Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
The Relevant of Sex Hormone Levels and Acne Grades in Patients with Acne Vulgaris: A Cross-Sectional Study in Beijing
Authors Zhang R , Zhou L , Lv M , Yue N , Fei W , Wang L, Liu Z, Zhang J
Received 15 August 2022
Accepted for publication 13 October 2022
Published 18 October 2022 Volume 2022:15 Pages 2211—2219
DOI https://doi.org/10.2147/CCID.S385376
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Rui Zhang1 *, Lanhua Zhou2 *, Meiyu Lv,1 Na Yue,1 Wenting Fei,3 Linyuan Wang,3 Zhaolan Liu,1 Jianjun Zhang1
1School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, 102488, People’s Republic of China; 2Department of Dermatology, Beijing Jingcheng Skin Hospital, Beijing, 100192, People’s Republic of China; 3School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianjun Zhang, School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Liangxiang Town, Fangshan District, Beijing, 102488, People’s Republic of China, Email [email protected]
Background: The tests of sex hormones play pivotal roles in the clinical diagnosis and treatment of acne vulgaris, but the majority of patients with acne vulgaris present regular sex hormone levels within the normal reference range.
Objective: To determine the correlation among levels of sex hormones, ratio of androgen to estrogen and acne grades in patients with acne vulgaris.
Methods: A cross-sectional study was applied to collect 693 patients with acne vulgaris. All samples were screened by cluster sampling among those who underwent tests of sex hormones at Beijing Jingcheng Skin Hospital from July 2021 to June 2022. A gender stratified analysis was performed to classify acne grades I–IV. Spearman correlation analysis was used to analyze the relationship between age, sex hormones, ratio of androgen to estrogen and acne grades, with multinomial logistic regression to analyze the association of sex hormones with acne grades in patients with acne.
Results: (1) The testosterone levels were mostly within normal reference values for both males and females with varying degrees of acne. For females, the serum follicle-stimulating hormone, estradiol, progesterone, testosterone, and ratio of androgen to estrogen were significantly different between acne grades. For males, there were significant differences in serum estradiol, testosterone, and ratio of androgen to estrogen across acne grades. (2) The acne grade was negatively correlated with estradiol and positively correlated with the ratio of androgen to estrogen; the female acne grade was also negatively correlated with age and progesterone, but positively correlated with follicle-stimulating hormone. (3) Multivariate logistic regression analysis indicated that the ratio of androgen to estrogen was independently correlated with the grade of acne and that acne grade worsened as the ratio increased.
Conclusion: The increase in the ratio of androgen to estrogen may aggravate the acne grade in patients with acne vulgaris.
Keywords: acne vulgaris, sex hormones, testosterone, estradiol, ratio of androgen to estrogen
Introduction
Acne vulgaris is the eighth most common non-fatal disease in the world, with a prevalence of 9.38% in the world’s population, with a prevalence of approximately 8.96% in men and 9.81% in women.1 Acne vulgaris is a chronic inflammatory skin disease that occurs mostly in areas with a high density of pilosebaceous glands, including face, back, chest and shoulders.2 Acne in adolescence, on a larger scale, reveals different clinical characteristic by the appearance of skin eruptions such as comedones, papules, nodules, pustules, and seborrhea.3 In China, the prevalence of acne is about 39.2%,4 compared to 42.2–73.5% in some European countries, with the highest prevalence between the ages of 15–17 years.5 Additionally, about 33% of people aged 15 to 44 suffer from acne,6 and the prevalence of acne in adolescents is about 80 to 90%, while it usually subsides by the age of 30.7 The acne scarring and hyperpigmentation formed by inflammatory lesions exert considerable physical and psychological stress on the patient.8 The pathogenesis of acne has not been fully elucidated, and multiply pathogenesis is currently thought to be involved, including genetic factors, androgen-induced sebaceous gland oversecretion of lipids, abnormal keratinization of pilosebaceous ducts, proliferation of skin microorganisms such as Cutibacterium acnes (formerly known as Propionibacterium acnes),9 and local inflammation as well as immune response.10 The treatment in clinical practice is currently focused on targeting inflammatory and bacterial therapies, adjusting hormone levels, exfoliating and other treatments that may achieve favorable efficacy, but will undergo a long course. The sense of patients are hopelessness and social isolation.11 Notably, patients need to maintain the therapy for a long time even during the recovery period, as drug withdrawal may lead to a relapse of the disease.12
As the sex hormone levels, especially the serum testosterone levels, are mostly within the normal reference range, a number of acne patients fail to account for elevated androgen levels in clinical practice, but the occurrence and development of acne are closely related to androgen levels.13 Androgens can be converted to estrogens in vivo with the help of aromatase, suggesting that the ratio of androgens to estrogens should be paid attention to, which can be of great benefit in understanding the occurrence and development of acne.14 Therefore, this study investigated the case reports from July 2021 to June 2022 at the Beijing Jingcheng Skin Hospital, with the main diagnosis of acne vulgaris, and took six tests of sex hormones. The correlation between acne and sex hormones, especially the ratio between men and women, was analyzed to explore the underlying mechanisms.
