Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
The Renoprotective Effects of Naringenin (NGN) in Gestational Pregnancy
Authors Zhao C, Yin X , Zhao C
Received 20 September 2019
Accepted for publication 22 December 2019
Published 8 January 2020 Volume 2020:13 Pages 53—63
DOI https://doi.org/10.2147/DMSO.S231851
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Chunrong Zhao,1 Xiufeng Yin,2 Chunping Zhao1
1Department of Obstetrics, Linyi Central Hospital of Shandong Province, Linyi, Shandong 276400, People’s Republic of China; 2Department of Obstetrics and Gynecology, Yixing People’s Hospital, Wuxi, Jiangsu 214200, People’s Republic of China
Correspondence: Xiufeng Yin
Department of Obstetrics and Gynecology, Yixing People’s Hospital, No. 75 Tongzhen Guan Road, Yicheng, Yixing, Jiangsu 214200, People’s Republic of China
Tel +86-510-8733 0741
Email [email protected]
Introduction: Gestational diabetes mellitus (GDM) is defined as glucose intolerance that is first diagnosed during pregnancy, a condition risking the health of both the mother and the baby. Naringenin (NGN) has been demonstrated to have multiple therapeutic functions, while it is also considered to exhibit antidiabetic properties. The present study aimed to investigate the protective effects of NGN in pregnant diabetic rats.
Methods: GDM was induced by feeding the rats with a high-fat diet for 5 weeks, followed by intraperitoneal injection of streptozotocin (35 mg/kg). The fasting blood glucose were determined with a glucometer and the 24-h urine protein (24-UPro) were determined by the sulfonyl salicylic acid method. The pathological morphological changes and apoptosis of glomeruli cells of kidney tissue using hematoxylin and eosin (H&E) staining and TUNEL analysis. Enzyme-linked immunosorbent assay (ELISA) kits were used to detect the serum T-AOC, the activity of SOD, the levels of GSH-Px, CAT and MDA, TNF-α, IL-6, TGF-β, ICAM-1.The expression of related genes were measured by RT-qPCR and Western blot analyses.
Results: In the NGN-treated group, it was observed that the general status of the rats was improved, while the levels of blood glucose and 24-UPro were significantly decreased. In addition, the histopathological changes in renal tissues and renal cell apoptosis were significantly improved upon treatment with NGN. The expression levels of oxidative stress and inflammation-associated factors also differed signifigcantly between the model and NGN-treated groups. Upon treatment with NGN, the levels of peroxisome proliferator-activated receptor α were significantly increased, while the activity of enzymes involved in the oxidative metabolism of fatty acids was significantly decreased.
Conclusion: These preliminary experimental findings demonstrate that NGN has a certain renoprotective effect on GDM, which provides a novel therapeutic option for this condition.
Keywords: gestational diabetes mellitus, Naringenin, streptozotocin, oxidative stress, inflammation
Introduction
Gestational diabetes mellitus (GDM) is identified as glucose intolerance that is first diagnosed during pregnancy, a common condition risking the health of both the mother and the baby.1 During pregnancy, women with GDM have an increased risk of preeclampsia, hypertension and premature delivery, with increasing possibility of developing into type 2 diabetes in the long term.1–4 Therefore, timely and effective control of the occurrence and development of GDM and its complications is of great importance for the mother and baby.
