Back to Journals » OncoTargets and Therapy » Volume 13
The Research Progress on the Prognostic Value of the Common Hematological Parameters in Peripheral Venous Blood in Breast Cancer
Authors Chen L , Kong X, Yan C, Fang Y, Wang J
Received 14 August 2019
Accepted for publication 6 January 2020
Published 14 February 2020 Volume 2020:13 Pages 1397—1412
DOI https://doi.org/10.2147/OTT.S227171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Li Chen,1,* Xiangyi Kong,1,* Chengrui Yan,2,* Yi Fang,1 Jing Wang1
1Department of Breast Surgical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China; 2Department of Neurosurgery, Peking University International Hospital, Beijing 100021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Wang; Yi Fang
Department of Breast Surgical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China
Email [email protected]; [email protected]
Abstract: Breast carcinoma is one of the most malignant tumors, severely influencing the physical and mental health of people. The latest epidemiological and clinical studies have found that breast tumor and inflammation are determinate relationships with each other. Inflammation is an essential component of the tumor microenvironment, and the change of inflammatory cells might influence tumor progression, such as neoplastic cell proliferation, migration, invasion, the collapse of antitumor immunity, metastasis and so forth. Peripheral blood tests at the time of diagnosis and treatment can reflect inflammatory conditions within the neoplasm. Evaluation of peripheral blood parameters including white blood cell, neutrophil, lymphocyte, monocyte, platelet counts, as well as neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (d-NLR) (neutrophil count divided by the result of white blood cell count minus neutrophil count), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR), which are indicators of systematic inflammatory response, have been widely proposed as prognostic factors for many malignancies. To intensively study the relationship between the common markers in peripheral blood and the treatment or prognosis of breast cancer will have critical clinical significance and application prospect, and can provide useful information for the clinicians. Herein, we review the research progress in the prognostic role of the peripheral blood in breast cancer to provide a new method for the treatment and prognosis of breast cancer.
Keywords: breast cancer, prognosis, neutrophil-to-lymphocyte ratio, NLR, platelet to lymphocyte ratio, PLR, lymphocyte-to-monocyte ratio, LMR
Introduction
Worldwide, breast cancer is one of the most common female malignant tumors, which seriously affects people’s quality of life and harms their health. It has become a major public health problem in the current society.1 There are about 1 million new breast cancer patients every year in the world, and the age of onset tends to be younger.2 According to the National Cancer Center, the incidence of breast cancer in developed coastal cities is increasing rapidly. It is expected that the incidence of breast cancer in postmenopausal women in China will reach 100/100,000.3 Studies have shown that,4,5 some immunological and histological indicators are closely related to the prognosis of breast cancer, but obtaining these indicators is time-consuming and laborious, and relatively expensive, greatly limiting its clinical application. Recent epidemiological and clinical studies have found that inflammatory responses are associated with breast tumors.6,7 Inflammation constitutes part of the tumor microenvironment, and changes in inflammatory cells affect tumor progression, including breast cancer cell proliferation, invasion, decreased immunity, metastasis, and so on.8,9 Tumor inflammatory response may be a potential target for treating tumors. Examination of peripheral blood, leukocytes, neutrophils, lymphocytes, monocytes, platelets, and derived NLR (dNLR), PLR, LMR, etc. can reflect the inflammatory state of many malignant cells.10,11 It can be seen that these common peripheral blood markers are related to the treatment and prognosis of breast cancer patients, but the specific mechanism of action is still unclear. At present, the diagnosis of breast cancer is based on the core needle aspiration and pathological examination as the gold standard, assisting B-ultrasound, mammography, breast magnetic resonance (MR) and other imaging examinations. Relatively speaking, the routine examination of peripheral blood is simple, operability, cheap, and better promotion. Therefore, an in-depth study of the relationship between common markers in peripheral blood and the treatment and prognosis of breast cancer patients provides a valuable reference for clinicians, which will have important clinical significance and application prospects. This article discusses the common markers in peripheral blood in order to provide a new method for the treatment and prognosis of breast cancer.
