Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
The Relationship of Drug Therapy to Aplastic Anemia in Pakistan: A Hospital-Based Case Control Study
Authors Syed MA , Atta Ur Rahman A, Syed MNS , Memon NM
Received 22 June 2021
Accepted for publication 9 August 2021
Published 27 August 2021 Volume 2021:17 Pages 903—908
DOI https://doi.org/10.2147/TCRM.S325742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Muhammad Asif Syed,1 Aneela Atta Ur Rahman,2 Muhammad Nadeem Shah Syed,3 Naveed Masood Memon4
1Field Epidemiology & Laboratory Training Program (FELTP), Karachi, Pakistan; 2Department of Community Medicine & Public Health Sciences, Liaquat University of Medical and Health Sciences, Jamshoro, Sindh, Pakistan; 3Federal NSTOP Officer, National Emergency Operation Center, Islamabad, Pakistan; 4Provincial Disease Surveillance and Response Unit, Hyderabad-Sindh, Field Epidemiology & Laboratory Training Program (FELTP), Karachi, Pakistan
Correspondence: Muhammad Asif Syed
Field Epidemiology Laboratory Training Program (FELTP) Pakistan, Karachi, Sindh, Pakistan
Tel +0092-286-34-77
Email [email protected]
Introduction: Drug-induced aplastic anemia has long been a menacing outcome of modern pharmacotherapy. The incidence of idiosyncratic, drug-induced aplastic anemia varies depending on the genetic susceptibility and the associated drug. Only scarce studies have explained the epidemiology and actual incidence of this reaction.
Purpose: The aim of the study was to establish the association between drugs and aplastic anemia.
Methods: A case-control study was conducted with 191 cases and 696 controls at a tertiary hospital for blood diseases in Karachi-Pakistan. Cases were patients of aplastic anemia diagnosed through bone marrow biopsy. The controls did not have either AA or chronic diseases. Each case was paired with four sex and age group match controls. Cases and controls were compared with respect to the drugs used. Univariate and multivariate analysis were performed in order to delineate the association.
Results: Median age of the study-participants was 27 years (04– 69 years). The majority 84 (44%) were from age group 16 to 30 years. The male-to-female ratio was 2:1. Among study participants, various drugs were significantly associated with aplastic anemia. Treatment of epilepsy with carbamazepine showed a positive association (OR=2.7, 95% C.I, 1.0– 6.8). An increased risk of aplastic anemia was noted with exposure to thiazide (OR=3.1, 95% C.I, 1.3– 7.4) and mebendazole (OR=3.7, 95% C.I, 1.5– 9.2). However, risks were not increased with chloramphenicol, trimethoprim/sulfamethoxazole, benzodiazepines, antihistamines, oral contraceptives, and herbal medicine.
Conclusion: This large-scale case–control study provide association of aplastic anemia with exposure to carbamazepine, thiazides and mebendazole in population of Pakistan. Patients should be monitored with complete blood indices for early detection of drug toxicity.