This study aims to evaluate the correlation between serum sex hormone levels and acne grade in patients with acne vulgaris and to explore the possibility of a relationship between testosterone/estradiol levels (A/E, ratio of androgen to estrogen) and acne vulgaris grade. To our knowledge, this study is the first to address this question in Beijing, China.
Methods
Calculation of Sample Size
The sample size was calculated according to the factor variables of multivariate logistic regression and each univariate variable has a minimum of 15 valid sample sizes.15 A total of nine factor variables were considered in this study including sex, age, six sex hormones, and ratio of androgen to estrogen. Given that there are 4 levels in the outcome variable, which multiply by 4, at least 540 cases are required.
General Information and Ethics
Cluster sampling collected the medical records of patients tested for sex hormones with a primary diagnosis of acne vulgaris from July 2021 to June 2022 at Beijing Jingcheng Skin Hospital. In the case of the same patient, the first test data will prevail and those with incomplete basic information will be excluded. All the data were collected by the information engineer of Beijing Jingcheng Skin Hospital, and informed consent for observation was obtained from the Hospital and each of the participants. This study was approved by the Ethics Committee of the Beijing University of Chinese Medicine and conducted according to the principles of the Declaration of Helsinki. Study Registration Scheme ChiCTR2100054470.
Study Methodology
Our study documented clinical data through the hospital’s electronic pathology data system and data control center, and the patient’s medical records and test report data were retrieved to gather the baseline data (including age, gender), laboratory data (luteinizing hormone, follicle-stimulating hormone, estradiol, total testosterone, and progesterone). The participants were classified into 4 groups, according to the clinical diagnosis and Pillsbury acne grade I–IV. Given that gender is responsible for the variations in hormone levels, they were divided into two subgroups, male and female, for statistical analysis.
Diagnostic Criteria and Grading Criteria
The diagnosis of acne vulgaris was based on the diagnostic criteria established in Zhao Bian’s China Clinical Dermatology.16 All the included cases are in line with the diagnostic points of acne: (1) it mostly occurred in adolescence; (2) it mostly involved areas with developed sebaceous glands, including the face, chest, and back (3) the lesions were scattered comedones, papules, pustules, etc., mostly symmetrically distributed (4) there were blackheads discharged after squeezing the comedones with yellow-white translucent fat plugs left (5) patients are usually asymptomatic and may sometimes have pain and tenderness.
Grading is performed according to the Pillsbury Acne Scale:17 Grade I: comedones and occasional small cysts confined to the face; Grade II: comedones, occasional pustules, and small cysts confined to the face; Grade III: many comedones, small and large inflammatory papules and pustules, more extensive but confined to the face; Grade IV: many comedones and deep lesions tend to merge and form tubules, involving the face and upper trunk.
Blood Sample Collection Specifications, Collection Requirements, Testing Reagents, and Equipment
All blood samples in the hospital were collected in the following manner: venous blood was obtained by antecubital venipuncture and analyzed within 1 hour after sampling using a KWS-5mL-PET vacuum blood collection tube. The concentrations of luteinizing hormone, follicle-stimulating hormone, estradiol, progesterone, testosterone, and prolactin were detected by magnetic particle chemiluminescence method using AutoLumo A2000 plus automatic chemiluminescence analyzer following the guidance and reference values of the normal human. All the kits were purchased from AUTOBIO DIAGNOSTICS. It is noted that all female blood samples were collected during the follicular phase of the menstrual cycle. The ratio of androgen to estrogen (A/E) = The concentrations of testosterone (nmol/L) ÷ The concentrations of estradiol (pmol/L) ×1000.
