Back to Journals » Cancer Management and Research » Volume 12
The Role of Adjuvant Chemoradiotherapy Over Radiotherapy After R0 Resection for Stage II-III Esophageal Squamous Cell Carcinoma
Authors Song T , Chen P, Fang M, Zhang X, Du D, Wu S
Received 30 September 2019
Accepted for publication 22 January 2020
Published 5 March 2020 Volume 2020:12 Pages 1631—1639
DOI https://doi.org/10.2147/CMAR.S232930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Tao Song,1,* Peng Chen,2,* Min Fang,1 Xuebang Zhang,3 Dexi Du,4 Shixiu Wu2
1Department of Radiation Oncology, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, Guangdong, People’s Republic of China; 3Department of Radiation Oncology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 4Department of Radiation Oncology, The Central Hospital of Lishui City, Lishui, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shixiu Wu
Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 113, Baohe Avenue, Longgang District, Shenzhen, Guangdong, People’s Republic of China
Tel +86-755-66619865
Email [email protected]
Purpose: This study compared the effectiveness and safety of postoperative concurrent chemoradiotherapy (POCRT) containing paclitaxel (PTX) and cisplatin (DDP) with postoperative radiotherapy (PORT) after R0 resection for stage II–III thoracic esophageal squamous cell carcinoma (TESCC).
Materials and Methods: After propensity score matching (PSM) analysis, 87 TESCC patients treated with PORT were matched 1:1 to 87 patients who received POCRT between July 2012 and December 2018. Radiotherapy was delivered at a dose of 200 cGy per day to a total dose of 5000 cGy. Concurrent chemotherapy consisted of DDP (25 mg/m2) for 3 days plus PTX (135 mg/m2) on day 1 every 3 weeks.
Results: Patient- and disease-related characteristics were well-balanced between the two groups. The median overall survival (OS) and disease-free survival (DFS) times were 39.2 and 31.0 months, respectively. The 5-year OS and DFS rates were 31.9% and 19.1% in the PORT group and 45.1% and 35.1% in the POCRT group, respectively. Statistical significance was demonstrated by comparing OS and DFS (P=0.022 and 0.016, respectively). Additionally, subgroup analysis revealed that in node positive TESCC patients, the POCRT group was significantly different from the PORT group regarding OS and DFS (P=0.049 and 0.039, respectively). POCRT decreased distant metastasis over PORT (P=0.044) with manageable toxicities. Multivariate analysis revealed that aside from factors associated with tumor stages, treatment modality was another strong prognostic factor for both OS and DFS (P=0.015 and 0.010, respectively).
Conclusion: Stage II–III TESCC patients could benefit from POCRT with manageable toxicities. Future well-designed prospective studies are highly warranted to confirm the findings in our report.
Keywords: esophageal squamous cell carcinoma, surgery, chemoradiotherapy, survival
Background
Esophageal carcinoma (EC) remains difficult to cure with poor overall 5-year survival rates for locally advanced stages.1,2 Traditionally, esophagectomy plays a pivotal role in the treatment of early-stage and localized EC, but as a single modality, surgical resection was associated with great in-hospital risk, an unacceptably high local recurrence rate and a poor survival rate in a series of randomized controlled trials,3–5 leading to the integration of radiotherapy (RT) and chemotherapy as neoadjuvant or adjuvant chemoradiotherapy modalities. In comparison with numerous studies investigating the efficacy of neoadjuvant chemoradiotherapy (nCRT), fewer studies have evaluated the effectiveness of postoperative concurrent chemoradiotherapy (POCRT) for patients with EC so far, especially for thoracic esophageal squamous cell carcinoma (TESCC). However, it was reported that there are differences in tumor histology and practice regarding nCRT or POCRT between eastern and western countries. It is indicated that adjuvant therapy is more commonly used in Asia than in Europe and North America based on the results from a questionnaire administered in 2009 by 250 participants representing 41 countries across six continents.6 Ten years later in 2019, results from an international survey still indicated that there was no uniform, worldwide strategy for surgical treatment of EC.7 Additionally, given the growing recognition that even after being clinically well staged using ultrasound, some T2N0 esophageal cancers (between 20–25%) may be upstaged to pathologic T3 and/or lymph node-positive disease; thus, numerous patients could be referred for postoperative therapy for TESCC.8