Keywords: drug induced aplastic anemia, case–control study, Karachi, Pakistan
Introduction
Drug-induced aplastic anemia is a life-threatening reactions associated with certain drugs which have the potential to be toxic to the bone marrow. Dose-dependent and idiosyncratic toxicity are the two of the probable mechanisms for drug-induced aplastic anemia.1 Direct, metabolite-driven and immune-mediated toxic product damaged the hematopoietic stem cells before their differentiation to stem cells.2 The mean lag time from onset of symptoms to therapy is 6.5 weeks (range few days to months).3 The degree of bone marrow suppression and pancytopenia depends on the nature of the particular drug and its potential for toxicity.4
Chloramphenicol seems to be the biggest culprit.5–8 The estimated incidence of aplastic anemia is 1 in 20,000 among the patients treated with chloramphenicol.9 From 1950 to 1965, the chloramphenicol-induced aplastic anemia accounts for 22% of the total cases. The overall prevalence has declined with decreased use of chloramphenicol in recent decades. The time between exposure and onset of the symptoms of aplastic anemia spans to months.10
Antiepileptic drugs carbamazepine, phenytoin, and valproic acid induced aplastic anemia occurs via toxic metabolites.11–13 The risk of aplastic anemia ranging from 1.4 to 60 cases/million, was detected among users of carbamazepine (within a 5-month period).14 Felbamate proved to be a risk factor for aplastic anemia with an incidence of 127 cases/million per year.6 Handoko et al reported that poly-therapy with the antiepileptic drug was more strongly associated with aplastic anemia than the mono-therapy.15 Other higher-risk agents for aplastic anemia include Thiazides,6,16,17 and Mebendazole.6–16
The other most cited drugs for aplastic anemia are, thiazides, gold salts, and penicillamine, amidopyrine, trimethoprim/sulfamethoxazole and antithyroid medications, in addition to the miscellaneous agents.5–14
Before prescribing any such drug that is known to cause aplastic anemia; the benefits (control or cure the illness) always need to be weighed against the risks of the aplastic anemia in addition to other adverse effects. The expected side effects (minimal to life-threatening) can be avoided by the selection of another therapeutic agent with very few to no side effects, lifestyle changes, medication discontinuation and monitoring of the health of the patients.
In Pakistan, there is a scarcity of data about aplastic anemia and its association with drugs. In the present study, we have the objective to determine the linkage of different drugs with the risk of aplastic anemia.
Methodology
A case-control study was conducted from 2015 to 2018 in Karachi-Pakistan. All newly diagnosed cases were selected from the National Institute of Blood Disease and Bone Marrow Transplantation.
A potential case was a patient having at least two of the following three criteria: (hemoglobin <10g/100 mL, reticulocytes <50 × 109/L, granulocytes <1.5 × 109/L, platelets <100 × l09/L). Bone marrow biopsy is also required with decreased cellularity and the absence of significant fibrosis or neoplastic infiltration. Exclusion criteria included the presence of other hematologic diseases such as other neoplasia, neural tube defects, hypersplenism or pancytopenia associated systemic diseases (myelodysplastic syndrome, Fanconi’s anemia, and paroxysmal nocturnal hemoglobinuria). In addition history of congenital forms of aplastic anemia, AIDS, previous organ transplantation, Felty’s syndrome and Kostmann’s syndrome; chemotherapy, immunotherapy or radiotherapy were also excluded.
A person devoid of aplastic anemia or other blood-related disorders considered as a control. For each case, four gender and age (range + 5 years) matched controls were enrolled. All controls were selected from the patients who were admitted in or have visited the same institute. Acceptable diagnosis for control were the cases of minor road traffic accident, acute abdominal emergencies, acute infections, and other conditions such as cataract, glaucoma, etc. The same exclusion criteria were used for the cases as for the controls.
In-person interviews were conducted with the cases and controls to collect information regarding demographic, socioeconomic status, history of illness, and drugs used, with the help of a questionnaire. The drugs included Chloramphenicol, Beta-lactam antibiotics (ampicillin/amoxicillin, penicillin, and cephalosporins), Trimethoprim/sulfamethoxazole, Tetracycline, Thiazides, certain non-steroidal anti-inflammatory drugs, Benzodiazepines, Chlorpheniramine, Mebendazole, antiepileptic, and herbal preparations. All selected drugs were identified through literature review and expert opinion. A person who had a history of drug intake between 29 and 180 days before the diagnosis of aplastic anemia, is considered as an exposed person. Exposure to drugs less than 29 days was excluded because temporality could not be determined. Information on the timing, frequency, and period of use (including use ranging back beyond 180 days) was recorded. Dosage information was not collected due to the poor recall of study participants.
To estimate aplastic anemia risk associated with drugs, odds ratios (OR) with 95% confidence intervals were calculated by using univariate regression models. Additionally, a multivariate logistic regression model was also used to control the effects of age, gender, region, total household income, years of education, history of occupational exposure to solvents, and pesticides. P < 0.05 was considered statistically significant.