Bias and Confounders Control
Several studies have shown that body mass index,18 the number of smoking frequency,19,20 and serum 25-hydroxyvitamin D levels21 are correlated with acne grades. Given that the collected cases were derived from the medical outpatient management system, and the specificity of outpatient medical diagnosis and treatment (the statistical information on living habits is not perfect) and survey recall bias, the aforementioned information bias and confounding factors were not within the scope of this statistical study. Our study expanded the sample size of the survey population to control for the aforementioned bias. Considering diseases with a large impact on the hormone level, exclude patients with secondary diagnosis of polycystic ovary syndrome, seborrheic alopecia, seborrheic dermatitis and chloasma.
Statistical Analysis
SPSS 22.0 statistical software was used for data processing. The normally distributed measurement data were expressed as mean ± standard deviation (SD), and one-way ANOVA was used for comparison between groups. In addition, the non-normally distributed measurement data were expressed as the median (Q1-Q3), and Kruskal–Wallis test was used for the rank sum test to describe the difference among groups. Non-normally distributed measurement data were analyzed using Spearman correlation, while the enumeration data were expressed as [n (%)] via the χ2 test. Multinomial logistic regression was used to analyze the relationship between the relevant variables and acne grade, with differences considered statistically significant at p <0.05.
Results
Study Participants
A total of 696 clinical medical records were enrolled in our study, with no duplicate patients. There is no patient with secondary diagnosis of polycystic ovary syndrome, seborrheic alopecia, seborrheic demateria and chloroma. Three patients with incomplete basic information were excluded, leaving 693 patients, including 565 females and 128 males, generally between the ages of 20 and 30 years. Moderate acne (Grade II and III) accounted for a large proportion among patients with varying degrees of acne with mild acne (Grade I) in few numbers. (Tables 1 and 2)
 |
Table 1 Acne Vulgaris Patients Study Participants |
 |
Table 2 Acne Vulgaris Patients Pillsbury Grades |
Comparison of Biochemical Results of Serum Sex Hormone Concentrations in Males and Females
The data showed that serum follicle-stimulating hormone, estradiol, progesterone, testosterone, and the ratio of androgen to estrogen among different acne grades were statistically significant in female acne patients (p <0.05), but there was no difference in luteinizing hormone and prolactin. Additionally, serum estradiol, testosterone, and the ratio of androgen to estrogen among different acne grades were statistically significant in male acne patients (p <0.05), but follicle-stimulating hormone, luteinizing hormone, progesterone, and prolactin did not differ among acne grades. Intriguingly, it was found that the hormone levels of male acne patients were mostly within the normal reference range. And, notably, only 2 of 128 male acne patients showed higher testosterone levels, while 4 (4.3%) of 565 female acne patients were at lower values. Furthermore, estradiol was observed to increase in 49 (8.7%) patients and decline in 210 (37.2%) patients (Tables 3–5).
 |
Table 3 Associations Between Acne Grades and Serum Sex Hormone Levels in Female Acne Patients |
 |
Table 4 Associations Between Acne Grades and Serum Sex Hormone Levels in Male Acne Patients |
 |
Table 5 The Reference Ranges of Serum Sex Hormones |
Univariate Correlation Analysis Between Sex Hormone Levels and Acne Grades
The Spearman analyses were carried out considering all influencing factors and found that the acne grades of female and male were negatively correlated with estradiol expression (p <0.05), and positively correlated with testosterone and the ratio of androgen to estrogen (p <0.05). Female acne grades presented negatively correlated with age and progesterone (p <0.05), and positively association with follicle-stimulating hormone (p <0.05) (Table 6).
 |
Table 6 Correlation Analysis Between Hormone Level and Acne Grade |
Multivariate Logistic Regression Analysis of Multiple Factors Affecting Acne Grade
Based on the results of the above correlation analysis, variables with p <0.05 were subjected to multinomial logistic regression and analyzed according to the −2-fold log-likelihood value. Once selected, they were again included in the multinomial logistic regression affecting acne grade. Taking Grade I (mild) acne as a reference comparison in the analysis, the ratio of androgen to estrogen served as an independent risk factor for acne grade with acne worsening as the ratio of androgen to estrogen increased (Tables 7 and 8).