Compared with the classic combination of 5-Fu and cisplatin (DDP) with RT for EC, preclinical data have shown that paclitaxel (PTX) could enhance the radiation sensitivity of tumor cells, potentiate the antitumor response rate and increase the therapeutic ratio of RT.9 Furthermore, ease of administration and decreased gastrointestinal toxic effects were also key advantages with platinum and paclitaxel therapy for EC when PTX was substituted for 5-Fu in a series of prospective studies.10,11,12,13
Previously, Chen et al reported that in patients with node positive TESCC, POCRT was significantly more effective than postoperative radiotherapy (PORT) in increasing overall survival (53.5 vs 41.7 months, respectively; P=0.030) and decreasing the rates of metastasis and recurrence. Although severe toxic reactions were more common in POCRT than in PORT, patients could generally tolerate POCRT.14 It is noteworthy that some imbalances in the distribution of confounders, such as sex and number of positive lymph nodes, existed between the POCRT and PORT groups in this report. Therefore, we further performed this propensity score matching (PSM) analysis to evaluate the effectiveness and safety of POCRT vs PORT for stage II–III TESCC in four cancer centers.
Patients and Methods
Patients
A retrospective review of 356 TESCC patients treated from July 2012 to December 2018 was conducted at the four cancer centers (Figure S1). The criteria for inclusion in our review included the following: I) pathological diagnosis of TESCC; II) pathological stages II–III disease according to the International Union Against Cancer (UICC, 2009) TNM stage criteria; III). esophagectomy with 2-field lymphadenectomy (2FL, consisting of mediastinal and upper abdominal nodes); IV) ECOG PS of at least 2; V) no evidence of severe organ dysfunction; and VI) adequate bone marrow, renal, hepatic, cardiac, and respiratory function. The choice for postoperative treatment is mainly based on the doctor’s choice and the patient’s willingness. 97 patients were excluded based on the inclusion criteria. After correcting for bias in the baseline factors, 174 TESCC patients (87 patients in each group) were included in the final analysis (Table 1). This study was approved by the institutional review board of Hangzhou Cancer Hospital (HZCH-2016-02). Written informed consent was obtained from all participants which included toxicities, survival outcomes and other medical records relevant to treatment. We confirmed that this report was conducted in accordance with the Declaration of Helsinki.
 |
Table 1 Patient and Tumor Characteristics Before and After Propensity Score Matching |
Treatment Details
Surgery
All patients underwent transhiatal esophagectomy with anastomosis in the neck. The entire thoracic esophagus was resected from the level of the clavicles to the gastric cardia with an R0 margin, which was defined as the microscopic negative margin of the UICC criteria. The total group of patients received 2FL and all technically accessible lymph nodes were removed. Four to eight weeks later (median time: four weeks), available patients received PORT or POCRT.
Radiation Treatment
All patients received intensity-modulated radiotherapy. The definition of clinical target volume (CTV) was defined as the primary esophageal tumor bed and the drainage areas of the lymph nodes at high risk. The upper TESCC, it included station 1, 2, 4, 5 and 7 involved lymph nodes. The middle TESCC included stations 2, 4, 5 and 7 lymph nodes. The lower TESCC included stations 7 and 8, cardia and left gastric lymph nodes. In addition to this definition, CTV was manually modified to include the 3 cm margin above and below the anastomotic site. The planning tumor volume (PTV) was defined as the CTV plus an additional 5–8 mm margin around the CTV. Dose-volume constraints of normal tissues and organs at risk (OARs) were described previously.15 The total radiation dose was 50 Gy in 25 fractions within 5 weeks. RT was interrupted for grade ≥3 esophagitis, grade 3 neutropenia with fever, or grade 4 neutropenia. RT was restarted when toxicities recovered to grade ≤2.
