Back to Journals » Clinical Interventions in Aging » Volume 14
The relationship between plasma lipids, oxidant–antioxidant status, and glycated proteins in individuals at risk for atherosclerosis
Authors Dzięgielewska-Gęsiak S , Płóciniczak A , Wilemska-Kucharzewska K, Kokot T, Muc-Wierzgoń M , Wysocka E
Received 26 November 2018
Accepted for publication 1 March 2019
Published 9 May 2019 Volume 2019:14 Pages 789—796
DOI https://doi.org/10.2147/CIA.S196016
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Sylwia Dzięgielewska-Gęsiak,1 Alicja Płóciniczak,2 Katarzyna Wilemska-Kucharzewska,1 Teresa Kokot,1 Małgorzata Muc-Wierzgoń,1 Ewa Wysocka2
1Department of Internal Medicine, Medical University of Silesia, 44-902 Bytom, Poland; 2Department of Laboratory Diagnostics, Poznan University of Medical Science, 60-569 Poznan, Poland
Objective: Ageing is one of the major risks for atherosclerosis. The age-related changes of interactions between plasma lipids, oxidative stress, antioxidant defense, and glycation processes are still not established while we age. Thus, the aim of the study was to analyze such relationships in individuals at risk for atherosclerosis due to their age.
Methods: Elderly and middle-aged persons with no acute disease or severe chronic disorder were assessed. Fasting plasma lipids (total cholesterol (T-C), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol, and triacylglycerols), thiobarbituric acid reacting substances (TBARS), plasma total antioxidant status (TAS), and glucose and glycated proteins (fructosamine (FA) and glycated hemoglobin (HbA1c)) were determined. An oral glucose tolerance test allowed exclusion of persons with type 2 diabetes.
Results: Lipid profiles were significantly profitable, increased HDL-C especially (p<0.0001), in the elderly versus middle-aged group. Decreased TBARS and TAS were found in the elderly versus middle-aged group (p=0.0001 and p=0.00002, respectively). Increased fructosamine was found in the elderly (255±30 μmol/L) versus middle-aged (236±33 μmol/L) group (p=0.006). Multiple regression analysis showed that in the middle-aged group TBARS correlated with T-C and HDL-C, and in the elderly group with HbA1c and FA independently of other factors.
Conclusion: The factors which have an impact on oxidant–antioxidant status are crucial to understanding the pathomechanisms of senescence as well as the development of chronic diseases. Healthy aging may be maintained throughout proper lipid control. Moreover, data support the premise that the balance between lipid metabolism and oxidative stress may play a role in the initial phases of glycation plasma proteins particularly among elderly persons.
Keywords: plasma lipids, oxidant-antioxidant markers, glycated proteins, aging, atherosclerosis risk
Introduction
Ageing is one of the major risks for atherosclerosis. Aging is accompanied by an increase in oxidative damage due to an impaired physiological function.1 On the other hand, the aging process – both at the cellular and tissue levels – increases the risk of diseases and death, which could be related to the improper lipid metabolism, oxidative stress, protein glycation, accumulation of DNA damage, and failure of protein repair.2–5
The identification of high-risk cardiovascular disease (CVD) patients should be performed using risk score charts.6–8 Increased low-density lipoprotein-cholesterol (LDL-C) and reduced high-density lipoprotein-cholesterol (HDL-C) levels in plasma are well-known risk factors for CVD.9 Hyperlipidemia is included in all scales mentioned above. What is more, it increases the CVD risk fiercely.10 Previous studies have indicated the benefit from lipid lowering, in almost every analysis and for almost every outcome.11,12 In the elderly population, hypercholesterolemia may be the only major CVD risk factor13,14 or one out of several major risk factors.15,16 Lipids tend to change while we age, altering the risk of CVD.17,18 Moreover, dyslipidemia increases the production of reactive oxygen species and consequently is a major stimulus for DNA damage. Genomic instability can directly affect vascular function by causing cell cycle arrest, apoptosis, and senescence and can play a role in the development and progression of atherosclerosis.19 Additionally, prolonged exposure to excess production of reactive oxygen species raises the oxidation of lipid products, which in turn leads to endothelium dysfunction, cardiovascular problems, and other chronic diseases related to aging.20,21 Oxidative stress is characterized by deregulation between oxidant and antioxidant balance in which many enzymatic and nonenzymatic factors are involved.22,23 Protein glycation depends on the duration of hyperglycemia, advanced aging, and metabolic diseases.24 A high amount of glucose, regardless of its source, is toxic at the molecular, cellular, and tissue levels. Nonenzymatic glycation of proteins leads through Schiff base to Amadori products such as glycated albumin (fructosamine) or glycated hemoglobin (HbA1c). The amount of glycated proteins (fructosamine and HbA1c) depends on the time-averaged glucose concentration. Thus, fructosamine and HbA1c reflect the extent of exposure to glucose in the 2–4 and 8–12 weeks before testing, respectively.25,26 The routes for hyperglycemia vary and involve numerous pathways such as protein glycation and formation of advanced glycation end products, the polyol pathway, as well as glucose autoxidation. Glucose control also plays an important role in the prooxidant/antioxidant balance and may increase the risk for atherosclerosis. The selected mechanisms are summarized in Figure 1.