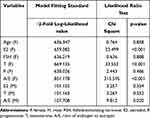 |
Table 7 Multivariable Analysis of the Relationship Among Sex Hormone Levels and Acne |
 |
Table 8 Multiple Logistic Regression Analysis of Sex Hormone Level and Acne Grade (Taking Grade I Acne Patients as a Reference Comparison) |
Discussion
Previous researches have not documented the effect of the ratio of androgen to estrogen on acne aggravation, it is the first time in this work to investigate their correlations in Beijing region. As compared to women, it has been reported that the incidence of acne in men is slightly lower, with the adolescence and early adulthood in the supreme risk. Notably, this trend reverses with age and women are more susceptible in adult.22 In this study, fewer male acne patients received formal treatment than female acne patients, which may attribute to the fact that women place more importance on appearance than men, as acne is considered a non-lethal disease that is often overlooked by male patients in its mild stages. The age of the patients for clinical therapy was mostly concentrated between 20 to 30 years old. In addition, given that acne is a self-limiting disease, most patients are reluctant to be diagnosed and treated due to mild symptoms in Grade I, which could explain the inferior number of patients with Grade I acne coming to the hospital for treatment. It was observed that abnormally elevated testosterone values were closely associated with acne in only 2 men and 4 women from the serum sex hormone results, accounting for a minority of the total number investigated, which was consistent with the findings of Egyptian scholars23 and Pakistani scholars,24 despite contrary to the studies of Indian scholars.25 Diminished levels of estradiol occupied 37.2% of female acne patients, which was in line with Turkish scholars.26 The acne grades were significantly correlated with estradiol and the ratio of androgen to estrogen, the latter being an independent risk factor through multivariate logistic regression. Notably, there was a tendency for the disease to exacerbate as the ratio of androgen to estrogen increased.
Many women experience periodic exacerbations of premenstrual acne. A survey of 3065 young Egyptian women found that 56.7% of acne sufferers experienced the exacerbations during menstruation.27 Another survey of 400 American women found that 44% experienced a worsening of premenstrual acne.28 The periodic exacerbations of premenstrual acne may attribute to the variations in the level of sex hormones in the body caused by various factors. During ovulation, the increase in progesterone promotes the synthesis of a large amount of testosterone, the increase in the number of sebaceous glands, and changes in the composition of sebum. Considering that estrogen and progesterone exert a reduction in pro-inflammatory cytokine activity and anti-inflammatory effects,29 the decrease in premenstrual hormone levels attenuated the inhibitory capacity of androgens, thereby weakening the anti-inflammatory effects and contributing to greater susceptibility to inflammatory responses.30 The role of progesterone in acne pathogenesis is controversial as it, as a competitive inhibitor of 5α reductase, may play a role in reducing sebaceous gland activity, but its inhibitory effect on sebum is minimal in humans.31 However, Kanda’s research32 reported that progesterone aggravates acne by stimulating sebum secretion and keratinocyte proliferation. Therefore, there is a lack of consensus on the relationship between acne vulgaris and serum progesterone levels in women. In addition, the role of estrogen-rich foods and drugs containing a large amount of estrogen in the treatment of acne also generated much discussion. It is currently believed that high levels of estrogen can protect acne by reducing endogenous androgen secretion and leading to sebaceous gland suppression.