The study protocol was approved by the Ethical Review Committee of the Liaquat University of Medical and Health Sciences, Jamshoro NO. LUMHS/REC/-122 dated 02–01-2014. Written informed consent was obtained from each participant (or parents and caregivers if case was a minor) before interviews. All participants’ information was anonymized and de-identified prior to the analysis by using codes. This study complies with the Declaration of Helsinki.
Results
Age group and genders of the cases and controls were the same as the matching was done. There were slightly more males than females. The median age of cases was 27 years (ranging between 04 and 69 years). Predominate cases 84 (44%) were belongs from the age group 16–30 years. The odds of aplastic anemia increase with the age group 31–50 years (OR=1.6, 95% C.I 1.0–2.7) and >50 years 50 (OR=2.0, 95% C.I 1.0–3.7). Participants living in rural environments were at a higher (OR=2.1, 95% C.I 1.5–3.0) as compare to those belong from urban areas (Table 1).
The monthly income of the participants was used as an index of socioeconomic status. We reported no association of income with the risk of aplastic anemia. An association with having no education (OR=1.9, 95% C.I 1.3–2.6) and involvement in any type of occupation (OR=1.4, 95% C.I 1.0–1.9) was observed in this study (Table 2).
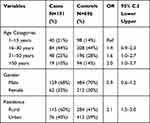 |
Table 1 Distribution of 191 Cases of Aplastic Anemia and 696 Controls by Region According to Age, Gender and Residence |
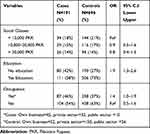 |
Table 2 Monthly Income and Employment Status of 191 Cases of Aplastic Anemia and 696 Controls |
The use of antiepileptic drugs in cases and controls is shown in Table 3. Antiepileptic drugs were more prevalent among cases n=11 (6%) than controls n=19 (3%) yielding a crude OR of 2.1 (95% C.I, 1.0–4.6). In 11 cases of aplastic anemia 8 (4%) were using carbamazepine and showed significant association (OR= 2.7; 95% C.I, 1.0–6.8). After adjustment for confounders, the use of antiepileptic drugs was significantly associated with aplastic anemia (adjusted odds ratio (aOR) 2; 95% C.I 2.1–4.7).
 |
Table 3 History of Drugs Exposure Among 191 Aplastic Anemia Cases (29–180 Days Before Diagnosis) and 696 Controls |
The use of chloramphenicol (topical) was identified among n=13 (7) cases and n=26 (4%) of the controls; there was no association of drug with aplastic anemia (OR=1.8; 95% C.I, 0.94–3.73). Likewise, no risk was observed with use of trimethoprim/sulfamethoxazole (OR=1.5, 95% C.I 0.97–2.62). With few users of tetracycline the estimated odds ratio was greater than one with a non-significant confidence interval (OR=1.8, 95% C.I 0.9–3.6). The observed odds ratio for B lactam antibiotics (penicillin, ampicillin/amoxicillin, and cephalosporin) were (OR=1.9, 95% C.I 0.93–3.69) (Table 3).
Ten (5%) patients with aplastic anemia and twelve controls (2%) had been exposed to Thiazides before the diagnosis of aplastic anemia. The calculated odds ratio with thiazides was (OR=3.1; 95% C.I 1.3–7.4). The multivariate odds ratio for thiazide after adjustment of confounders was (aOR= 1.7; 95% C.I 1.2–5.1). No risk was observed with the used of furosemide (Table 3).
The other drugs that were also associated with aplastic anemia are mebendazole (OR=3.7; 95% C.I, 1.5–9.2). The adjusted odds ratio for mebendazole was 2.5 (95% C.I 1.5–4.3). A number of drugs that have been commonly associated with aplastic anemia have not been used by sufficient cases or controls, therefore, no evidence of association was found with antihistaminic drugs, oral antidiabetics, interferon, benzodiazepine, and herbal preparations. No cases as well as controls were reported to use acetazolamide, Antithyroid drugs, and gold (Table 3).
