shown that the CCL20-CCR6 axis promotes cancer cell proliferation, invasion and/or migration, probably by promoting AKT,42,43 MAPK,43 PI3K or NFκB signaling pathways,39,42,43 or by promoting the expression of EMT-related proteins or matrix metalloproteinases (MMPs).33,34,40 In our study, the RNA-sequencing analysis demonstrated that rhCCL20 treatment did not cause a widespread change of gene transcriptome in ovarian cancer cells, and the differentially expressed genes were enrichment in cell adhesion molecules. qRT-PCR confirmed that the crucial genes that mediate cell motility, cell-cell adhesion and epithelial–mesenchymal transition, including VCAN (versican) and CDH2 (N-cadherin), were greatly up-regulated after rhCCL20 treatment. In addition, the expression of CDH1 (E-cadherin), whose loss is considered the key process of epithelial–mesenchymal transition, was strikingly suppressed after rhCCL20 treatment. Furthermore, immunohistochemical analysis of tumor tissues from in vivo experiment verified that rhCCL20 significantly promoted the expression of N-cadherin and versican in metastatic tumors, and inhibited the expression of E-cadherin, while knockout of CCR6 successfully blocked the expression changes induced by rhCCL20. Collectively, the result suggests that CCL20 could promote the expression of EMT markers in ovarian cancer cells.
It is well known that EMT is a crucial mechanism of tumor metastasis. EMT is a reversible dynamic process that allows epithelial cells temporarily shedding their epithelial cell phenotype and acquiring the mesenchymal cell phenotype, which is characterized by upregulation of mesenchymal markers and molecules that induce EMT, and downregulation of epithelial markers.44 The expression changes of EMT-related genes could lead to enhanced migration and invasion capacity, increased resistance to apoptosis, and a remarkable increased production of extracellular matrix components.45 In ovarian cancer, EMT contributes to chemotherapy resistance and stemness, making it a potential target to reverse therapy resistance. For example, blockade of type II TGF-β receptor could reverse EMT and thereby improving the response to carboplatin in metastatic ovarian cancer models.46
In conclusion, the above results suggest that CCL20-CCR6 axis promotes the metastasis of ovarian cancer, probably through increasing cancer cell mobility and epithelial–mesenchymal transition. Combined with our previous research, CCL20-CCR6 blockade may provide a novel therapeutic strategy for ovarian cancer treatment.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81974454 and 81772770) and the Shanghai Municipal Key Clinical Specialty.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Koonings PP, Campbell K, Mishell DR
3. Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–44. doi:10.1097/01.pgp.0000101080.35393.16
4. Piver MS. Treatment of ovarian cancer at the crossroads: 50 years after single-agent melphalan chemotherapy. Oncology. 2006;20:1156, 1158.
5. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–1388. doi:10.1016/S0140-6736(13)62146-7
6. Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69:280–304.
7. Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a Phase 3 randomised trial. Lancet Oncol. 2015;16(8):928–936. doi:10.1016/S1470-2045(15)00086-8
8. Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised Phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi:10.1016/S1470-2045(14)70391-2
9. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505. doi:10.1056/NEJMoa1810858
10. Al-Salama ZT, Keam SJ. Entrectinib: first global approval. Drugs. 2019;79(13):1477–1483. doi:10.1007/s40265-019-01177-y
11. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three Phase 1–2 trials. Lancet Oncol. 2020;21(2):271–282. doi:10.1016/S1470-2045(19)30691-6
12. Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the Phase II KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080–1087. doi:10.1093/annonc/mdz135
13. Do HTT, Lee CH, Cho J. Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers. 2020;12:287.
14. Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006;25(3):357–371. doi:10.1007/s10555-006-9003-5
15. Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12(1):593–633. doi:10.1146/annurev.iy.12.040194.003113
16. Jones VS, Huang RY, Chen LP, Chen ZS, Fu L, Huang RP. Cytokines in cancer drug resistance: cues to new therapeutic strategies. Biochim Biophys Acta. 2016;1865:255–265.
17. Vilgelm AE, Richmond A. Chemokines modulate immune surveillance in tumorigenesis, metastasis, and response to immunotherapy. Front Immunol. 2019;10:333. doi:10.3389/fimmu.2019.00333
18. O’Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. 2008;409:635–649.
19. Lupi LA, Delella FK, Cucielo MS, et al. P-MAPA and interleukin-12 reduce cell migration/invasion and attenuate the toll-like receptor-mediated inflammatory response in ovarian cancer SKOV-3 cells: a Preliminary Study. Molecules. 2019;25(1):5. doi:10.3390/molecules25010005
20. Silveira HS, Lupi LA, Romagnoli GG, et al. P-MAPA activates TLR2 and TLR4 signaling while its combination with IL-12 stimulates CD4+ and CD8+ effector T cells in ovarian cancer. Life Sci. 2020;254:117786. doi:10.1016/j.lfs.2020.117786
21. You Y, Li Y, Li M, et al. Ovarian cancer stem cells promote tumour immune privilege and invasion via CCL5 and regulatory T cells. Clin Exp Immunol. 2018;191(1):60–73. doi:10.1111/cei.13044
22. Zhu M, Xu W, Wei C, et al. CCL14 serves as a novel prognostic factor and tumor suppressor of HCC by modulating cell cycle and promoting apoptosis. Cell Death Dis. 2019;10(11):796. doi:10.1038/s41419-019-1966-6
23. Zhang X, Wan JX, Ke ZP, Wang F, Chai HX, Liu JQ. TMEM88, CCL14 and CLEC3B as prognostic biomarkers for prognosis and palindromia of human hepatocellular carcinoma. Tumour Biol. 2017;39:1010428317708900. doi:10.1177/1010428317708900
24. Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta. J Immunol. 1997;158:1033–1036.