In clinical practice, the ratio of aspartate aminotransferase/alanine aminotransferase is used to reflect the damage to liver cells. Specifically, the ratio of direct bilirubin / total bilirubin indicates the type of jaundice, while the ratio of albumin/globulin indicates liver cirrhosis, both of which are essential for clinical diagnosis. Another study pointed out that adjusting the ratio of estrogen to androgen in the body can alter the lipid metabolism in the male liver.33 Changes in the ratio of androgen to estrogen are associated with the development of benign and malignant prostate disease, and the imbalanced ratio of estrogen and androgen may affect prostate fibrosis.34,35 Experimental data suggested that modulation of the androgen to estrogen ratio was expected as a potential target for diabetic nephropathy.36 Therefore, it is speculated that the development of acne is related to the ratio of androgen to estrogen. Serum luteinizing hormone, follicle-stimulating hormone, estradiol, testosterone, progesterone, and prolactin are the most frequently used indicators of endocrine function. Luteinizing hormone Genitin and follicle-stimulating hormone act primarily on the follicles to accelerate follicle development and regulate estrogen secretion. Mature follicles secrete a large amount of estradiol, participating in the rapid increase in serum estradiol levels, which in turn has a feedback effect on the hypothalamus.37,38 Then, the estradiol stimulates the hypothalamus to release a large amount of gonadotropin, driving the pituitary to secrete luteinizing hormone and follicle-stimulating hormone. Testosterone is an androgen mostly regulated by luteinizing hormone and has a negative feedback regulation effect on the hypothalamus-pituitary gland. Given its role as an essential intermediate in sex steroid synthesis, testosterone can be further converted into estradiol by aromatase. The appearance of acne and bisexual sebum production in prepubertal girls correlates with serum dehydroepiandrosterone levels, whereas patients with acne vulgaris present with elevated serum progesterone levels and reduced estrogen levels,39 suggesting that the onset or extent of acne cannot be determined by a single change in estrogen or androgenic. The conversion of androgens to estrogens via aromatase in vivo, and the regulation ability of rate-limiting enzymes to be altered by external or pharmacological stimuli, resulting in an imbalance in the conversion from androgens to estrogens, might be a central process in the development of acne.
This study found that the ratio of androgen to estrogen of patients with acne vulgaris was an independent risk factor for acne grade, highlighting the importance of screening for the ratio of androgen to estrogen in acne patients. Larger clinical trials are needed to identify the significance of sex ratio on acne grades.
Conclusion
The ratio of androgen to estrogen was identified as an independent risk factor for acne grade, and acne grade escalated as the ratio increased. Therefore, the ratio of androgen to estrogen has the potential to be an indicator of acne grades.
Study Limitations
There still existed some limitations in our study as the research used the cross-sectional design with the scope of the survey confined to one dermatology hospital, which leads to the finite sample size with only the age and gender in the medical records of the acne patients. However, the secondary diagnosis of the patients, body mass index, lifestyle habits, and genetic factors were not further analyzed, thus resulting in selection bias. Therefore, larger sample sizes will be urgently warranted to confirm the current results in the future. In our study, we found that the number of women who diagnosed and treated acne vulgaris in hospitals was 4.4 times that of men, which was not due to the different prevalence of acne vulgaris in men and women. Therefore, in future clinical trials, the sample impact caused by this imbalance between men and women should be considered.
Abbreviations
A/E, ratio of androgen to estrogen; E2, estradiol; F, female; FSH, follicle-stimulating hormone; LH, luteinizing hormone; M, male; N, numbers; P, progesterone; PRL, prolactin; SD, standard deviation; T, testosterone.
Data Sharing Statement
No additional data are available. The authors intend to share individual identified participant data except for basic information of participants. The data can be obtained from each author.
Funding
This study was funded by the National Key R&D Program of China, Grant/Award Number: 2018YFC1706800.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi:10.1016/S0140-6736(12)61729-2
2. Cooper AJ, Harris VR. Modern management of acne. Med J Aust. 2017;206(1):41–45. doi:10.5694/mja16.00516
3. Chilicka K, Rogowska AM, Szyguła R, Rusztowicz M, Nowicka D. Efficacy of oxybrasion in the treatment of acne vulgaris: a preliminary report. J Clin Med. 2022;11(13):3824. doi:10.3390/jcm11133824
4. Li D, Chen Q, Liu Y, Liu T, Tang W, Li S. The prevalence of acne in Mainland China: a systematic review and meta-analysis. BMJ Open. 2017;7(4):e015354. doi:10.1136/bmjopen-2016-015354
5. Wolkenstein P, Machovcová A, Szepietowski JC, Tennstedt D, Veraldi S, Delarue A. Acne prevalence and associations with lifestyle: a cross-sectional online survey of adolescents/young adults in 7 European countries. J Eur Acad Dermatol Venereol. 2018;32(2):298–306. doi:10.1111/jdv.14475
6. Stern RS. The prevalence of acne on the basis of physical examination. J Am Acad Dermatol. 1992;26(6):931–935. doi:10.1016/0190-9622(92)70135-3
7. Dash G, Patil A, Kroumpouzos G, et al. Hormonal therapies in the management of acne vulgaris. J Drugs Dermatol. 2022;21(6):618–623. doi:10.36849/JDD.6494