Common Hematological Parameters in Peripheral Blood
White Blood Cells
There are five types of white blood cells, which are neutrophils, eosinophils, basophils, lymphocytes and monocytes. The increase or decrease in the total number is greatly affected by the number of neutrophils, and lymphocytes also cause changes in the number of white blood cells. At present, there are not many studies on white blood cells in breast cancer. Most studies indicate that white blood cells are mainly changed by changes in neutrophils and lymphocytes in peripheral blood.12,13
An increase in the number of neutrophils can cause an increase in the total number of white blood cells. Under normal conditions, peripheral blood leukocytes and neutrophils are dynamically changing within 24 hrs, lower in the morning and higher in the afternoon. Pregnancy or childbirth, excessive exercise or labor, shower or full meal, cold or hot can temporarily increase white blood cells and neutrophils. In pathological conditions, neutrophils are elevated in acute infection, severe trauma, blood cell destruction, hemorrhage, acute poisoning, malignant tumors, etc. Neutrophils are reduced by Gram-negative bacilli infection, blood system diseases, biophysics. Chemical factors such as damage and autoimmune diseases are common. Studies have shown that tumor-associated neutrophils can promote tumor development.14 Xue et al found that preoperative neutrophil elevation, patients with poor prognosis, short survival time.15 In Li Sen’s study, they found that neutrophils enhance the migration, invasiveness and epithelial-mesenchymal transition (EMT) of gastric cancer (GC) cells through IL-17a (Figure 1A–C).16
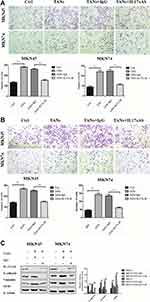 |
Figure 1 Neutrophils enhance the migration, invasiveness and EMT of GC cells through IL-17a. (A) The effect of neutrophils on the migration ability of GC cells (MKN45 and MKN74) was determined 24 h when IL-17a neutralizing antibody or IgG isotype control antibody was added to Transwell co-culture chamber. Magnifications: × 100. *P < 0.05; **P < 0.001. (B) The effect of neutrophils on the invasion ability of GC cells (MKN45 and MKN74) was determined 24 h when IL-17a neutralizing antibody or IgG isotype control antibody was added to Transwell co-culture chamber. Magnifications: × 100. *, P < 0.05; **, P < 0.001. (C) Protein expression of E-cadherin, Vimentin, and ZEB1 in GC cells (MKN45 and MKN74) co-cultured with neutrophils was analyzed by Western blot when IL-17a neutralizing antibody or IgG isotype control antibody was added to the Transwell co-culture system. Densitometric analysis of E-cadherin, Vimentin, and ZEB1 expression was shown. **P < 0.001.16 Epithelial to mesenchymal transition (EMT) can contribute to gastric cancer (GC) progression and recurrence following therapy. The results indicated that neutrophils were widely distributed in gastric tissues of patients with GC and were enriched predominantly at the invasion margin, and was the independent predictor of poor disease-free survival (DFS) and disease-specific survival (DSS). Moreover, IL-17a was produced at the highest levels in co-culture compared with that in TANs not undergoing co-culture, and the TANs enhanced the migration, invasion and EMT of GC cells through the secretion of IL-17a. The conclusions prove that neutrophils correlate with tumor stage and predict poor prognosis in GC. TANs produce IL-17a and the IL-17a-targeted therapy might be used to treat patients with GC. Reprinted from Li et al.16 Copyright © The Author(s). 2019. (This work was published and licensed by BioMed Central). |
Under normal circumstances, lymphocytosis is more common in children. When the baby is born, lymphocytes account for 35%, granulocytes account for 65%; 4 to 6 days lymphocytes can reach 50%, which is roughly equal to the number of granulocytes. And 4 to 6 years old, the proportion of lymphocytes decreases, and the proportion of granulocytes increased, close to the adult level. The pathological increase of lymphocytes is common in infectious diseases, acute and chronic lymphocytic leukemia, lymphoma and transplant rejection. The pathological reduction of lymphocytes is more common with radiation damage, immunodeficiency diseases, and lack of c-ball. Studies have shown that tumor-infiltrating lymphocyte changes are associated with the prognosis of gastric cancer.17
Under physiological conditions, mononuclear cells are more common in infants and young children. Pathological increases are more common in infections and blood diseases than other illnesses. Huang JJ and other scholars found that peripheral blood mononuclear cells can affect the progression of malignant tumors and is a related factor in tumorigenesis.18 Goto W and other scholars have found that changes in monocytes and lymphocytes, the ratio of lymphocytes to monocytes is an independent prognostic factor for breast cancer and can affect the patient’s disease-free survival time.19
C-Reactive Protein (CRP)
CRP is an acute phase protein with a highly sensitive marker of inflammatory and tissue damage. CRP is elevated in peripheral blood and is common in infections, tissue damage, and acute and chronic inflammatory diseases. In the peripheral blood of various tumor patients, CRP will increase to varying degrees. The change of CRP in peripheral blood has a certain relationship with the occurrence and progression of breast cancer.20 As a non-specific protein, CRP has many functions, and the detection method is simple, which may play a certain role in predicting and evaluating the survival of tumor patients.