Naringenin (NGN) is a common dietary flavanone in citric fruits, including grapefruits, lemons and oranges.5 It is a flavanone glycoside which has a molecular formula of C15H12O5 and molecular weight of 272.26 g/mol,6,7 and the molecular formula is shown in Figure 1A. NGN has been reported to have multiple therapeutics properties, including antioxidant, antithrombotic, antihypertensive, anti-hyperlipidemic and anti-inflammatory properties.8–12 Furthermore, it has been suggested that NGN regulates lipoprotein metabolism and may be used in the management of diabetes, insulin resistance and atherosclerosis, which has been widely discussed in a previous study.13 In diabetes mellitus, NGN is considered to reduce the plasma glucose levels.14
Previous study suggested that in a type 2 diabetes rat model, NGN was able to ameliorate cognitive deficits via oxidative stress, pro-inflammatory factors and PPAR signaling.15 In high-cholesterol fed rats, NGN was able to attenuate renal and platelet purinergic signaling perturbations by inhibiting ROS and NF-κB signaling pathways.16 NGN exhibits a potential cardio-protective effect via the regulation of oxidative stress and inflammatory markers in doxorubicin and isoproterenol-induced cardiotoxicity.17 It has been reported that NGN exhibits a protective effect on glycerol-induced acute renal failure in the kidney of rats.18 However, the role of NGN in GDM has not been reported thus far.
Therefore, the present study aimed to explore the renoprotective effect of NGN on GDM in rats, as well as its effects on oxidative stress, PPAR signaling pathway, inflammatory response and cell apoptosis in rats.
Materials and Methods
Animals
A total of 63 female Sprague-Dawley rats (age, 6–8 weeks; weight, 220–250g) were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences (Shanghai, China). The animals were housed in a specific pathogen free animal facility and maintained under controlled room temperature (22–26 °C) and humidity (40–70%) with a 12:12 h light: dark cycle. All animal experiments and the use of mice were approved by the Ethics Committee of Yixing People’s Hospital. All experimental protocols conducted in the rats were carried out in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health.
Establishment of a GDM Animal Model
Female rats were fed with high fat (HIF) feeding for 5 weeks and kept in the same cage with male rats in the stage of estrus. Upon the presence of a vaginal mucous plug the next morning, pregnant rats could be confirmed, and that day was marked gestation day 1. After 5 days, the pregnant rats were weighed, injected STZ (35 mg/kg, S0130; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) by which destroyed pancreatic islet β cells, in order to generate gestational diabetic models. After 24 and 72 h, the results of fasting blood glucose test was above 13.5 mmol/l stably, which was considered to indicate a successful model. NGN (SN8020, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was dispersed in 0.9% saline solution. A total of 45 model rats were selected and randomly divided into a GDM group (n=9), an NGN gavage therapy group, which was administered p.o. with 30 (n=9), 50 (n=9) or 100 mg/kg (n=9) NGN and a metformin hydrochloride therapy group (n=9) (which received 200 mg/kg metformin hydrochloride; Zhonghui Pharmaceutical Co., Ltd. Beijing, China). Rats in each treatment group were administered the corresponding drug once a day for 2–3 weeks. In addition, untreated gestational rats were used as the normal group (n=9), while non-gestational female rats of the same age were used as the control group (n=9). Control and normal gestation rats were fed on a normal diet, not injected with STZ. Rats in the control, normal and GDM groups received the same volume of saline solution (NaCl 0.9%).
General Observation
The mental state (spontaneous activity), appearance (coat color), eating, drinking, urine volume and weight of rats were observed and recorded carefully every day prior and subsequent to treatment for 2–3 weeks.
Detection of Blood Glucose and 24-h Urine Protein (24h-UPro) Levels
Upon drug treatment for 1 week and 2 weeks, blood was extracted from the caudal vein of the rats in each group in the fasting state. The levels of fasting blood glucose were determined with a glucometer (OneTouch Basic 200–200; LifeScan, Inc., Milpitas, CA, USA). Subsequently, 24h-UPro levels were determined by the sulfonyl salicylic acid method according to the manufacturer’s protocol (Shenzhen Mindray Bio-Medical Electronics Co. Ltd. Shenzhen, China).
Determination of Serum Total Antioxidant Capacity (T-AOC)
After drug treatment, rats were sacrificed by cervical dislocation and blood was collected from the abdominal aorta, and blood serum was extracted by centrifugation at 15,000 x g at 4 °C for 10 min. Then, the T-AOC was determined with an ultraviolet-visible (UV-VIS) spectrophotometer (UV-5100, Shanghai Precision Instrument Co. Ltd., Shanghai, China) according to the procedure of the Total Antioxidant Capacity Assay Kit (Sigma-Aldrich; Merck KGaA).