Chemotherapy
For TESCC patients who were treated with POCRT, two cycles of concurrent chemotherapy were administered. PTX 135 mg/m2 was delivered on day 1 of the first and fourth weeks and DDP 25 mg/m2 was administered on days 1–3 and days 22–24 of RT. Chemotherapy was delayed for acute toxicities until recovery to grade ≤2, and/or the dose was reduced for grade 3 or higher hematological toxicity. PTX was reduced to 80% in the second course if either of the following occurred: grade 3 neutropenia with fever or grade 4 neutropenia. Granulocyte colony-stimulating factor (G-CSF) was used to treat the occurrence of febrile neutropenia. If the creatinine clearance further decreased to less than 50 mL/min, the DDP dose was also reduced to 80%.
Evaluation and Follow-Up
Physician-reported hematological, esophageal and pulmonary toxicities were evaluated according to the common toxicity criteria for adverse events version 4.0 (CTCAE v4.0). Follow-up modalities included physical examination, routine blood tests, and enhanced computed tomography (CT). Integrated positron emission tomography/CT was performed based on the patient’s choice if clinically indicated. Patients were followed up every month during the first half of the year, every 2 months during the second half of the year, every 3 months during the second year, and then at 6-month intervals after 2 years. Treatment failure was defined as any sign of recurrent disease, which could be local, distant, and/or both.
Statistical Analysis
To reduce the imbalance in potential confounders between the POCRT group and PORT group, propensity score matching was applied to create two treatment cohorts with balanced distributions of baseline characteristics. The matching factors consisted of age, sex, ECOG PS, tumor location, differentiation, T stage and clinical stage (Table 1). N stage that was not statistically significant in the original cohort was removed. The method of using PSM between the two groups has been described previously.16 Only patients matched by propensity scores were included in the subsequent analyses.
All statistical analyses were performed using SPSS version 23.0 software (SPSS Inc., Chicago, IL). Overall survival (OS) was calculated as the time (in months) between the first day of esophagectomy and the date of the last follow-up or the date of death. Disease-free survival (DFS) was calculated from the date of surgery initiation to the date of documented tumor recurrence (radiologic or pathologic) or the date of the last follow-up for those remaining. OS and DFS were calculated using the Kaplan-Meier method and compared using the Log rank test. Categorical variables were compared using the χ2 or Fisher’s exact tests. The parameters were also analyzed with respect to OS and DFS using univariate and multivariate Cox regression analysis. Variables identified with a 2-sided P value <0.05 on univariate analysis were included in further multivariate analyses. All statistical tests were two-sided, with the threshold for significance set at P<0.05.
Results
Clinicopathologic background
After PSM, 87 TESCC patients with R0 resection in each group were available for outcome comparison (Table 1). There were no significant differences between the two groups regarding age, gender, ECOG PS, tumor location or histological differentiation. There was also no significant difference in pT, pN and clinical stage between the PORT group and the POCRT group.
Grade ≥3 Acute and Late Toxicities
In the POCRT group, five patients refused the second cycle of chemotherapy for occurring grade 4 leukocytopenia, and these patients also refused RT. 10 (12.6%) patients required a dose reduction in the second cycle for hematological toxicities. 81 patients completed RT, including four patients with radiation delay, one patient with severe esophagitis and one patient with grade 4 nausea/vomiting. Thus, 66 (75.9%) patients completed the POCRT on schedule.
Among the 87 patients in the PORT group, 75 (86.2%) patients received the full dose of RT, whereas 12 patients required radiation delay or dose reduction due to grade ≥3 toxicities. The difference in treatment compliance between the two groups was not statistically significant (P = 0.082).