Platelets
Thrombocytopenia is mainly seen in two cases, namely, increased primary and increased reactivity. The primary increase is more common in myeloproliferative diseases; the increase in reactivity is more common in acute infection, acute hemolysis and neoplastic diseases. Thrombocytopenia is mainly seen in hematological diseases, splenomegaly, and blood dilution. The occurrence of tumors is often accompanied by an increase in platelets, which in turn leads to disseminated intravascular coagulation, which causes tumor metastasis, which is found in tumors such as gastric cancer, colon cancer, lung cancer, kidney cancer, and prostate cancer.21 Johnson et al found that platelets promote the distant metastasis of breast cancer by inducing IL-8 uptake by tumor cells.22 Platelets can promote the development and development of inflammatory reactions, which in turn stimulate the production of inflammatory factors. In Jiang L’ s research, their results showed that platelet released promote breast cancer cell proliferation through PI3K/PKC signaling (Figure 2A–D).23
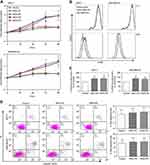 |
Figure 2 Platelet releasates enhance cell proliferation without affecting apoptosis of breast cancer cells. Breast cancer cell lines MCF-7 and MDA-MB-231 were treated with or without 10% (final concentration) of PAR1-PR or PAR4-PR under a serum-free culture condition. (A) Cell proliferation rate was detected at indicated time points using a CCK assay. The fold increases of OD values, proportional to the increase of cell numbers, were referred to those at time 0. Data are presented as mean ± SEM from at least five independent experiments. #P<0.05, †P<0.001 vs control at corresponding time points. (B) CFSE fluorescence intensities of breast cancer cells were determined by flow cytometry after MCF-7 and MDA-MB-231 cells were cultured without (control; black solid lines) or with PAR1-PR (blue dash lines) or PAR4-PR (red dot lines) for 96 h. (C) Quantification of CFSE dilution. #P<0.05 vs control. (D) The MCF-7 and MDA-MB-231 cells were stained with FITC-conjugated Annexin V and PE-conjugated PI after 72 h of culture, and were examined using the Beckman Coulter F500 flow cytometer. Percentages of total apoptotic cells, that is, all Annexin-V-positive cells, were plotted. Images in (B and D) are the representatives from three independent experiments.23 A full color version of this figure is available at the British Journal of Cancer journal online. Selective platelet release of pro- or anti-angiogenic factors distinctly regulated angiogenesis. The results indicated that the PAR1-PR and PAR4-PR supplementation similarly enhanced cell proliferation of MCF-7 and MDA-MB-231 breast cancer cells, and the VEGF receptor blockade abolished PAR1-PR/PAR4-PR-enhanced cancer cell proliferation. Moreover, the Src and ERK inhibition diminished, and PI3K and PKC blockade abolished platelet releasate-enhanced cancer cell proliferation. The PAR1-PR enhanced tumour growth and angiogenesis more markedly than PAR4-PR. The conclusions prove that platelet releasate increases breast cancer cell proliferation through VEGF–integrin cooperative signaling, and proangiogenic factor-rich platelet releasate enhances cancer cell-induced angiogenesis more markedly. Reprinted from Jiang et al.23 Copyright © The Author(s). 2019. (This work was published and licensed by British Journal of Cancer.) |
D-Dimer and Plasma Fibrin Degradation Products (FDPs)
Positive D-dimer is more common in pulmonary thromboembolism and deep vein thrombosis. The positive or increased FDPs are mainly seen in primary fibrinolysis and secondary fibrinolysis. However, secondary fibrinolysis is caused by diffuse intravascular coagulation, malignant tumors, pulmonary thromboembolism, organ transplant rejection, blood system diseases, and thrombolytic therapy. Studies have shown that the level of D-dimer is related to the occurrence of breast cancer, is an independent factor in the prognosis of breast cancer, and is associated with distant metastasis of breast cancer.24 Levitan and other scholars have pointed out that thromboembolism in cancer patients is 6 times that of normal people. Thromboembolism can lead to hypercoagulability of blood, damage to blood vessels and slow blood flow.25
Correlation Ratio of Common Hematological Parameters in Peripheral Blood
A single peripheral hematological parameter has certain research value for the prognosis of breast cancer patients. In recent years, more and more studies have begun to pay attention to the study of the correlation ratio of different hematological parameters, in order to further understand its relationship with breast cancer prognosis. Tumor cells release a variety of inflammatory substances, causing cell damage, DNA mutations, affecting the changes of the tumor microenvironment, thereby enhancing the proliferation and invasion ability of tumor cells. In the cellular microenvironment, the interaction of different inflammatory cells and extracellular matrix can also affect tumorigenesis and development.