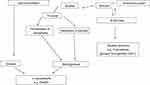 | Figure 1 Mechanism of lipid peroxidation and protein glycation.Abbreviations: HbA1c, glycated hemoglobin; TBARS, thiobarbituric acid-reacting substances. |
The comparison of oxidative stress parameters between middle-aged and older subjects revealed a progressive and slow decline of antioxidant status in healthy free-living elderly people.27 Therefore, as seen above, the proper balance in the prooxidant–antioxidant compounds can contribute to successful aging, free of cardiac problems. The oxidative stress theory of aging states that oxidative damage is associated with age-related disease and may determine successful aging.28
Furthermore, recent studies have shown that oxidative stress and glycation have a significant impact on the development of metabolic complications.29,30 There is evidence which indicates that oxidative stress and protein glycation may join and explain metabolic complications throughout the life span.31,32
Thus, this study analyzed the relationship between plasma lipids, oxidant–antioxidant status, and glycated proteins in individuals at risk for atherosclerosis due to their age.
Methods
The aim of the study was to evaluate and compare concentrations of plasma lipids, peroxidation products, and total antioxidant status as well as glycated proteins in healthy, nondiabetic persons due to their age (middle-aged and elderly).
The study was performed in accordance with the Declaration of Helsinki of 1975 for Human Research revised in 2008, and the study protocol was approved by the Bioethics Committee of Medical University of Silesia in Katowice (statement numbers KNW/0022/KB1/38/IV/16/17/18) and Poznan University of Medical Sciences in Poznan; Poland (statement number 595/11).
All participants gave informed, signed consent to participate in the study.
Subjects and settings
Nonsmoking, drug-naive white Europids, using no special diet, no supplements, and no alcohol, without acute or chronic disease, were invited to participate in the study. Elderly (65 years old or more, according to the World Health Organization (WHO) statement) (E) persons (n=42, mean age 72±6 years) and middle-aged (n=35, mean age 48±10 years) (MA) persons from the western region of Poland were assessed.
The exclusion criteria were the presence of the following conditions: coronary artery disease (accompanied by current steady-state electrocardiography), positive history of stroke, diabetes, cancer, inflammatory disease, liver cirrhosis, and kidney failure. Moreover, those who previously used drugs with antioxidant capacity were excluded (including vitamins and supplements).
A thorough physical examination, including measurement of systolic and diastolic arterial blood pressure and calculation of the body mass index (BMI), was performed. Arterial blood pressure was measured by validated sphygmomanometer (M10-IT model; Omron Health Care, Kyoto, Japan), following the recommendations of the European Society of Hypertension. The average of three measurements was used to characterize subjects.33 The BMI was derived from the height and weight measurements.
Cardiovascular risk score
To evaluate the 10-year risk of cardiovascular and coronary heart disease, the Pol-SCORE risk chart was used.34
Blood sampling and biochemical analysis
Blood was collected by ulnar vein puncture. All studied persons qualified for an oral glucose tolerance test (OGTT) according to WHO recommendation.35 The newly diagnosed type 2 diabetes patients were excluded.
Glucose and lipid assays
Concentrations of glucose at 0 min and 120 min of the 75-g OGTT, and fasting plasma lipids (total cholesterol (T-C), HDL-C, LDL-C, and triacylglycerols (TAG)), were measured using enzymatic methods (bioMerieux, Marcy I’Etoile, France) and the UV-160A Shimadzu spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Low-density lipoprotein was calculated using the Friedewald formula: [LDL-C] = [T-C] − [HDL-C] − [0.45·TAG], if TAG <4.56 mmol·L−1.