All patients were evaluated for acute toxicities. In general, patients who received POCRT suffered more treatment toxicities than those who received PORT (Table 2). The incidence of grade 3 or higher leukocytopenia was significantly higher in the POCRT group compared with the PORT group (17.2% vs 3.4%, P=0.003; respectively). The incidences of grade ≥3 anemia and thrombocytopenia were 5.7% and 8.0% for the POCRT group, and 2.3% and 2.3% for the PORT group, respectively. For nonhematologic toxicity, nausea/vomiting also showed a significant difference between the POCRT group and the PORT group (10.3% for the POCRT group and 2.0% for the PORT group, P=0.009). Other causes of severe non-hematologic toxicities included anorexia, esophagitis, anastomosis and dysphagia. There were no adjuvant treatment-related toxic deaths during the treatment course. Late grade ≥3 toxicities occurred in 83 and 84 TESCC patients in the PORT group and POCRT group, respectively. Radiation-induced pneumonia was the most common late toxicity in both groups. However, no significant difference in the incidence of late toxic reactions was found between the two groups.
 |
Table 2 Acute and Late Grade ≥3 Adverse Events |
Survival Outcomes
The median follow-up period was 35.7 months (range, 4.0 to 72.0 months). Loss to follow-up occurred in four patients (one in the PORT group and three in the POCRT group; the overall follow-up rate, 97.7%). For all TESCC patients, the median OS was 39.2 months (95% CI: 30.9–47.3 months). The 1-, 3-, and 5-year OS rates for the PORT group were 81.1% (95% CI: 0.709–0.913), 44.9% (95% CI: 0.341–0.557), and 31.9% (95% CI: 0.211–0.427), respectively. The 1-, 3-, and 5-year OS rates for the POCRT group were 88.6% (95% CI: 0.819–0.953), 59.7% (95% CI: 0.493–0.701), and 45.1% (95% CI: 0.332–0.570), respectively. A statistically significant difference was demonstrated when comparing group survival times for OS (P=0.022, Figure 1A).
At the time of the last follow-up, treatment failure was evaluated in 65 (74.7%) patients in the PORT group and 50 (57.5%) patients in the POCRT group (P=0.016, Table 3). No significant difference was found between the two groups regarding local/regional recurrence (P=0.124). However, the POCRT group had a significant difference (P=0.044) compared to the PORT group in distant metastasis. The most frequent sites of distant metastases were the peritoneal cavity (10 in the PORT group and 5 in the POCRT group) and liver (9 in the PORT group and 5 in the POCRT group) for the entire group. Five patients in the PORT group and six patients in the POCRT group had both local/regional and distant failure; however, this difference did not reach statistical significance (P=0.646). The median disease-free survival (DFS) time for the entire cohort was 31.0 months. The 1-, 3-, and 5-year DFS rates for the PORT group were 76.1% (95% CI: 0.671–0.851), 35.6% (95% CI: 0.252–0.460), and 19.1% (95% CI: 0.097–0.285), respectively. The 1-, 3-, and 5-year DFS rates for the POCRT group were 81.8% (95% CI: 0.738–0.898), 50.5% (95% CI: 0.399–0.611), and 35.1% (95% CI: 0.226–0.476), respectively. There was also a statistically significant difference in DFS between the PORT group and the POCRT group (P=0.016, Figure 1B).
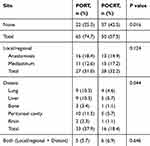 |
Table 3 Patterns of Treatment Failure |
For patients with negative lymph nodes (N0), the median OS and DFS times for the PORT group were 61.9 months and 41.6 months, respectively. The estimated median OS for the POCRT group was not reached, and the median DFS was 59.8 months. No statistically significant differences were observed in OS and DFS between the two groups (P=0.173 and 0.192, respectively, Figure 1C and D). In patients with positive lymph nodes (N+), the median OS and DFS times for the PORT group were 24.5 months and 20.3 months, respectively. The corresponding median OS and DFS times for the POCRT group were 36.3 months and 27.7 months, respectively. There were significant differences in OS and DFS between the two subgroups (P=0.049 and 0.039, respectively, Figure 1E and F).