Neutrophil-to-Lymphocyte Ratio (NLR)
In clinical first-line treatment, peripheral blood neutrophil levels can be used to respond to systemic inflammatory responses. Lymphocytes are an important component of tumor-specific immune responses and play a role in immune surveillance and killing of tumor cells. Most studies have shown that preoperative high NLR ratio suggests a poor prognosis in breast cancer patients, and can be used as an independent factor in the prognosis of breast cancer. The mechanism of elevated NLR can be explained by the relative decrease of lymphocytes and the relative increase of neutrophils, which causes the balance of cell microenvironment to be broken, and the inflammatory reaction occurs and develops toward the tumor, which leads to poor prognosis. Elyasinia and other scholars have shown that NLR is associated with TNM staging of breast cancer and has a certain reference value for the prognosis of breast cancer, while the high NLR value indicates poor prognosis.26 A meta-analysis study showed that NLR is associated with the prognosis of breast cancer, with higher values, shorter survival time for disease-free survival (DFS) and overall survival (OS), and a poorer prognosis.27 The high NLR value may be related to the number of lymph node metastasis and metastasis, and the incidence of lymph node metastasis is 1.7 times higher than that of normal people.28 Recent published studies on the correlation between neutrophil-to-lymphocyte ratio and breast cancer are listed in Table 1.29–34
 |
Table 1 Recently Published Studies on the Correlation Between Neutrophil-to-Lymphocyte Ratio and Breast Cancer |
Lymphocyte-to-Monocyte Ratio (LMR)/Monocyte-to-Lymphocyte Ratio (MLR)
Both monocytes and neutrophils are differentiated from granulocyte-mononuclear progenitor cells, which have a similar effect on the development of inflammation. Monocytes can produce a variety of inflammatory mediators, such as vascular endothelial growth factor, epidermal growth factor, and tumor necrosis factor, through macrophages, thereby promoting tumor cell growth and angiogenesis.35 Marín Hernández C and other scholars have pointed out that preoperative LMR is associated with the prognosis of breast cancer in breast cancer patients receiving neoadjuvant chemotherapy. The higher the value, the longer the survival time and the better prognosis.36 Hu RJ and other scholars have pointed out that preoperative LMR is associated with DFS and OS in breast cancer patients and can be used as a reference index for breast cancer prognosis. The higher the value, the better the prognosis.37 Recently published studies on the correlation between monocyte-to-lymphocyte ratio/lymphocyte-to-monocyte ratio and breast cancer are listed in Table 2.19,38–42
 |
Table 2 Recently Published Studies on the Correlation Between Monocyte-to-Lymphocyte Ratio/Lymphocyte-to-Monocyte Ratio and Breast Cancer |
Platelet-to-Lymphocyte Ratio (PLR)
Tumor development is often accompanied by thrombocytosis, and platelets themselves can also affect tumorigenesis and development. Most of the blood of patients with tumors is hypercoagulable, and high PLR usually indicates that the prognosis of patients with cancer is not good. According to scholars such as Chen L, PLR is an effective predictor of gastric cancer patients receiving neoadjuvant chemotherapy.43 Vernieri C and other studies have pointed out that PLR is a predictor of triple-negative breast cancer after receiving neoadjuvant chemotherapy, and it is also a valid reference for patients to benefit from it.44 Yersal Ö and other scholars have pointed out that PLR is positively correlated with lymph node-positive metastasis rate, and the higher the value, the higher the positive rate of lymph node metastasis.45 Recently published studies on the correlation between platelet-to-lymphocyte ratio and breast cancer are listed in Table 3.44,46–50
 |
Table 3 Recently Published Studies on the Correlation Between Platelet-to-Lymphocyte Ratio and Breast Cancer |
Combined Application of Common Hematological Parameters in Peripheral Blood
NLR and LMR/MLR
Studies have shown that NLR and LMR can provide reference information for the prognosis of pancreatic cancer. LMR was the benign predictor of luminal A and HER-2 overexpression; the WBC and NLR were the adverse predictors of TNBC. These peripheral blood parameters can play an important role in the diagnosis and treatment of patients with different molecular subtypes of BC.38 Losada B and other scholars have found low PLR and longer DFS in elderly breast cancer patients are in line with findings in patients with a wider range of ages, and NLR contrast with those of other studies, indicating a potential differential effect in elderly patients.51 The application of NLR and LMR/MLR in breast cancer has been reported to be rare. In C. Marín Hernández's study, he proved that High LMR and low NLR could be considered as favourable prognostic factors in breast cancer (BC) patients treated with nCT (Figure 3).36
 |
Figure 3 DFS and OS curves according to ratios.36 The comparison between the DFS of the groups classified by ratios revealed that those patients with both the patients with higher LMR and the ones with lower NLR had a better prognosis. The comparison between the OS of the groups classified by ratios revealed that those patients with low NLR had a better prognosis. Neoadjuvant chemotherapy in breast cancer is more and more standardized, and systemic inflammatory response is associated with the tumors. The results indicated that patients with high LMR and low NLR were associated with a lower percentage of relapse, and NLR was associated with a better survival. The conclusions prove that high LMR and low NLR can be considered as favourable prognostic factors in BC patients treated with neoadjuvant chemotherapy.36 Reprinted by permission from Springer Nature. Marín Hernández C, Piñero Madrona A, Gil Vázquez PJ, Galindo Fernández PJ, Ruiz Merino G, Alonso Romero JL, Parrilla Paricio P. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol. 2018; 20(4):476–483. Copyright © 2018, Clinical and Translational Oncology Available from: https://link.springer.com/journal/12094. |
NLR and PLR