Pathology Examination
Upon blood collection, the tissue of the left kidney was collected. Samples were then fixed in 4% paraformaldehyde overnight and embedded in paraffin. Tissue paraffin sections of 5-μm thickness were cut for histological analysis. Hematoxylin and eosin (H&E) staining was performed using a standard protocol.19 Then, the pathological morphological changes of kidney tissue were observed under an inverted light microscope (CKX41; Olympus Corporation, Tokyo, Japan).
Apoptosis Assay
After treatment, rats were sacrificed by cervical dislocation. Then, in order to investigate apoptosis, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) analysis were performed on 5 μm paraffin sections using the in situ Cell Death Detection kit (Roche Molecular Biochemicals) following the manufacturer’s protocal. Apoptosis of rat glomeruli cells was observed under light microscopy. The number of apoptotic cells and the total number of cells were calculated respectively. A poptosis index was calculated.
Oxidative Stress Assessment
After drug treatment, the tissue of the right kidney was extracted, shredded and homogenized in cold phosphate buffered saline (PBS; ThermoFisher Scientific). After centrifugation at 15,000 x g for 10 min, the clear supernatant was collected and analyzed for markers of oxidative stress. Following the manufacturer’s instructions, the activity of superoxide dismutase (SOD), the levels of glutathione peroxidase (GSH-Px), catalase (CAT) and malondialdehyde (MDA) were measured with a UV-VIS spectrophotometer (UV-5100; Shanghai Precision Instrument Co.,Ltd.).
Cytokine Analyses
After drug treatment, the concentrations of plasma tumor necrosis factor α (TNF-α; Cat. No. 550734), interleukin (IL)-6 (Cat. No.550319), transforming growth factor β (TGF-β; Cat. No. 559119) and intercellular adhesion molecule 1 (ICAM-1; Cat. No. 551424) were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (BD Pharmingen; BD Biosciences, San Jose, CA, USA) according to the manufacturer’s protocols. All samples were analyzed in duplicate.
Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
Total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s protocol. The concentration and purity were measured using an ultraviolet spectrophotometer, and the RNA purity (A260/A280 = 1.8–2.0) was satisfactory for the experiments. Next, the total RNA was reverse-transcribed into cDNA using RT kit (Takara, Biotechnology Co., Ltd. Dalian, China) following the manufacturer’s instructions. qPCR was performed at 95 °C for an initial 10-min period, followed by 40 cycles of denaturation for 30 secs at 95 °C, annealing at 60 °C for 30 secs and extension at 72 °C for 30 secs with SYBR Premix Ex Taq (Applied Biosystems; Thermo Fisher Scientific, Inc.). The relative expression levels were calculated using the 2−ΔΔCq method. GAPDH was used as the endogenous controls. Following primers were used: PPARα forward 5′-GCAGCCTGTGAGTCTTGTGAGTGA-3′, reverse 5′- CTCCATCAGGTCTCCACACAGC-3′; Acox1 forward 5′-GCCCAACTGTGACTTCCATT-3′, reverse 5′-GGCATGTAACCCGTAGCACT-3′; L-PBE forward 5′-GGTCGTTGGAGTTCCTGTTGCT-3′, reverse 5′- TGGGCAAGCTTGGGACTGGC-3′; MCAD forward 5′- GCTCGTGAGCACATTGAAAA −3′, reverse 5′- CATTGTCCAAAAGCCAAACC −3′; GADPH forward 5′-ATCAAGAAGGTGGTGAAGCA-3′, reverse 5′-AAGGTGGAAGAATGGGAGTTG-3′. GAPDH was used as the endogenous control. The relative mRNA expression levels were calculated using the 2−ΔΔCq method.