Univariate and Multivariate Analysis
Univariate and multivariate analyses were performed to assess the predictive ability of each variable (Table 4). The results suggested that several covariates were significantly associated with OS: treatment modality (P=0.024), pT stage (P<0.001), pN stage (P=0.001), differentiation (P=0.002), tumor location (P=0.020) and clinical stage (P<0.001). The variables significantly associated with DFS were treatment modality (P=0.017), pT stage (P<0.001), pN stage (P=0.003), differentiation (P=0.001), tumor location (P=0.025) and clinical stage (P<0.001).
 |
Table 4 Predictive Factors of Overall Survival and Disease-Free Survival in Univariate and Multivariate Analysis |
Factors that were found to be significant (P<0.05) in univariate analysis were included in the multivariate analysis. Multivariate analysis revealed that except for pT, pN, differentiation and clinical stages, treatment modality (P=0.015 and 0.010, respectively) was another independent factor affecting OS and DFS (Table 4).
Discussion
In the current study, we compared the safety and efficiency of POCRT using PTX plus DDP versus PORT for stage II–III TESCC patients. After balancing basic factors through PSM, our results showed that adjuvant POCRT was significantly more effective than PORT. POCRT increased the OS and DFS rates, especially for patients with positive lymph nodes and decreased the rate of distant metastasis. Grade 3 or higher acute toxic reactions, including leukocytopenia and nausea/vomiting, were significantly more common in the POCRT group than in the PORT group. However, patients were able to tolerate these toxic reactions, and no significant differences in the incidence of late toxic reactions were found between the two groups. Additionally, multivariate analysis demonstrated that POCRT was an independent prognostic factor for survival outcomes.
The extent of lymphadenectomy and its effect on postoperative complications and long-term survival are still controversial in EC. Based on a meta-analysis comparing 3-field lymphadenectomy (3FL) with 2FL in over 7000 patients, there was a clear benefit of 3FL in the 1-, 3-, and 5-year OS rates. However, for postoperative complications, 3FL was associated with significantly more recurrent nerve palsy and anastomosis leakage. Thus, how to best manage TESCC in the viewpoint of surgery is still controversial.17 Previously, an analysis of the Surveillance Epidemiology and End Results (SEER) database evaluated the impact of adjuvant RT in 1046 EC patients,18 and the results indicated that there were significant improvements in median survival, 3-year OS rate, and DFS for patients who received PORT. Multivariate analysis confirmed that the addition of PORT was associated with improved survival (HR 0.70, 95% CI: 0.59–0.83, P<0.001) than surgery alone. Similar results were found in another large, prospective study conducted by Xiao et al.19 Their results demonstrated that PORT significantly improved survival outcomes for stage III tumors (P=0.0027) compared with surgery alone. In another large-scale meta-analysis evaluating the effect of POCRT with non-POCRT regimen for esophageal cancer patients, they found that POCRT yielded significant survival benefit (HR: 1.66, 95% CI 1.30–2.11; P<0.0001) and improved local-regional control rate with tolerable toxicities.20 However, selecting bias and difference in treatment regimens should be given into account for the final application of this meta-analysis.