Graziano V and other scholars found that in patients with breast cancer receiving neoadjuvant chemotherapy, combined with NLR and PLR, low NLR and low PLR can improve the sensitivity to chemotherapy, while high NLR and PLR have no significant effect on chemotherapy and postoperative prognosis was relatively poor.46 Kim HY and other scholars determined that the combination of NLR and PLR showed improved prediction of NAC response, and revealed their potential as screening tools, significant prognostic role in breast cancer patients who receive neoadjuvant chemotherapy.52 Sut A and other scholars indicated that the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), medium platelet volume/lymphocyte ratio (MPVRL) are associated to the process of inflammation in breast cancer patients, and these biomarkers have important clinical implications.53 In Xu et al research, their study showed that the patients with lower pretreatment NLR (<2.05) or PLR (<159.01) will have higher ER-positive expression, the patients with low NLR or low PLR would have a higher rate of complete remission (CR) and partial remission (PR) (Figure 4A–D).7
 |
Figure 4 The relationship between NLR, PLR and ER, PR. (A) A higher ER-positive proportion was observed primarily in those patients with lower NLR before treatment; (B) A higher ER-positive proportion was observed primarily in those patients with lower PLR before treatment; (C) No statistical difference was found between the NLRlow group and the NLRhigh group in the expression of PR; (D) The patients with lower PLR (PLR < 159.01) showed higher PR-positive expression when compared to the patients with higher PLR.7 Proinflammatory markers, including neutrophil/lymphocyte ratio and platelet/lymphocyte ratio, are associated with many aspects of different malignancies. The results indicated that the proportion of ER-positive breast cancers before NAC was higher both in low NLR group and low PLR group. And the patients with low pretreatment NLR or PLR had better responses to NAC than those with high NLR or PLR. The conclusions prove that the patients with low pretreatment NLR or PLR had higher ER expression, and Pretreatment NLR and PLR may be important predictive indicators for neoadjuvant chemotherapy response in breast cancer patients.7 Reprinted by permission from Springer Nature. Xu J, Ni C, Ma C, Zhang L, Jing X, Li C, Liu Y, Qu X. Association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with ER and PR in breast cancer patients and their changes after neoadjuvant chemotherapy. Clin Transl Oncol. 2017;19(8): 989–996. Copyright © 2017, Clinical and Translational Oncology. Available from: https://link.springer.com/journal/12094. |
NLR, PLR, LMR/MLR and Other Joint Applications
Losada et al pointed out that the combination of NLR, PLR, LMR and NMR in the prognosis of breast cancer, PLR can be used as an independent prognostic factor for breast cancer. Patients with higher PLR values have shorter DFS survival time and poorer prognosis.51 Sun et al showed that hematological parameters of RDW, MPV, NLR, and PLR can be used as an adjuvant tool for the diagnosis of BC, and the value of MPV can reflect the Ki67 proliferation index before surgery and identify patients with positive lymph node metastasis.54 Wariss BR and other scholars studied that the neutrophil–lymphocyte ratio (NLR), a derived neutrophil-to-lymphocyte ratio (dNLR), absolute neutrophil count (ANC) and platelet-to-lymphocyte ratio (PLR) are independent markers of prognosis in breast cancer.55 Combined with these markers for a comprehensive assessment of breast cancer prognosis, we found that high NLR and high PLR are adverse prognostic factors for breast cancer patients, and postoperative survival time will be shortened. In Sun et al's study, they examined the roles of pretreatment systemic immune-inflammation index (SII), established as neutrophil x platelet/lymphocyte counts, in hormone receptor-negative, human epidermal growth factor receptor 2+ (HER2+) breast cancer patients. Analyses showed that HER2+ patients with high and low SII had median DFS of 15.1 and 31.5 months, respectively (P<0.001), while the median DMFS in HER2+ patients with high SII was 18.4 months and in patients with low SII was 33.0 months (P=0.001), and the median OS was 54.5 and 71.1 months respectively in high and low SII patients, respectively (P=0.002) (Figure 5A–C).56 Multivariate analysis had revealed increased SII independently linked to poor DFS (HR =1.46, 95% CI: 1.01–2.11, P=0.045), suggesting that increased SII independently predicts poor survival for hormone receptor-negative, HER2+ breast cancer patients.
 |
Figure 5 (A) Disease-free survival rates of 155 patients with hormone receptor-negative, HER2+ breast cancer according to low and high SII; (B) Distant metastasis-free survival rates of 155 patients with hormone receptor-negative, HER2+ breast cancer according to low and high SII; (C) Overall survival rates of 155 patients with hormone receptor-negative, HER2+ breast cancer according to low and high SII. The pretreatment systemic immune-inflammation index (SII) in hormone receptor-negative, human epidermal growth factor receptor 2+ (HER2+) breast cancer patients was used to predict the survival. The results showed that HER2+ patients with high and low SII had median DFS of 15.1 and 31.5 months, respectively; and the median OS of 54.5 and 71.1 months, respectively.56 The multivariate analysis had revealed increased SII independently linked to poor DFS. The conclusions prove that SII independently predicts poor survival for hormone receptor-negative, HER2+ breast cancer patients.56 Reprinted from Sun Y, Li W, Li AJ, Su H, Yue J, Yu J. Increased systemic immune-inflammation index independently predicts poor survival for hormone receptor-negative, HER2-positive breast cancer patients. Cancer Manag Res. 2019;11:3153–3162. © 2019 Sun et al. This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution – Non Commercial (unported, v3.0) License (http://creativecommons.org/licenses/by-nc/3.0/). |
Research Value and Limitations of Common Hematological Parameters in Peripheral Blood
Common hematological parameters in peripheral blood are used to evaluate the prognosis of breast cancer patients. They are simple, reproducible, etc., and can provide a reference for hospitals at various levels, in line with the current imbalance of medical resources in China. However, current research on these indicators lack uniform research standards, and large-scale multi-center, large sample imbalances are rare. At the same time, the specific mechanism of action of these indicators and breast cancer is still unclear, and further research is needed.