Discussion
Drugs are the important risk factors believed to be linked with aplastic anemia. Published literature identified a range of pharmaceutical agents (as mention earlier) related with development of aplastic anemia. Avoidance of suspected drugs will reduce the incidence of aplastic anemia and improve the quality of life.
In this study, we found an association between aplastic anemia and exposure to carbamazepine with odds of 2.7. A similar chance of disease was also documented by Handoko et.al.15 Carbamazepine is the most widely prescribed drug that had a relationship with aplastic anemia as documented in a different case report.18–20 Carbamazepine, phenytoin, and phenobarbitone are the first choice antiepileptic drugs. Regardless of the availability of newer antiepileptic drugs, these drugs are widely used because of their effectiveness and low cost. All three drugs induce both dose-related toxicity and hypersensitivity, adverse effects on liver, brain, kidney, gastrointestinal and haemopoietic systems.18–21 The risk of aplastic anemia with the use of other antiepileptic drug like sodium valproate11 phenytoin12 and felbamate22 were found in several case reports. We did not find any significant association of Phenytoin with aplastic anemia.
Sulfa-containing drugs are of particular interest because they seem to be strongly associated with aplastic anemia in certain regions of the world.3–23 In our study 13% of the cases were exposed to sulfa-containing drugs and showed non-significant association. A strong relationship was also documented in a study of Thailand.6–16 IAAAS data showed that the risk of aplastic anemia increased with combination of trimethoprim.24 In our setting, increased use of sulfa-containing drugs is linked with over the counter availability of the drugs and self-medication to treat infections.
Among all the drugs, chloramphenicol has been the most common cause of aplastic anemia and attributed to 20% to 30% of total cases25,26 in the United States near fifty percent of aplastic anemia cases were caused by these drugs from 1949 to 1952.27 Globally, despite its effectiveness, low cost, and few numbers of side effects, the use of chloramphenicol has been limited. However, withdrawal from markets was not followed by a decline in the incidence of aplastic anemia. Based on this discussion, we agree with the view of Surapol Issaragrisil et al16 that aplastic anemia risk among chloramphenicol users was probably overrated and biased in the past by a selective tendency to report chloramphenicol-exposed cases. The current study did not find any significant association of chloramphenicol with aplastic anemia as only 1.1% of the cases and controls had a history of a topical use (eye drops) of chloramphenicol and not for systematic use. Similar uses with no significant association were also reported in studies conducted in Thailand.6–16
For diuretics agents, the current study identified association with thiazide diuretics; a similar association was reported in studies done in a different part of the world.16–28 However, in IAAAS29 use of thiazide diuretics was not related to aplastic anemia which might be due to the difference of study region. Mebendazole, an anthelmintic drug, is of particular interest because it appears to be significantly connected with aplastic anemia according to the results of this study. A similar association was confirmed by globally.6–17
In Pakistan, herbal medicines or home remedies used are common because people believe that they might be effective in the treatment of different diseases. These herbal medicines are formulated with well-known or unknown drugs or chemicals30 that might be associated with aplastic anemia.31 In this study, herbal medicines were used in 7% of cases and 1% of controls that indicates no association between aplastic anemia and the use of herbal medicines. A similar finding was also reported by S Issaragrisil et al in Thailand.6
In the management of drug induced aplastic anemia, the first step is to withdraw the suspected drug, as early removal can lead to the reversal of the bone marrow suppression effect. In the supportive care, transfuse the erythrocytes and platelets and give prophylactic antimicrobials and antifungals.32 Allogeneic hematopoietic stem cell transplantation (HSCT) and immunosuppressive therapy are the major treatment options for drug-induced aplastic anemia. Antithymocyte globulin and cyclosporine is also used for the treatment of aplastic anemia.33
Conclusion and Recommendations
Our study replicates the well-known association between carbamazepine, thiazides, and mebendazole. The risk of this idiosyncratic reaction differs for different individuals. It is suggested that clinicians be aware of the possibility of drug-related aplastic anemia and revise their prescribing habits accordingly. There is an urgent need to develop the capacity building of clinicians for continuous surveillance of the safety and efficacy of the pharmaceutical products which are used in their clinical practice. Formulation of new legislation for drug safety and national guidelines for pharmacovigilance is also recommended.