Western Blotting
Western blot analysis was conducted to evaluate the expression levels of associated proteins. Proteins were isolated from liver tissue using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) and protein concentration was measured with a BCA protein assay kit (Beyotime Institute of Biotechnology). Subsequently, proteins (20 µg/per lane) were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (EMD Millipore, Billerica, MA, USA). Then, the PVDF membranes were blocked with 5% nonfat milk for 2 h at room temperature, and incubated with the following primary antibodies: antibody-PPARα (1:1000, ab24509), antibody-Acox1 (1:1000, ab184032), antibody-L-PBE (1:1000, ab123490) and antibody-MCAD (1:1000, ab156036). Then, the membranes were incubated with a secondary goat anti-rabbit immunoglobulin G antibody (1:3000, ab214880, Abcam, Cambridge, UK) for 1 h at room temperature. An enhanced chemiluminescence kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for visualization and the images were analyzed using ImageJ v1.8.0 (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
Statistical analysis was performed with SPSS v 20.0 software (IBM Corp., Armonk, NY, USA). Each experiment was repeated in triplicate and all data were presented as means ± standard deviations. Differences among multiple groups were analyzed using variance ANOVA followed by the Dunnett’s post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
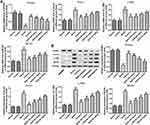
Effects of NGN on Body Weight, Blood Glucose and 24h-UPro Levels
Following NGN treatment for 1 and 2 weeks, a glucometer was used to detect blood glucose levels, while the sulfonyl salicylic acid method was used to determine the 24h-UPro levels. The results indicated that, compared with the normal gestation group, the blood glucose and 24h-UPro levels were significantly increased in the GDM model group (Figure 1B and C, P<0.001). Upon treatment with NGN, the blood glucose and 24h-UPro levels among the NGN groups were significantly decreased in a dose-dependent manner, while metformin treated group has a significant difference compared with non-treatment group. Overall, the general status of the rats in the NGN-treated groups appeared to improve.
Effects of NGN on T-AOC in the Serum
T-AOC level was determined by UV-VIS spectrophotometry. As shown in Figure 2A, compared with the normal gestation group, the T-AOC in the serum was markedly decreased in the GDM group (P<0.01). Following treatment with NGN, the T-AOC in the NGN (30, 50 and 100 mg/kg) groups was significantly increased compared with that in the model group in a dose-dependent manner, while the metformin-treated group (positive control) also exhibited a significant difference compared with the model group (P<0.01).
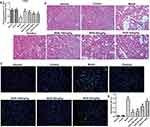
Effects of NGN on Renal Tissue Histopathological Changes in Gestational Diabetic Rats
H&E staining was performed to detect pathological morphological changes in the kidney tissue of rats. As shown in Figure 2B, the renal tissues in the model group exhibited evident histopathological changes. The glomerular volume was increased, the renal tubular epithelial cells exhibited vacuolar degeneration, and there was basement membrane thickening, as well as lymphocytes infiltration. Upon treatment with NGN, the histopathological morphologies in the renal tissues were evidently improved in a concentration-dependent fashion, while the metformin-treated group also exhibited marked improvement compared with the model group.
Effects of NGN on Renal Cell Apoptosis in Gestational Diabetic Rats
To explore the effect of NGN on glomerular cell apoptosis, renal cell apoptosis was observed by TUNEL assay, and the apoptosis index was calculated. As shown in Figure 2C, the number of renal cells undergoing apoptosis was significantly increased in GDM model group than that in control group (p<0.001, Figure 2D). In contrast, in the three NGN groups, renal cell apoptosis was evidently improved, and the apoptosis rate was markedly decreased as compared with the model group, which were also dose-dependent (Figure 2D). Similarly, the metformin-treated positive control group also exhibited a significant reduction in the apoptosis rate compared with the GDM model group (p<0.001, Figure 2D). These results revealed that NGN was able to significantly inhibit the apoptosis of renal cells in GDM rats.