Conclusion and Perspectives
In summary, neutrophils, lymphocytes, NLR, LMR, PLR and other indicators can provide valuable information for the prognosis and diagnosis of breast cancer, especially lymph node metastasis and prognosis. A peripheral blood test is a common clinical detection method, which is very convenient, cheap, reproducible, etc. It is more convenient and cheaper than imaging examination, pathological examination and blood markers. Therefore, with the further development of medical research and research, the common parameters of peripheral blood will have a good development prospect for the prognosis of breast cancer, and patients will benefit from it.
Abbreviations
NLR, Neutrophil-to-lymphocyte ratio; dNLR, Derived neutrophil-to-lymphocyte ratio; PLR, Platelet-to-lymphocyte ratio; LMR, Lymphocyte-to-monocyte ratio; MR, Magnetic resonance; EMT, Epithelial-mesenchymal transition; GC, Gastric cancer; IL, Interleukin; CRP, C-reactive protein; FDPs, Fibrin degradation products; DFS, Disease-free survival; OS, Overall survival; pCR, pathologic complete response; BC, Breast cancer; SII, Systemic immune-inflammation index.
Data Sharing Statement
The material supporting the conclusion of this review has been included within the article.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.v68.6
3. Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;29(1):1–10. doi:10.21147/j.issn.1000-9604.2017.01.01
4. Harano K, Kogawa T, Wu J, et al. Thrombocytosis as a prognostic factor in inflammatory breast cancer. Breast Cancer Res Treat. 2017;166(3):819–832. doi:10.1007/s10549-017-4463-6
5. Jeong H, Hwang I, Kang SH, Shin HC, Kwon SY. Tumor-associated macrophages as potential prognostic biomarkers of invasive breast cancer. J Breast Cancer. 2019;22(1):38–51. doi:10.4048/jbc.2019.22.e5
6. Li QX, Shi DJ, Zhang LX, et al. Association of body mass and systemic immune-inflammation indices with endocrine therapy resistance in luminal breast cancers. J Int Med Res. 2019;47(5):1936–1947. doi:10.1177/0300060519831570
7. Xu J, Ni C, Ma C, et al. Association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with ER and PR in breast cancer patients and their changes after neoadjuvant chemotherapy. Clin Transl Oncol. 2017;19(8):989–996. doi:10.1007/s12094-017-1630-5
8. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi:10.1016/j.cell.2010.01.025
9. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi:10.1016/S0140-6736(00)04046-0
10. Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis-tracing the accessory. Oncogene. 2014;33(25):3217–3224. doi:10.1038/onc.2013.272
11. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumors, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771.