With the development of minimally invasive esophagectomy and earlier recovery from surgery, attempts including chemoradiotherapy in the sequence of surgery were gaining more attention. As noted earlier, differences in histology and practice existed around the world, and POCRT did have certain advantages over nCRT for EC in some respects.6 The most encouraging results came from the landmark MacDonald trial21 which compared POCRT versus surgery alone in patients with gastric and gastroesophageal junction tumors. The median OS in the surgery alone group was 27 months, compared with 36 months in the POCRT group (HR 1.35, 95% CI: 1.09–1.66, P=0.005). The incidence of tumor recurrence was also significantly different between the two groups (P<0.001). However, it should be pointed out that, this Phase III trial enrolled all adenocarcinoma of the stomach or gastroesophageal junction tumors. It is still questionable to apply these findings into TESCC patients. To date, only one prospective non-randomized trial has compared the advantages of POCRT with those for PORT for the treatment of T3–4 and N0–1 ESCC.22 In this study, patients received either POCRT with weekly DDP followed by systemic adjuvant chemotherapy or PORT alone. The preplanned RT dose was 55–60 Gy for all patients (n=30 per group). They found that POCRT was well tolerated, with significantly better OS (30.9 months vs 20.7 months; 95% CI, 27.5–36.4 vs 15.2–26.1) and 3-year survival rate (70.0% vs 33.7%; P=0.003). However, because only 80% (24/30) of patients in the PORT group completed the planned course compared with 100% treatment compliance in the POCRT group and because of the small sample size in this trial, the conclusion was relatively controversial. In another retrospective analysis, as mentioned above,14 164 node-positive TESCC patients underwent POCRT, with 140 patients receiving PORT. The 5-year OS rates for the POCRT and PORT groups were 47.4% and 38.6%, respectively (P=0.03). The distant metastasis rate, the mixed (regional lymph node and distant) metastasis rate, and the overall recurrence rate were also significantly lower in the POCRT group than in the PORT group (P<0.05). Our results were consistent with their findings. For patients who received POCRT, the total incidence of distant metastasis was significantly lower than patients in the PORT group after PSM (P=0.044). The application of chemotherapy might one proper reason to explain this difference.
Another important concern for the application of POCRT is treatment toxicity. 15 (17.2%) patients in the POCRT group got severe leukocytopenia, compared with 3 (3.4%) patients in the PORT group had grade 3 leukocytopenia (P=0.003) in this report. Compared with the results in the former retrospective analysis,14 33 (20.1%) and 3 (2.1%) patients had grade ≥3 neutropenia in the POCRT group and PORT group, respectively. They concluded that severe early toxic reactions were more common with POCRT than with PORT, but patients could tolerate POCRT generally. In another phase III trial which used the TP regimen in the setting of definitive concurrent chemoradiotherapy,10 the results also showed that the most frequent acute adverse events were grade ≥3 leukocytopenia (41/166). Additionally, as 32% (16/50) of patients in the POCRT group subsequently developed distant metastases compared with 50.8% (33/65) in the PORT group, it seemed reasonable to suggest that systemic adjuvant chemoradiotherapy had a role in controlling distant metastasis.
This study is subject to some limitations. One pitfall of the current study is the nature of the retrospective design with small sample size, some potential, unmeasured factors might influence the final results. Secondly, lacking the direct comparison between preoperative chemoradiotherapy and POCRT made it hard to conclude any advantages for postoperative therapies.
Conclusion
In modern daily practice, it would be reasonable to add chemotherapy to postoperative RT as per NCCN guidelines to maximize the benefit of radiosensitization with systemic therapy. Our results support opinions indicating adjuvant POCRT use for stage II–III TESCC patients, but attention should be paid to the relatively high incidence of toxicities. We recommended POCRT for TESCC patients, especially for node-positive patients. Future prospective trials in large cohorts are needed to confirm the findings in our report.
Abbreviations
TESCC, thoracic esophageal squamous cell carcinoma; PO, postoperative; RT, radiotherapy; CRT, concurrent chemoradiotherapy; PTX, paclitaxel; DDP, cisplatin; PSM, propensity score matching; 2FL, 2-field lymphadenectomy; CTV, clinical target volume; PTV, planning tumor volume; SEER, surveillance epidemiology and end results; OARs, organs at risk.