Glycated protein assays
Parameters were assessed in fasting blood samples.
Fructosamine assay. Glycated albumin (fructosamine) was measured using a colorimetric method based on the ability of ketoamines to reduce nitrotetrazolium-blue to formazan in an alkaline solution. The rate of formation of formazan is directly proportional to the concentration of fructosamine. The measurement was done on a Cobas 400 analyzer (Roche Diagnostics, Mannheim, Germany). The sensitivity of this assay was 0.14 μmol/L with an intra-assay coefficient of variation (CV) and inter-assay CV of 2.80% and 0.65%, respectively.
HbA1c assay. Glycated hemoglobin (HbA1c) was measured by ion-exchange high-performance liquid chromatography in a D-10 system (BioRad Laboratories Inc., Hercules, CA, USA) using a specific standardized measurement set established through the National Glycohemoglobin Standardization Program. The sensitivity of this assay was 0.05% with an intra-assay CV and inter-assay CV of 2.35% and 2.66%, respectively.
Oxidative stress markers
Oxidant–antioxidant balance was estimated in fasting blood samples.
Total antioxidant status (TAS). The concentration of plasma TAS was assessed in all serum samples by a colorimetric assay based on the decrease of the optical density of the blank produced by each sample in analogy to its antioxidant property, using a Randox reagent kit (Randox Laboratories Ltd., Crumlin, UK) and a STATFAXTM 1904 Plus spectrophotometer (Awareness Technology, Inc., Palm City, FL, USA). Optical density was read at 600 nm. The intra-assay CV and inter-assay CV for plasma TAS concentrations was 2.50% and 4.80%, respectively.
Thiobarbituric acid-reacting substances (TBARS). The concentration of plasma TBARS, reflecting plasma lipid peroxidation products, was determined by Okhawa et al's method36 using Sigma-Aldrich reagents (Sigma-Aldrich Co., Saint Louis, MO, USA) and a Specord M40 spectrometer (Carl Zeiss Meditec AG, Jena, Germany). The intra-assay CV and inter-assay CV for TBARS was 2.80% and 4.70%, respectively.
Statistical analysis
Statistica (version 13.0) for Windows was used for statistical analysis. The normality of value distribution was checked by the Shapiro–Wilk test. Then, the results with a Gaussian distribution were analyzed with Student’s t-test, and those with a non-Gaussian distribution were verified by a nonparametric Mann–Whitney U-test to assess the differences between the studied age groups. The Spearman rank correlation test was used to evaluate the strength of association between two variables. The multiple regression analysis between oxidative stress markers and biochemical parameters was performed in the whole study population as well as in the MA and E groups (LDL-C was not included as a derivative of analyzed variables). p<0.05 or lower was considered statistically significant. The obtained data are presented as the mean±SD for Gaussian distribution and the median with interquartile range for non-Gaussian distribution.
Results
The clinical and laboratory data of the studied groups are shown in Table 1. There were no gender differences in the MA and E populations, except higher HDL-C observed in MA females (p=0.0437). Therefore, males and females were investigated in both groups together. No significant differences in BMI, blood pressure, and glucose concentration were noted among the investigated age groups. The Pol-SCORE risk was higher in the E group but not statistically significant. Lipid profiles (T-C, HDL-C, LDL-C, and TAG) were significantly profitable, increased HDL-C especially (p<0.0001), in the otherwise healthy E group versus the healthy MA group. Concerning oxidant–antioxidant status, decreased TAS was found in the E versus MA group (p=0.00002) and decreased TBARS was found in the E versus MA group (p=0.0001). Concerning glycated proteins, glycated hemoglobin levels did not differ between the investigated groups. The only thing noticed, however, was the increased fructosamine concentration in the E versus MA group (p=0.006).
 | Table 1 Characteristics of the studied groups |
Also, correlation analysis regarding lipids, oxidative stress markers, and glycated proteins in the whole studied healthy population was performed. In the case of all healthy participants of the study (both middle-aged and elderly, MA+E), TBARS correlated positively with T-C (R=0.4205; p<0.05) and TAG (R=0.3466; p<0.05), and negatively with fructosamine (R=−0.4433; p<0.05) and HDL-C (R=−0.5178; p<0.05); TAS correlated negatively with fasting glucose (R=−0.3261; p<0.05) and HDL-C (R=−0.3085; p<0.05). In the MA group, TBARS correlated positively with T-C (R=0.5484; p<0.05) and TAG (R=0.4724; p<0.05), and negatively with HDL-C (R=−0.4205; p<0.05). In the E group, TBARS correlated positively with glucose at 120 min during the OGTT (R=0.3328; p<0.05) and HbA1c (R=0.4922; p<0.05), and negatively with fructosamine (R=−0.4859; p<0.05).