12. Park B, Lee HS, Lee JW, Park S. Association of white blood cell count with breast cancer burden varies according to menopausal status, body mass index, and hormone receptor status: a case-control study. Sci Rep. 2019;9(1):5762. doi:10.1038/s41598-019-42234-6
13. Lohman AC, Van Rijn I, Lindhardt CL, Vonthein R, Rades D, Holländer NH. Preliminary results from a prospective study comparing white blood cell and neutrophil counts from a laboratory to those measured with a new device in patients with breast cancer. In Vivo. 2018;32(5):1283–1288. doi:10.21873/invivo.11378
14. Mouchemore KA, Anderson RL, Hamilton JA. Neutrophils, G-CSF and their contribution to breast cancer metastasis. FEBS J. 2018;285(4):665–679. doi:10.1111/febs.2018.285.issue-4
15. Xue LB, Liu YH, Zhang B, et al. Prognostic role of high neutrophil to lymphocyte ratio in breast cancer patients receiving neoadjuvant chemotherapy: meta-analysis. Medicine (Baltimore). 2019;98(1):e13842. doi:10.1097/MD.0000000000013842
16. Li S, Cong X, Gao H, et al. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. 2019;38(1):6. doi:10.1186/s13046-018-1003-0
17. Lee M, Park IA, Heo SH, Kim YA, Gong G, Lee HJ. Association between p53 expression and amount of tumor-infiltrating lymphocytes in triple-negative breast cancer. J Pathol Transl Med. 2019;53(3):180–187. doi:10.4132/jptm.2019.02.08
18. Huang JJ, Li YJ, Xia Y, et al. Prognostic significance of peripheral monocyte count in patients with extranodal natural killer/T-cell lymphoma. BMC Cancer. 2013;13:222. doi:10.1186/1471-2407-13-222
19. Goto W, Kashiwagi S, Asano Y, et al. Predictive value of lymphocyte to monocyte ratio in the preoperative setting for progression of patients with breast cancer. BMC Cancer. 2018;18(1):1137. doi:10.1186/s12885-018-5051-9
20. El-Abd E, El-Sheikh M, Zaky S, Fayed W, El-Zoghby S. Plasma TuM2-PK correlates with tumor size, CRP and CA 15-3 in metastatic breast carcinomas; short versus long term follow up study of the Egyptian breast cancer patients. Cancer Biomark. 2017;20(2):123–133. doi:10.3233/CBM-160482
21. Matowicka-Karna J, Kamocki Z, Polińska B, Osada J, Kemona H. Platelets and inflammatory markers in patients with gastric cancer. Clin Dev Immunol. 2013;2013:401623. doi:10.1155/2013/401623
22. Johnson KE, Ceglowski JR, Roweth HG, et al. Aspirin inhibits platelets from reprogramming breast tumor cells and promoting metastasis. Blood Adv. 2019;3(2):198–211. doi:10.1182/bloodadvances.2018026161
23. Jiang L, Luan Y, Miao X, et al. Platelet releasate promotes breast cancer growth and angiogenesis via VEGF-integrin cooperative signalling. Br J Cancer. 2017;117(5):695–703. doi:10.1038/bjc.2017.214
24. Sringeri RR, Chandra PS. Role of plasma D-dimer levels in breast cancer patients and its correlation with clinical and histopathological stage. Indian J Surg Oncol. 2018;9(3):307–311. doi:10.1007/s13193-017-0682-x
25. Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using medicare claims data. Medicine (Baltimore). 1999;78(5):285–291. doi:10.1097/00005792-199909000-00001
26. Elyasinia F, Keramati MR, Ahmadi F, et al. Neutrophil-lymphocyte ratio in different stages of breast cancer. Acta Med Iran. 2017;55(4):228–232.
27. Ethier J-L, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. doi:10.1186/s13058-016-0794-1
28. Xinji Z, Yonggang L, Xiaojun S, Xiaowu C, Dong Z, Dajian Z. The prognostic role of neutrophils to lymphocytes ratio and platelet count in gastric cancer: a meta-analysis. Int J Surg. 2015;21:84–91. doi:10.1016/j.ijsu.2015.07.681
29. Ibrahim A, Serkan YF, Tuba A, Erol B, Lütfi P. Can neutrophil to lymphocyte ratio be a predictor tool for the non-sentinel lymph node metastasis in breast cancer? Chirurgia (Bucur). 2019;114(1):83–88. doi:10.21614/chirurgia.114.1.83
30. Imamura M, Morimoto T, Egawa C, et al. Significance of baseline neutrophil-to-lymphocyte ratio for progression-free survival of patients with HER2-positive breast cancer treated with trastuzumab emtansine. Sci Rep. 2019;9(1):1811. doi:10.1038/s41598-018-37633-0
31. Fujimoto Y, Ozawa H, Higuchi T, et al. Improved prognosis of low baseline neutrophil-to-lymphocyte ratio is significantly exclusive in breast cancer patients with high absolute counts of lymphocytes. Mol Clin Oncol. 2019;10(2):275–284. doi:10.3892/mco.2018.1783
32. Patel DA, Xi J, Luo J, et al. Neutrophil-to-lymphocyte ratio as a predictor of survival in patients with triple-negative breast cancer. Breast Cancer Res Treat. 2019;174(2):443–452. doi:10.1007/s10549-018-05106-7
33. Ren K, Yin Y, He F, Shao Y, Wang S. Prognostic role of derived neutrophil-to-lymphocyte ratio in surgical triple-negative breast cancer. Cancer Manag Res. 2018;10:4891–4898. doi:10.2147/CMAR
34. Mandó P, Rizzo M, Roberti MP, et al. High neutrophil to lymphocyte ratio and decreased CD69+NK cells represent a phenotype of high risk in early-stage breast cancer patients. Onco Targets Ther. 2018;11:2901–2910. doi:10.2147/OTT.S160911
35. Diaz-Montero CM, Finke J, Montero AJ. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol. 2014;41(2):174–184. doi:10.1053/j.seminoncol.2014.02.003
36. Marín Hernández C, Piñero Madrona A, Gil Vázquez PJ, et al. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol. 2018;20(4):476–483. doi:10.1007/s12094-017-1732-0
37. Hu RJ, Liu Q, Ma JY, Zhou J, Liu G. Preoperative lymphocyte-to-monocyte ratio predicts breast cancer outcome: a meta-analysis. Clin Chim Acta. 2018;484:1–6. doi:10.1016/j.cca.2018.05.031
38. Zenan H, Zixiong L, Zhicheng Y, et al. Clinical prognostic evaluation of immunocytes in different molecular subtypes of breast cancer. J Cell Physiol. 2019. doi:10.1002/jcp.28662.