Disclosure
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–1092. doi:10.1200/JCO.2007.12.9593
4. Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled Phase III trial. Lancet Oncol. 2005;6(9):659–668. doi:10.1016/S1470-2045(05)70288-6
5. Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19(2):305–313. doi:10.1200/JCO.2001.19.2.305
6. Boone J, Livestro DP, Elias SG, Borel Rinkes IH, van Hillegersberg R. International survey on esophageal cancer: part II staging and neoadjuvant therapy. Dis Esophagus. 2009;22(3):203–210. doi:10.1111/des.2009.22.issue-3
7. van Rijswijk AS, Hagens ERC, van der Peet DL, van Berge Henegouwen MI, Gisbertz SS. Differences in esophageal cancer surgery in terms of surgical approach and extent of lymphadenectomy: findings of an international survey. Ann Surg Oncol. 2019;26(7):2063–2072. doi:10.1245/s10434-019-07316-9
8. Jabbour SK, Thomas CR Radiation therapy in the postoperative management of esophageal cancer. J Gastrointest Oncol. 2010;1(2):102–111. doi:10.3978/j.issn.2078-6891.2010.013
9. Milas L, Milas MM, Mason KA. Combination of taxanes with radiation: preclinical studies. Semin Radiat Oncol. 1999;9(2 Suppl 1):12–26.
10. Suntharalingam M, Winter K, Ilson D, et al. Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: the NRG oncology RTOG 0436 Phase 3 randomized clinical trial. JAMA Oncol. 2017;3(11):1520–1528. doi:10.1001/jamaoncol.2017.1598
11. Honing J, Smit JK, Muijs CT, et al. A comparison of carboplatin and paclitaxel with cisplatinum and 5-fluorouracil in definitive chemoradiation in esophageal cancer patients. Ann Oncol. 2014;25(3):638–643. doi:10.1093/annonc/mdt589
12. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi:10.1056/NEJMoa1112088
13. Kleinberg LR, Catalano PJ, Forastiere AA, et al. Eastern Cooperative Oncology Group and American College of Radiology imaging network randomized Phase 2 trial of neoadjuvant preoperative paclitaxel/cisplatin/radiation therapy (RT) or irinotecan/cisplatin/RT in esophageal adenocarcinoma: long-term outcome and implications for trial design. Int J Radiat Oncol Biol Phys. 2016;94(4):738–746. doi:10.1016/j.ijrobp.2015.12.009
14. Chen J, Pan J, Liu J, et al. Postoperative radiation therapy with or without concurrent chemotherapy for node-positive thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;86(4):671–677. doi:10.1016/j.ijrobp.2013.03.026
15. Li G, Hu W, Wang J, et al. Phase II study of concurrent chemoradiation in combination with erlotinib for locally advanced esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2010;78(5):1407–1412. doi:10.1016/j.ijrobp.2009.10.012
16. Song T, Du D, Zhang X, Fang M, Wu S. Comparative study of radiotherapy plus erlotinib versus chemoradiotherapy for elderly patients with esophageal cancer: a propensity score-matched analysis. Dis Esophagus. 2017;30(9):1–10. doi:10.1093/dote/dox060
17. Ma GW, Situ DR, Ma QL, et al. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World J Gastroenterol. 2014;20(47):18022–18030. doi:10.3748/wjg.v20.i47.18022
18. Schreiber D, Rineer J, Vongtama D, et al. Impact of postoperative radiation after esophagectomy for esophageal cancer. J Thorac Oncol. 2010;5(2):244–250. doi:10.1097/JTO.0b013e3181c5e34f
19. Xiao ZF, Yang ZY, Liang J, et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg. 2003;75(2):331–336. doi:10.1016/S0003-4975(02)04401-6
20. Kang J, Chang JY, Sun X, Men Y, Zeng H, Hui Z. Role of postoperative concurrent chemoradiotherapy for esophageal carcinoma: a meta-analysis of 2165 patients. J Cancer. 2018;9(3):584–593. doi:10.7150/jca.20940
21. Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–730. doi:10.1056/NEJMoa010187
22. Liu HC, Hung SK, Huang CJ, et al. Esophagectomy for locally advanced esophageal cancer, followed by chemoradiotherapy and adjuvant chemotherapy. World J Gastroenterol. 2005;11(34):5367–5372. doi:10.3748/wjg.v11.i34.5367
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