8. Tan JK. Psychosocial impact of acne vulgaris: evaluating the evidence. Skin Therapy Lett. 2004;9(7):1–3, 9.
9. Chilicka K, Rogowska AM, Rusztowicz M, et al. The effects of green tea (camellia sinensis), bamboo extract (Bambusa vulgaris) and lactic acid on sebum production in young women with acne vulgaris using sonophoresis treatment. Healthcare. 2022;10(4):684. doi:10.3390/healthcare10040684
10. Knutsen-Larson S, Dawson AL, Dunnick CA, Dellavalle RP. Acne vulgaris: pathogenesis, treatment, and needs assessment. Dermatol Clin. 2012;30(1):99–106, viii–ix. doi:10.1016/j.det.2011.09.001
11. Chilicka K, Rogowska AM, Szyguła R. Effects of topical hydrogen purification on skin parameters and acne vulgaris in adult women. Healthcare. 2021;9(2):144. doi:10.3390/healthcare9020144
12. Fox L, Csongradi C, Aucamp M, du Plessis J, Gerber M. Treatment modalities for acne. Molecules. 2016;21(8):1063. doi:10.3390/molecules21081063
13. Hu T, Wei Z, Ju Q, Chen W. Sex hormones and acne: state of the art. J Dtsch Dermatol Ges. 2021;19(4):509–515. doi:10.1111/ddg.14426
14. Li X, Rahman N. Impact of androgen/estrogen ratio: lessons learned from the aromatase over-expression mice. Gen Comp Endocrinol. 2008;159(1):1–9. doi:10.1016/j.ygcen.2008.07.025
15. Gao Y, Zhang J. Determination of sample size in logistic regression analysis [In Chinese]. J Evid Based Med. 2018;18(2):122–124.
16. Zhao B. China Clinical Dermatology. Jiangsu Science and Technology Press; 2017.
17. Witkowski JA, Parish LC. The assessment of acne: an evaluation of grading and lesion counting in the measurement of acne. Clin Dermatol. 2004;22(5):394–397. doi:10.1016/j.clindermatol.2004.03.008
18. Chaudhary S, Ameer A, Sarwar MZ, Naqi SA, Butt AI. A cross-sectional study of body mass index and sleep quality as risk factors to severity of acne. J Pak Med Assoc. 2021;71(9):2148–2150. doi:10.47391/JPMA.404
19. Zhang JZ, Xiang F, Yu SR, Luo D, Li TT, Kang XJ. Association between acne and smoking: systematic review and meta-analysis of observational studies. Chin Med J. 2021;134(15):1887–1888. doi:10.1097/CM9.0000000000001286
20. Rombouts S, Nijsten T, Lambert J. Cigarette smoking and acne in adolescents: results from a cross-sectional study. J Eur Acad Dermatol Venereol. 2007;21(3):326–333. doi:10.1111/j.1468-3083.2006.01915.x
21. Rasti SD, Dewinta NR, Kamal RH, Adissadah AF, Madanny AE, Dewanti L. Correlation between serum 25-hydroxy vitamin D levels and the severity of acne vulgaris: a systematic review. Indian J Dermatol. 2022;67(1):31–36. doi:10.4103/ijd.ijd_871_21
22. Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172(Suppl 1):3–12. doi:10.1111/bjd.13462
23. Bakry OA, El SR, El FS, Kotb D. Role of hormones and blood lipids in the pathogenesis of acne vulgaris in non-obese, non-hirsute females. Indian Dermatol Online J. 2014;5(Suppl 1):S9–S16. doi:10.4103/2229-5178.144506
24. Rahman M, Khondker L, Hazra S, Khan M. Association of serum testosterone with acne vulgaris in women-a case control study. J Pak Assoc Dermatol. 2012;2012(22):105–111.