39. Lee KH, Kim EY, Yun JS, et al. The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer. 2018;18(1):938. doi:10.1186/s12885-018-4832-5
40. Takeuchi H, Kawanaka H, Fukuyama S, Kubo N, Hiroshige S, Yano T. Comparison of the prognostic values of preoperative inflammation-based parameters in patients with breast cancer. PLoS One. 2017;12(5):e0177137. doi:10.1371/journal.pone.0177137
41. Ji H, Xuan Q, Yan C, Liu T, Nanding A, Zhang Q. The prognostic and predictive value of the lymphocyte to monocyte ratio in luminal-type breast cancer patients treated with CEF chemotherapy. Oncotarget. 2016;7(23):34881–34889. doi:10.18632/oncotarget.v7i23
42. He J, Lv P, Yang X, Chen Y, Liu C, Qiu X. Pretreatment lymphocyte to monocyte ratio as a predictor of prognosis in patients with early-stage triple-negative breast cancer. Tumour Biol. 2016;37(7):9037–9043. doi:10.1007/s13277-016-4793-8
43. Chen L, Hao Y, Cong X, et al. Peripheral venous blood platelet-to-lymphocyte ratio (PLR) for predicting the survival of patients with gastric cancer treated with SOX or XELOX regimen neoadjuvant chemotherapy. Technol Cancer Res Treat. 2019;18:1533033819829485. doi:10.1177/1533033819829485
44. Vernieri C, Mennitto A, Prisciandaro M, et al. The neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict efficacy of platinum-based chemotherapy in patients with metastatic triple negative breast cancer. Sci Rep. 2018;8(1):8703. doi:10.1038/s41598-018-27075-z
45. Yersal Ö, Çetinkünar S, Aktimur R, et al. Neutrophil/ lymphocyte and platelet/lymphocyte ratios are not different among breast cancer subtypes. Asian Pac J Cancer Prev. 2017;18(8):2227–2231. doi:10.22034/APJCP.2017.18.8.2227
46. Graziano V, Grassadonia A, Iezzi L, et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast. 2019;44:33–38. doi:10.1016/j.breast.2018.12.014
47. Cuello-López J, Fidalgo-Zapata A, López-Agudelo L, Vásquez-Trespalacios E. Platelet-to-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer. PLoS One. 2018;13(11):e0207224. doi:10.1371/journal.pone.0207224
48. Durhan A, Durhan G, Eroğlu A. Regression in local recurrence in the contralateral breast following mastectomy in bilateral locally advanced breast cancer: a comparison of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios. Turk J Surg. 2018;34(2):140–142. doi:10.5152/turkjsurg.
49. Liu C, Huang Z, Wang Q, et al. Usefulness of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in hormone-receptor-negative breast cancer. Onco Targets Ther. 2016;9:4653–4660.
50. Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524–2530. doi:10.1038/bjc.2014.163
51. Losada B, Guerra JA, Malón D, Jara C, Rodriguez L, Del Barco S. Pretreatment neutrophil/lymphocyte, platelet/lymphocyte, lymphocyte/monocyte, and neutrophil/monocyte ratios and outcome in elderly breast cancer patients. Clin Transl Oncol. 2019;21(7):855–863. doi:10.1007/s12094-018-1999-9
52. Kim HY, Kim TH, Yoon HK, Lee A. The role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in predicting neoadjuvant chemotherapy response in breast cancer. J Breast Cancer. 2019;22(3):425–438. doi:10.4048/jbc.2019.22.e41
53. Sut A, Pytel M, Zadrożny M, Golanski J, Rozalski M. Polyphenol-rich diet is associated with decreased level of inflammatory biomarkers in breast cancer patients. Rocz Panstw Zakl Hig. 2019;70(2):177–184. doi:10.32394/rpzh
54. Sun H, Yin CQ, Liu Q, Wang F, Yuan CH. Clinical significance of routine blood test-associated inflammatory index in breast cancer patients. Med Sci Monit. 2017;23:5090–5095. doi:10.12659/MSM.906709
55. Wariss BR, de Souza Abrahão K, de Aguiar SS, Bergmann A, Thuler LCS. Effectiveness of four inflammatory markers in predicting prognosis in 2374 women with breast cancer. Maturitas. 2017;101:51–56. doi:10.1016/j.maturitas.2017.04.015
56. Sun Y, Li W, Li AJ, Su H, Yue J, Yu J. Increased systemic immune-inflammation index independently predicts poor survival for hormone receptor-negative, HER2-positive breast cancer patients. Cancer Manag Res. 2019;11:3153–3162. doi:10.2147/CMAR.S190335
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