The multiple regression analysis showed, in the whole investigated healthy population, that TBARS correlated with T-C (β=0.34) and HDL-C (β=–0.38), independently of other factors (R=0.63; R2=0.40; p<0.00001). Yet the MA group multiple regression analysis clearly revealed that TBARS correlated with T-C (β=0.42) and HDL-C (β=−0.50), independently of other factors (R=0.71; R2=0.51; p<0.00001). In the E group, multiple regression analysis showed that TBARS correlated with HbA1c (β=0.42) and fructosamine (β=−0.52), independently of other factors (R=0.74; R2=0.55; p<0.00001).
Discussion
To begin with, it would be of worth to note that the exploration of the usefulness of various simple markers with respect to the identification of participants at risk for atherosclerosis is urgently needed. Of great significance is the fact that the suggested variables can be measured in any metabolic clinic and do not require a large financial outlay. Furthermore, in the discussion on cardiovascular risk factors, the lipid profile is said to play a pivotal role. Interestingly, in the performed study we observed that the elderly group had lower T-C, LDL-C, and TAG in comparison with the middle-aged population. That can, however, be explained by an age-related reduction in the cholesterol absorption, synthesis, and low-density lipoprotein apo-B transport and is additionally in agreement with cross-sectional and longitudinal studies.37,38
On the other hand, only healthy elderly individuals, with no history of CVD, were included in the study. The study population consisted of elderly individuals who had no history of CVD and healthy middle-aged people likely to develop the disease later. However, a lower T-C concentration indicates frailty and can predict functional decline while we age.39 Frailty and functional decline may be not only due to aging itself but could be associated with increased oxidative stress and decreased antioxidant defense.40 A combination of higher levels of oxidative stress as well as imbalance in the antioxidant defense system are likely to be involved in pathophysiological processes during aging.41
The much greater absolute risk in elderly people means that they need more complex and holistic care. In 2011, the American Heart Association made the concept of “ideal cardiovascular health”, which means that the prevention of CVD should focus not only on the control of traditional CVD risk factors.42 The present analyses provide support for the use of nontraditional CVD risk factors such as oxidant–antioxidant stress markers as well as glycated proteins. In the present study, TAS was lower in the investigated elderly group in comparison with the middle-aged group, indicating lower antioxidant properties. However, TBARS in the elderly investigated population also decreased. This may be due to a relatively high concentration of HDL-C and thus lower oxidative stress. In our previous study, elderly persons with high HDL-C had lower TBARS concentration and better antioxidant defense.43 On the other hand, in the work by Rogulj et al44 the older subjects with early type 2 diabetes mellitus had greater oxidative DNA damage, but none of the plasma oxidative stress and inflammation markers were different either between the middle-aged metabolic syndrome patients and the younger type 2 diabetes mellitus patients or between the two age groups. However, we must have in mind that in present study, in comparison with Rogulj et al's work, we investigated only healthy subjects without type 2 diabetes mellitus. Yet with age we may observe changes in body composition.45 Interestingly, our investigated age groups had comparable metabolic components such as BMI, waist circumference, and blood pressure.
The effects of oxidative stress on lipids are mainly expressed by the induction of lipid peroxidation. In the study by Block et al46 there were no apparent relationships of age and malondialdehyde (MDA), reflecting lipid peroxidation products. Yet Moreto et al47 found higher MDA concentration in middle-aged persons with higher values of waist circumference, fasting blood glucose, and TAG. In the present study, lower TBARS in the elderly group may be due to better TAG and HDL-C. The work by Hadij Ahmed et al48showed significantly higher levels of MDA in middle-aged patients with coronary artery disease than in controls; moreover, they found a significant positive correlation between MDA and levels of some trans fatty acids in those patients.The present study showed different TBARS in the investigated age groups and TBARS negatively correlated with HLD-C only in middle-aged persons independently of other metabolic factors. In middle-aged humans, the linear changes of plasma HDL-C support the suggestion about high-density lipoprotein function and counteract TBARS production by 51%. Moreover, the study further revealed that the comparison of lipid profile in healthy elderly versus middle-aged persons was worse in the latter. It also indicates that successful aging may be caused by well-balanced lipid profiles and this is in agreement with Cherubini et al's research,49 which proved higher lipid oxidation in octogenarians with carotid atherosclerosis than in those with successful vascular aging. On the other hand, we should remember the study by Augusti et al50 which demonstrated that some oxidative events initiate even with clinically acceptable lipid concentration.