Acknowledgments
We thank the cases of aplastic anemia and control who participated in this study. In addition, we would also like to acknowledge Dr Anum Vighio Department of Health, Sindh and Dr Abdul Ghani who provided us technical assistance for this research.
Disclosure
The authors stated that they have no potential conflicts of interests for this work.
References
1. Mintzer DM, Billet SN, Chmielewski L. Drug-induced hematologic syndromes. Adv Hematol. 2009;2009:1–11. doi:10.1155/2009/495863
2. Al Qahtani SA. Drug-induced megaloblastic, aplastic, and hemolytic anemias: current concepts of pathophysiology and treatment. Int J Clin Exp Med. 2018;11(6):5501–5512.
3. Vandendries ER, Drews RE. Drug-associated disease: hematologic dysfunction. Crit Care Clin. 2006;22(2):347–355. doi:10.1016/j.ccc.2006.02.002
4. Greene EM, Hagemann TM. Drug-Induced Hematologic Disorders. Pharmacotherapy: A Pathophysiologic Approach. New York, USA: McGraw-Hill Education; 2017.
5. Segel GB, Lichtman MA. Aplastic Anemia: Acquired and Inherited. Williams Hematology.
6. Issaragrisil S, Kaufman DW, Anderson T, et al. Low drug attributability of aplastic anemia in Thailand. Blood J Am Soc Hematol. 1997;89(11):4034–4039.
7. Isenberg SJ. The fall and rise of chloramphenicol. J Am Assoc Pediatric Ophthalmol Strabismus. 2003;7(5):307–308.
8. Durosinmi MA, Ajayi AA. A prospective study of chloramphenicol induced aplastic anaemia in Nigerians. Trop Geogr Med. 1993;45(4):159–161.
9. Yunis A. Chloramphenicol toxicity: 25 years of research. Am J Med. 1989;87(3N):44N–8N.
10. Maluf E, Hamerschlak N, Cavalcanti AB, et al. Incidence and risk factors of aplastic anemia in Latin American countries: the LATIN case-control study. Haematologica. 2009;94(9):1220. doi:10.3324/haematol.2008.002642
11. Mintzer DM, Billet SN, Chmielewski L. Drug-induced hematologic syndromes. Adv Hematol. 2009;2009:1–11.
12. Blain H, Hamdan KA, Blain A, Jeandel C. Aplastic anemia induced by phenytoin: a geriatric case with severe folic acid deficiency. J Am Geriatr Soc. 2002;50(2):396–397. doi:10.1046/j.1532-5415.2002.50083.x
13. Aliyu H, Ayo J, Ambali S, Kawu M, Aluwong T. Heamatobiochemical alterations induced by carbamazepine and phenytoin: mini review. Biochem Pharmacol. 2016;5(219):2167–0501.1000219. doi:10.4172/2167-0501.1000219
14. Kaufman DW, Kelly JP, Jurgelon JM, et al. Drugs in the aetiology of agranulocytosis and aplastic anaemia. Eur J Haematol. 1996;57(S60):23–30. doi:10.1111/j.1600-0609.1996.tb01641.x
15. Handoko KB, Souverein PC, Van Staa TP, et al. Risk of aplastic anemia in patients using antiepileptic drugs. Epilepsia. 2006;47(7):1232–1236. doi:10.1111/j.1528-1167.2006.00596.x
16. Issaragrisil S, Kaufman DW, Anderson T, et al. The epidemiology of aplastic anemia in Thailand. Blood. 2006;107(4):1299–1307. doi:10.1182/blood-2005-01-0161
17. Issaragrisil S. Epidemiology of aplastic anemia in Thailand. Thai Aplastic Anemia Study Group. Int J Hematol. 1999;70(3):137–140.