Effects of NGN on Oxidative Stress
The activities of SOD, GSH-Px and CAT, and the content of MDA in renal tissues were determined by ELISA. As shown in Figure 3, compared with the normal gestation group, the activities of SOD, GSH-Px and CAT in renal tissues were significantly decreased (P<0.01 or P<0.001), while the content of MDA in renal tissue was significantly increased (P<0.01) in the GDM model group. Upon treatment with NGN, the activities of SOD, GSH-Px and CAT in the renal tissues of GDM rats were significantly increased, while the content of MDA in renal tissue was significantly decreased compared with model group in a dose-dependent manner. When compared with GDM model group, metformin-treated sharply elevated the activities of SOD, GSH-Px and CAT but decreased MDA in renal tissue (P<0.01 or P<0.001).
Effects of NGN on Inflammatory Cytokines
The levels of inflammation-associated factors were also determined by ELISA. The results revealed that, compared with the normal gestation group, the levels of plasma TNF-α, IL-6, TGF-β and ICAM-1 were significantly increased in the GDM model group (Figure 4, P<0.001). Upon treatment with NGN, the levels of TNF-α, IL-6, TGF-β and ICAM-1 were significantly decreased with increasing NGN dosages (Figure 4), as well as the metformin-treated group also exhibited a significant difference compared with the model group (P<0.001).
Effects of NGN on PPARα Signaling and Fatty Acid Oxidative Metabolic Enzymes
To explore the effect of NGN on fatty acid metabolism in the liver, RT-qPCR and Western blotting were performed to evaluate the expression levels of PPARα, Acox1, L-PBE and MCAD. As shown in Figure 5, the mRNA and protein levels of PPARα were significantly decreased (P<0.001), but the mRNA and protein levels of Acox1, L-PBE and MCAD were significantly increased in the model group compared with the normal gestation group. Upon treatment with NGN, the expression of PPARα was significantly increased, while the expression levels of Acox1, L-PBE and MCAD were significantly decreased in a dose-dependent manner in the GDM rats when compared with GDM model group. Meanwhile, metformin-treated rats also exhibited a significant difference in expression levels of these genes when compared with the model group.
Discussion
Previous evidence suggested that NGN may reduce plasma glucose levels in diabetes.14 Several animal studies have shown that NGN is an anti-hyperglycemic drug, as it ameliorates the complications of diabetes.11,20 In the present study, blood glucose and 24h-UPro levels were significantly decreased following treatment with NGN in GDM rats. These results suggest that NGN may reduce blood sugar levels in gestational diabetes.
Hyperglycemia during pregnancy can induce complications in multiple tissues of the mother, including the kidney.21 In recent years, with the development of pathophysiology, oxidative stress injury and inflammatory response caused by abnormal glucose levels and lipid metabolism have been reported to play a key role in the development of nephrotic complications.22 Previous evidence suggested that oxidative stress plays an important role in the pathogenesis of diabetes mellitus.23 NGN is considered to be an oxidative stress reliever in the treatment of diabetes mellitus. Previous study indicated that NGN reduces MDA levels and controls the insult of lipid peroxidation in diabetes.24 Consistent with the aforementioned findings, the present study demonstrated that NGN could elevate the activities of SOD, GSH-Px and CAT and reduce the content of MDA in these tissues of GDM rats.
It was also observed that NGN improved pathological alterations in the organs of diabetic mice,25 while it significantly inhibited lipid peroxidation in the kidney and liver. In the present study, the results of H&E staining revealed evident histopathological damages in the GDM model group. However, upon treatment with NGN, the histopathological damages in renal tissues were markedly improved. This indicated that NGN had a protective effect on kidney tissue in GDM rats. In addition, it has been reported that NGN reduced hepatic triglyceride and cholesterol levels, and significantly upregulated the expression of PPARα.26 The present study results demonstrated that, following NGN treatment, the expression of PPARα was significantly increased, while the expression levels of Acox1, L-PBE and MCAD were significantly decreased in the GDM rats. Furthermore, it has previously been demonstrated that NGN normalized glucose levels and lipid metabolism, and improved vascular dysfunction in type 2 diabetic rats by reducing oxidative stress and inflammation.27 Similarly, the findings of the present study revealed that NGN promoted the activities of SOD, GSH-Px and CAT in renal tissues, while significantly decreasing the expression levels of inflammatory factors, including TNF-α, IL-6, TGF-β and ICAM-1.