The concentration of glycated proteins tends to increase through the life span and the process is independent of glucose concentration. The average rate of HbA1c increase ranges from 0.05 to 0.1% (0.55–1.09 mmol/mol) per decade.51 We did not notice differences in HbA1c level between the investigated age groups, although HbA1c correlated with oxidative stress markers in the elderly group. The correlations are in accordance with the scientific literature supporting the hypothesis that lower antioxidant defense is due to pathophysiological processes during aging or hyperglycemia.41,52
The AMORIS cohort study demonstrated that fructosamine is a strong predictor for myocardial infarction independently of hyperglycemia.53 The present study showed higher fructosamine concentration in elderly nondiabetics in comparison with the middle-aged nondiabetic group. Moreover, in the elderly population the multiple regression analysis showed negative correlation between fructosamine and TBARS independently, what suggests that oxidative stress in 55% of people accompanies glycation processes and may indicate that the elderly persons are at higher cardiovascular risk not only because of age. Besides, Mazidi et al54 suggest that diet may play an important role in chronic disease occurrence. However, we did not investigate the role of diet in the study although the investigated groups had a comparable diet style.
Limitations of the study
All in all, it can be clearly stated that the results of the present study have some limitations and should be interpreted with caution. The fact that a number of persons at atherosclerosis risk (both middle-aged and elderly) were on antioxidant medications and excluded from our study led to a relatively small sample size, and should therefore be considered a potential limit of this study. However, although the sample size gathered is larger than most sample sizes previously reported, it is still relatively small and may restrict the power to detect associations with statistical analyses. Thus, the inclusion of a greater number of subjects is warranted to improve the power of future studies. In addition, we only calculated risk for atherosclerosis or coronary artery diseases; therefore, the serum measurement of oxidant–antioxidant markers and glycated proteins does not necessarily reflect the influence of its balance in former diseased patients.
Conclusions
It would be worth mentioning that the factors which impact oxidant–antioxidant status are not only essential in understanding the pathomechanisms of senescence but also in the development of chronic diseases. Furthermore, lipid metabolism and oxidant–antioxidant balance are largely conditioned by a number of factors when we age. Healthy aging, however, may be maintained throughout proper lipid control. Moreover, data support the premise that lipid metabolism and oxidative stress may play an important role in the initial phases of glycation plasma proteins particularly among healthy elderly persons.
Acknowledgments
This study did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Romano AD, Serviddio G, de Matthaeis A, Bellanti F, Vendemiale G. Oxidative stress and aging. J Nephrol. 2010;23(Suppl 15):S29–S36.
2. Gautieri A, Passini FS, Silván U, et al. Advanced glycation end-products: mechanics of aged collagen from molecule to tissue. Matrix Biol. 2016:pii: S0945-053X(16)30168-8. doi:10.1016/j.matbio.2016.09.001.
3. Cesari M, Kritchevsky SB, Nicklas B,
4. Radman M. Protein damage, radiation sensitivity and aging. DNA Repair (Amst). 2016;44:186–192. doi:10.1016/j.dnarep.2016.05.025
5. Kanu N, Penicud K, Hristova M, et al. The ATM cofactor ATMIN protects against oxidative stress and accumulation of DNA damage in the aging brain. J Biol Chem. 2010;285:38534–38542. doi:10.1074/jbc.M110.145896
6. Rb D
7. Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105:310–315. doi:10.1161/hc0302.102575
8. Conroy RM, Pyorala K, Fitzgerald AP,
9. Martin-Ventura JL, Rodrigues-Diez R, Martinez-Lopez D, Salaices M, Blanco-Colio LM, Briones AM. Oxidative stress in human atherothrombosis: sources, markers and therapeutic targets. Int J Mol Sci. 2017;18(11):2315. doi:10.3390/ijms18112315
10.
11.