18. Yáñez-Rubal J, Estévez-Rodríguez J, Crespo-Lopez M, Martin-Herranz I. Carbamazepine-induced aplastic anaemia: a case report. Rev Neurol. 2002;35(7):647–649.
19. Franceschi M, Ciboddo G, Truci G, Borri A, Canal N. Fatal aplastic anemia in a patient treated with carbamazepine. Epilepsia. 1988;29(5):582–583. doi:10.1111/j.1528-1157.1988.tb03765.x
20. Misra U, Kalita J, Rathore C. Phenytoin and carbamazepine cross reactivity: report of a case and review of literature. Postgrad Med J. 2003;79(938):703–704.
21. Ibáñez L, Vidal X, Ballarín E, Laporte J-R. Population-based drug-induced agranulocytosis. Arch Intern Med. 2005;165(8):869–874. doi:10.1001/archinte.165.8.869
22. Smith CL, Powell K. Review of the sulfonamides and trimethoprim. Pediatrics Rev. 2000;21(11):368–371. doi:10.1542/pir.21-11-368
23. Heimpel H, Raghavachar A. Hematological side effects of co-trimoxazole. Infection. 1987;15(5):S248–S53. doi:10.1007/BF01643198
24. Agranulocytosis I, Study AA. Anti‐infective drug use in relation to the risk of agranulocytosis and aplastic anemia. Arch Intern Med. 1989;149(5):1036–1040.
25. Young NS, Alter BP. Aplastic Anemia, Acquired and Inherited. WB Saunders Company; 1994.
26. Scott JL, Cartwright GE, Wintrobe MM. Acquired aplastic anemia: an analysis of thirty-nine cases and review of the pertinent literature. Medicine. 1959;38(2):119–172. doi:10.1097/00005792-195905000-00003
27. Lewis C, Putnam L, Hendricks F, Kerlan I, Welch H. Chloramphenicol (Chloromycetin) in relation to Blood Dyscrasias with Observations on other Drugs. Special Survey Antibiotics Chemotherapy. 1952;2(12):601–609.
28. Kaufman D, Kelly J, Anderson T, Harmon D, Shapiro S. Evaluation of case reports of aplastic anemia among patients treated with felbamate. Epilepsia. 1997;38(12):1265–1269. doi:10.1111/j.1528-1157.1997.tb00062.x
29. Kaufman DW, Kelly JP, Levy M, Shapiro S. The Drug Etiology of Agranulocytosis and Aplastic Anemia. Oxford Acad Press; 1991.
30. Boqari DT, Al Faraj S, Arafah M, et al. Herb-induced acute bone marrow intoxication and interstitial nephritis superimposing glomerular C1q deposition in a patient with paroxysmal nocturnal hemoglobinuria. Saudi J Kidney Dis Transpl. 2015;26(3):572. doi:10.4103/1319-2442.157384
31. Jia Y, Du H, Yao M, et al. Chinese herbal medicine for myelosuppression induced by chemotherapy or radiotherapy: a systematic review of randomized controlled trials. Evi Based Complementary Alternative Med. 2015;2015:1–12. doi:10.1155/2015/690976
32. Carey PJ. Drug-induced myelosuppression. Drug Safety. 2003;26(10):691–706. doi:10.2165/00002018-200326100-00003
33. Zhu Y, Gao Q, Hu J, Liu X, Guan D, Zhang F. Allo-HSCT compared with immunosuppressive therapy for acquired aplastic anemia: a system review and meta-analysis. BMC Immunol. 2020;21(1):1–11. doi:10.1186/s12865-020-0340-x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