25. Chenglong Z, Dongsheng W, Liang T, et al. Stromal cell-derived CCL20 promotes tumor progression and osteolysis in giant cell tumor of bone. Cell Physiol Biochem. 2018;51:2472–2483. doi:10.1159/000495903
26. Jin P, Shin S-H, Chun Y-S, et al. Astrocyte-derived CCL20 reinforces HIF-1-mediated hypoxic responses in glioblastoma by stimulating the CCR6-NF-κB signaling pathway. Oncogene. 2018;37(23):3070–3087. doi:10.1038/s41388-018-0182-7
27. Samaniego R, Gutierrez-Gonzalez A, Gutierrez-Seijo A, et al. CCL20 expression by tumor-associated macrophages predicts progression of human primary cutaneous melanoma. Cancer Immunol Res. 2018;6(3):267–275. doi:10.1158/2326-6066.CIR-17-0198
28. Zhu CC, Chen C, Xu ZQ, et al. CCR6 promotes tumor angiogenesis via the AKT/NF-kappaB/VEGF pathway in colorectal cancer. Biochim Biophys Acta Mol Basis Dis. 2018;1864:387–397. doi:10.1016/j.bbadis.2017.10.033
29. Walch-Ruckheim B, Mavrova R, Henning M, et al. Stromal fibroblasts induce CCL20 through IL6/C/EBPbeta to support the recruitment of Th17 cells during cervical cancer progression. Cancer Res. 2015;75:5248–5259. doi:10.1158/0008-5472.CAN-15-0732
30. Chen KJ, Lin SZ, Zhou L, et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6:e24671. doi:10.1371/journal.pone.0024671
31. Zhang XP, Hu ZJ, Meng AH, Duan GC, Zhao QT, Yang J. Role of CCL20/CCR6 and the ERK signaling pathway in lung adenocarcinoma. Oncol Lett. 2017;14:8183–8189.
32. Win Maung HM, Chan-On W, Kunkeaw N, Khaenam P. Common transcriptional programs and the role of chemokine (C-C motif) ligand 20 (CCL20) in cell migration of cholangiocarcinoma. EXCLI J. 2020;19:154–166.
33. Kapur N, Mir H, Clark Iii CE, et al. CCR6 expression in colon cancer is associated with advanced disease and supports epithelial-to-mesenchymal transition. Br J Cancer. 2016;114(12):1343–1351. doi:10.1038/bjc.2016.113
34. Du D, Liu Y, Qian H, et al. The effects of the CCR6/CCL20 biological axis on the invasion and metastasis of hepatocellular carcinoma. Int J Mol Sci. 2014;15(4):6441–6452. doi:10.3390/ijms15046441
35. Liu W, Wang W, Wang X, Xu C, Zhang N, Di W. Cisplatin-stimulated macrophages promote ovarian cancer migration via the CCL20-CCR6 axis. Cancer Lett. 2020;472:59–69. doi:10.1016/j.canlet.2019.12.024
36. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–572.
37. Fuji S, Utsunomiya A, Inoue Y, et al. Outcomes of patients with relapsed aggressive adult T-cell leukemia-lymphoma: clinical effectiveness of anti-CCR4 antibody and allogeneic hematopoietic stem cell transplantation. Haematologica. 2018;103(5):e211–e214. doi:10.3324/haematol.2017.184564
38. Micallef IN, Stiff PJ, Nademanee AP, et al. Plerixafor plus granulocyte colony-stimulating factor for patients with non-hodgkin lymphoma and multiple myeloma: long-term follow-up report. Biol Blood Marrow Transplant. 2018;24(6):1187–1195. doi:10.1016/j.bbmt.2018.01.039
39. Eryong L, Jili S, Yanhui Z, Chao Z, Yuehui W. CCL20/CCR6 promotes cell proliferation and metastasis in laryngeal cancer by activating p38 pathway. Biomed Pharmacother. 2017;85:486–492. doi:10.1016/j.biopha.2016.11.055
40. Zeng W, Chang H, Ma M, Li Y. CCL20/CCR6 promotes the invasion and migration of thyroid cancer cells via NF-kappa B signaling-induced MMP-3 production. Exp Mol Pathol. 2014;97(1):184–190. doi:10.1016/j.yexmp.2014.06.012
41. Zhang XG, Song BT, Liu FJ, Sun D, Wang KX, Qu H. CCR6 overexpression predicted advanced biological behaviors and poor prognosis in patients with gastric cancer. Clin Transl Oncol. 2016;18(7):700–707. doi:10.1007/s12094-015-1420-x
42. Brand S, Olszak T, Beigel F, et al. Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells. J Cell Biochem. 2006;97(4):709–723. doi:10.1002/jcb.20672
43. Wang B, Shi L, Sun X, Wang L, Wang X, Chen C. Production of CCL20 from lung cancer cells induces the cell migration and proliferation through PI3K pathway. J Cell Mol Med. 2016;20:920–929. doi:10.1111/jcmm.12781
44. Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12:361–373. doi:10.1007/s11684-018-0656-6
45. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84.
46. Newsted D, Banerjee S, Watt K, et al. Blockade of TGF-β signaling with novel synthetic antibodies limits immune exclusion and improves chemotherapy response in metastatic ovarian cancer models. Oncoimmunology. 2019;8(2):e1539613. doi:10.1080/2162402X.2018.1539613
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.