In conclusion, the present study is the first to elucidate renoprotective effects of NGN in GDM. The results indicated that NGN improved blood glucose and 24h-UPro levels in GDM rats, and increased the T-AOC of ROS in the serum. Furthermore, NGN improved the histopathological damages in renal tissues, inhibited the apoptosis of renal cells, improved the activity of antioxidant enzymes, and reduced oxidative stress damage and the levels of inflammatory factors in the kidneys of GDM rats. These findings suggested that NGN was able to significantly improve the antioxidant and anti-inflammatory abilities of the kidneys in GDM rats, and may therefore serve a renoprotective role in GDM.
Ethics Approval and Consent to Participate
All animal experiments and the use of mice were approved by the Ethics Committee of Yixing People’s Hospital. All experimental protocols conducted in the rats were carried out in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgment
The authors would like to thank Mr. Yong Fei Tan, Dr. Hongdi Zhu, Dr. Huiyun Yang and Dr. Fengdi Yuan, and Dr. Tieliang Ma of the clinical laboratory of Yixing People’s Hospital (Wuxi, China).
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Nankervis A, Price S, Conn J. Gestational diabetes mellitus. Aust J Gen Pract. 2018;47:445–449. doi:10.31128/AJGP-01-18-4479
2. Reddy TNI, Kumar K, Lokanatha V, Reddy C, Jagetia G. Cardioprotective effect of naringin in mice treated with doxorubicin. Planta Med. 2008;74:337–348. doi:10.1055/s-2008-1075245
3. Damm P. Future risk of diabetes in mother and child after gestational diabetes mellitus. Int J Gynecology Obstetrics. 2009;104:S25–S26. doi:10.1016/j.ijgo.2008.11.025
4. Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30(Suppl 2):S169–S174. doi:10.2337/dc07-s211
5. Lu Y, Zhang C, Bucheli P, Wei D. Citrus flavonoids in fruit and traditional chinese medicinal food ingredients in China. Plant Foods Hum Nutr. 2006;61:55–63. doi:10.1007/s11130-006-0014-8
6. Harborne JB. Comparative biochemistry of flavonoids—II.: 3-Desoxyanthocyanins and their systematic distribution in ferns and gesnerads. Phytochemistry. 1966;5:589–600. doi:10.1016/S0031-9422(00)83637-7
7. Kuhnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–191.
8. van Acker FAA, Schouten O, Haenen GRMM, van der Vijgh WJF, Bast A. Flavonoids can replace α-tocopherol as an antioxidant. FEBS Lett. 2000;473:145–148. doi:10.1016/S0014-5793(00)01517-9
9. Priscilla MJ DH, Thirumurugan K. Flavanone naringenin: an effective antihyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J Funct Foods. 2015;14:363–373. doi:10.1016/j.jff.2015.02.005
10. Liu Y, An W, Gao A. Protective effects of naringenin in cardiorenal syndrome. J Surg Res. 2016;203:416–423. doi:10.1016/j.jss.2016.03.003
11. Visnagri A, Adil M, Kandhare AD, Bodhankar SL. Effect of naringin on hemodynamic changes and left ventricular function in renal artery occluded renovascular hypertension in rats. J Pharm Bioallied Sci. 2015;7:121–127. doi:10.4103/0975-7406.154437
12. Tsai S-J, Huang C-S, Mong M-C, Kam W-Y, Huang H-Y, Yin M-C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J Agric Food Chem. 2012;60:514–521. doi:10.1021/jf203259h
13. Mulvihill EE, Burke AC, Huff MW. Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu Rev Nutr. 2016;36:275–299. doi:10.1146/annurev-nutr-071715-050718
14. Ortiz-Andrade RR, Sánchez-Salgado JC, Navarrete-Vázquez G, et al. Antidiabetic and toxicological evaluations of naringenin in normoglycaemic and NIDDM rat models and its implications on extra-pancreatic glucose regulation. Diabetes Obesity Metab. 2008;10:1097–1104. doi:10.1111/dom.2008.10.issue-11
15. Qi Z, Xu Y, Liang Z, et al. Naringin ameliorates cognitive deficits via oxidative stress, proinflammatory factors and the PPARgamma signaling pathway in a type 2 diabetic rat model. Mol Med Rep. 2015;12:7093–7101. doi:10.3892/mmr.2015.4232
16. Chtourou Y, Kamoun Z, Zarrouk W, et al. Naringenin ameliorates renal and platelet purinergic signalling alterations in high-cholesterol fed rats through the suppression of ROS and NF-kappaB signaling pathways. Food Funct. 2016;7:183–193. doi:10.1039/C5FO00871A
17. Rajadurai M, Stanely Mainzen Prince P. Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology. 2007;230:178–188. doi:10.1016/j.tox.2006.11.053
18. Singh D, Chander V, Chopra K. Protective effect of naringin, a bioflavonoid on glycerol-induced acute renal failure in rat kidney. Toxicology. 2004;201:143–151. doi:10.1016/j.tox.2004.04.018
19. Guo Y, Wang L, Ma R, et al. JiangTang XiaoKe granule attenuates cathepsin K expression and improves IGF-1 expression in the bone of high fat diet induced KK-Ay diabetic mice. Life Sci. 2016;148:24–30. doi:10.1016/j.lfs.2016.02.056
20. Tsai SJ, Huang CS, Mong MC, Kam WY, Huang H-Y, Yin M-C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J Agric Food Chem. 2012;60:514–521. doi:10.1021/jf203259h
21. Khaki A, Khaki AA, Hajhosseini L, Golzar FS, Ainehchi N. The anti-oxidant effects of ginger and cinnamon on spermatogenesis Dys-function of diabetes rats. Afr J Traditional Complementary Altern Med. 2014;11:1–8. doi:10.4314/ajtcam.v11i4.1
22. Dalla Vestra M, Mussap M, Gallina P, et al. Acute-phase markers of inflammation and glomerular structure in patients with Type 2 diabetes. J Am Soc Nephrol. 2005;16:S78. doi:10.1681/ASN.2004110961
23. Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochimica Et Biophysica Acta (BBA) - Genl Subj. 2014;1840:2709–2729. doi:10.1016/j.bbagen.2014.05.017
24. Annadurai T, Muralidharan AR, Joseph T, Hsu MJ, Thomas PA, Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin–nicotinamide-induced experimental diabetic rats. J Physiol Biochem. 2012;68:307–318. doi:10.1007/s13105-011-0142-y
25. Sirovina D, Orsolic N, Gregorović G, Končić MZ. Naringenin ameliorates pathological changes in liver and kidney of diabetic mice: a preliminary study. Arh Hig Rada Toksikol. 2016;67:19–24. doi:10.1515/aiht-2016-67-2708
26. Cho KW, Kim YO, Andrade JE, Burgess JR, Kim Y-C. Dietary naringenin increases hepatic peroxisome proliferators–activated receptor α protein expression and decreases plasma triglyceride and adiposity in rats. Eur J Nutr. 2011;50:81–88. doi:10.1007/s00394-010-0117-8
27. Ren B, Qin W, Wu F, et al. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur J Pharmacol. 2016;773:13–23. doi:10.1016/j.ejphar.2016.01.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.