25. Gayen R, Podder I, Chakraborty I, Chowdhury SN. Sex hormones, metabolic status, and obesity in female patients with acne vulgaris along with clinical correlation: an observational cross-sectional study. Indian J Dermatol. 2021;66(1):60–66. doi:10.4103/ijd.IJD_82_20
26. Akdogan N, Dogan S, Atakan N, Yalin B. Association of serum hormone levels with acne vulgaris: low estradiol level can be a pathogenetic factor in female acne. Our Dermatol Online. 2018;9(3):249–256. doi:10.7241/ourd.20183.4
27. Arafa A, Mostafa A, Khamis Y. The association of acne and menstrual symptoms among young women (18–25 years) in Egypt: a population-based cross-sectional study. Int J Adolesc Med Health. 2020;33(6):463–468. doi:10.1515/ijamh-2019-0220
28. Stoll S, Shalita AR, Webster GF, Kaplan R, Danesh S, Penstein A. The effect of the menstrual cycle on acne. J Am Acad Dermatol. 2001;45(6):957–960. doi:10.1067/mjd.2001.117382
29. Saint-Jean M, Khammari A, Seite S, Moyal D, Dreno B. Characteristics of premenstrual acne flare-up and benefits of a dermocosmetic treatment: a double-blind randomised trial. Eur J Dermatol. 2017;27(2):144–149. doi:10.1684/ejd.2016.2952
30. Slowik A, Beyer C. Inflammasomes are neuroprotective targets for sex steroids. J Steroid Biochem Mol Biol. 2015;153:135–143. doi:10.1016/j.jsbmb.2015.02.013
31. Simpson NB, Cunliffe WJ. Disorders of the Sebaceous Glands.: Rook’s Textbook of Dermatology. Wiley Online Library; 2004.
32. Kanda N, Watanabe S. Regulatory roles of sex hormones in cutaneous biology and immunology. J Dermatol Sci. 2005;38(1):1–7. doi:10.1016/j.jdermsci.2004.10.011
33. Castagnetta LA, Carruba G, Granata OM, et al. Increased estrogen formation and estrogen to androgen ratio in the synovial fluid of patients with rheumatoid arthritis. J Rheumatol. 2003;30(12):2597–2605.
34. Ellem SJ, Risbridger GP. Aromatase and regulating the estrogen: androgenratio in the prostate gland. J Steroid Biochem Mol Biol. 2010;118(4–5):246–251. doi:10.1016/j.jsbmb.2009.10.015
35. Cao Y, Tian Y, Zhang H, Luo GH, Sun ZL, Xia SJ. Imbalance in the estrogen/androgen ratio may affect prostate fibrosis through the TGF-β/Smad signaling pathway. Int Urol Nephrol. 2022;54(3):499–508. doi:10.1007/s11255-021-03079-z
36. Inada A, Inada O, Fujii NL, et al. Adjusting the 17β-estradiol-to-androgen ratio ameliorates diabetic nephropathy. J Am Soc Nephrol. 2016;27(10):3035–3050. doi:10.1681/ASN.2015070741
37. Salehpour S, Hosseini S, Nazari L, Saharkhiz N, Zademodarres S. Effects of orlistat on serum androgen levels among iranian obese women with polycystic ovarian syndrome. JBRA Assist Reprod. 2018;22(3):180–184. doi:10.5935/1518-0557.20180033
38. Singh A, Bora P, Krishna A. Direct action of adiponectin ameliorates increased androgen synthesis and reduces insulin receptor expression in the polycystic ovary. Biochem Biophys Res Commun. 2017;488(3):509–515. doi:10.1016/j.bbrc.2017.05.076
39. Ju Q, Tao T, Hu T, Karadağ AS, Al-Khuzaei S, Chen W. Sex hormones and acne. Clin Dermatol. 2017;35(2):130–137. doi:10.1016/j.clindermatol.2016.10.004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