12. Shepherd J, Blauw GJ, Murphy MB, et al.;
13. Bruckert E, Lièvre M, Giral P, et al. Short-term efficacy and safety of extended-release fluvastatin in a large cohort of elderly patients. Am J Geriatr Cardiol. 2003;12:225–231. doi:10.1111/j.1076-7460.2003.02000.x
14. Nakaya N, Mizuno K, Ohashi Y, et al. Low-dose pravastatin and age related differences in risk factors for cardiovascular disease in hypercholesterolaemic Japanese: analysis of the management of elevated cholesterol in the primary prevention group of adult Japanese (MEGA study). Drugs Aging. 2011;28:681–692. doi:10.2165/11595620-000000000-00000
15. Neil HA, DeMicco DA, Luo D;
16. Collier DJ, Poulter NR, Dahlöf B;
17. Schubert CM, Rogers NL, Remsberg KE, et al. Lipids, lipoproteins, lifestyle, adiposity and fat-free mass during middle age: the Fels Longitudinal Study. Int J Obes (Lond). 2006;30(2):251–260. doi:10.1038/sj.ijo.0803129
18. Kapur NK, Ashen D, Blumenthal RS. High density lipoprotein cholesterol: an evolving target of therapy in the management of cardiovascular disease. Vasc Health Risk Manag. 2008;4:39–57.
19. Cervelli T, Borghini A, Galli A, Andreassi MG. DNA damage and repair in atherosclerosis: current insights and future perspectives. Int J Mol Sci. 2012;13:16929–16944. doi:10.3390/ijms131216929
20. Nitenberg A, Cosson E, Pham I. Postprandial endothelial dysfunction: role of glucose, lipids and insulin. Diabetes Metab. 2006;32(Spec No2):S28–S33. doi:10.1016/S1262-3636(06)70482-7
21. Karaouzene N, Merzouk H, Aribi M, et al. Effects of the association of aging and obesity on lipids, lipoproteins and oxidative stress biomarkers: a comparison of older with young men. Nutr Metab Cardiovasc Dis. 2011;21(10):792–999. doi:10.1016/j.numecd.2010.02.007
22. Maurya PK, Kumar P, Chandra P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J Methodol. 2015;5(4):216–222. doi:10.5662/wjm.v5.i4.216
23. Amir Aslani B, Ghobadi S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016;146:163–173. doi:10.1016/j.lfs.2016.01.014
24. Yamagishi S, Matsui T, Nakamura K. Possible link of food-derived advanced glycation end products (AGEs) to the development of diabetes. Med Hypotheses. 2008;71(6):876–878. doi:10.1016/j.mehy.2008.07.034
25. Sacks DB. Hemoglobin A1c in diabetes: panacea or pointless? Diabetes. 2013;62:41–43. doi:10.2337/db12-1485
26. True MW. Circulating biomarkers of glycemia in diabetes management and implications for personalized medicine. J Diabetes Sci Technol. 2009;3(4):743–747. doi:10.1177/193229680900300421
27. Andriollo-Sanchez M, Hininger-Favier I, Meunier N, et al. Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: the ZENITH study. Eur J Clin Nutr. 2005;59(Suppl 2):S58–S62. doi:10.1038/sj.ejcn.1602300
28. Buffenstein R, Edrey YH, Yang T, Mele J. The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. Age (Dordr). 2008;30(2–3):99–109. doi:10.1007/s11357-008-9058-z
29. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4): 851–863. doi:10.5114/aoms.2016.58928
30. Xu L, Yi-Ru W, Li PC, Feng B. Advanced glycation end products increase lipids accumulation in macrophages through upregulation of receptor of advanced glycation end products: increasing uptake, esterification and decreasing efflux of cholesterol. Lipids Health Dis. 2016;15:161. doi:10.1186/s12944-016-0334-0
31. Linnane AW, Kios M, Vitetta L. Healthy aging: regulation of the metabolome by cellular redox modulation and prooxidant signaling systems: the essential roles of superoxide anion and hydrogen peroxide. Biogerontology. 2007;8:445–467. doi:10.1007/s10522-007-9096-4
32. Santilli F, D’Ardes D, Davì G. Oxidative stress in chronic vascular disease: from prediction to prevention. Vascul Pharmacol. 2015;74:23–37. doi:10.1016/j.vph.2015.09.003
33. O’Brien E, Asmar R, Beilin L, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701. doi:10.1097/01.hjh.0000163132.84890.c4
34. Zdrojewski T, Jankowski P, Bandosz P, et al. Nowa wersja systemu oceny ryzyka sercowo- -naczyniowego i tablic SCORE dla populacji Polski. A new version of cardiovascular risk assessment system and risk charts calibrated for Polish population. Kard Pol. 2015;73(10):958–961. doi:10.5603/KP.2015.0182
35.
36. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi:10.1016/0003-2697(79)90738-3
37. Upmeier E, Lavonius S, Heinonen P, et al. Longitudinal changes in serum lipids in older people the Turku elderly study 1991–2006. Age Ageing. 2011;40:280–283. doi:10.1093/ageing/afg180
38. Walter M. Interrelationships among HDL metabolism, aging, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1244–1250. doi:10.1161/ATVBAHA.108.181438
39. Schalk BW, Visser M, Deeg DJ, Lm B. Lower levels of serum albumin and total cholesterol and future decline in functional performance in older persons: the Longitudinal Aging Study Amsterdam. Age Ageing. 2004;33:266–272. doi:10.1093/ageing/afh073
40. Rowiński R, Kozakiewicz M, Kędziora-Kornatowska K, Hubner-Wozniak E, Kedziora J. Markers of oxidative stress and erythrocyte antioxidant enzyme activity in older men and women with differing physical activity. Exp Gerontol. 2013;48(11):1141–1146. doi:10.1016/j.exger.2013.07.010
41. Distelmaier K, Goliasch G. Gone with the age(DL): high -density lipoprotein in senescence. Pol Arch Med Wewn. 2016;126(10):727–728. doi:10.20452/pamw.3594
42. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690–1696. doi:10.1016/j.jacc.2010.11.041
43. Dziegielewska-Gesiak S, Bielawska L, Zowczak-Drabarczyk M, et al. The impact of high density lipoprotein on oxidant-antioxidant balance in healthy elderly people. Pol Arch Med Wewn. 2016;126(10):731–738. doi:10.20452/pamw.3559
44. Rogulj D, El Aklouk I, Konjevoda P, et al. Age-dependent systemic DNA damage in early Type 2 diabetes mellitus. Acta Biochim Pol. 2017;64(2):233–238. doi:10.18388/abp.2016_1313
45. Malczyk E, Dzięgielewska-Gesiak S, Fatyga E, Ziółko E, Kokot T, Muc-Wierzgoń M. Body composition in healthy older persons: role of the ratio of extracellular/total body water. J Biol Regul Homeost Agents. 2016;30(6):447–452.
46. Block G, Dietrich M, Norkus EP, Packer L. Oxidative stress in human populations. In: Rg C, Rodriguez H, editors. Critical Reviews of Oxidative Stress and Aging. Advances in Basic Science, Diagnostics and Intervention. Vol. 2. Singapore: World Scientific Publishing; 2003:870–880.
47. Moreto F, de Oliveira EP, Manda RM, Burini RC. The higher plasma malondialdehyde concentrations are determined by metabolic syndrome-related glucolipotoxicity. Oxid Med Cell Longev. 2014;Article ID 505368. doi:10.1155/2014/505368
48. Hadj Ahmed S, Kharroubi W, Kaoubaa N, et al. Correlation of trans fatty acids with the severity of coronary artery disease lesions. Lipids Health Dis. 2018;17:52. doi:10.1186/s12944-018-0699-3
49. Cherubini A, Zuliani G, Costantini F, et al. High vitamin E plasma levels and low density lipoprotein oxidation are associated with the absence of atherosclerosis in octogenarians. J Am Geriatr Soc. 2001;49:651–654. doi:10.1046/j.1532-5415.2001.49128.x
50. Augusti PR, Ruviaro AR, Quatrin A, et al. Imbalance in superoxide dismutase/thioredoxin reductase activities in hypercholesterolemic subjects: relationship with low density lipoprotein oxidation. Lipids Health Dis. 2012;11:79. doi:10.1186/1476-511X-11-79
51. Dubowitz N, Xue W, Long Q, et al. Aging is associated with increased HbA1c levels, independently of glucose levels and insulin resistance, and also with decreased HbA1c diagnostic specificity. Diabet Med. 2014;31:927–935. doi:10.1111/dme.12459
52. Sushma V, Nibha S, Pushpank V, Shukla KN, Mohammad A, Monisha B. Antioxidant enzyme levels as markers for type 2 diabetes mellitus. Int J Bioassays. 2013;2:685–690.
53. Malmström H, Walldius G, Grill V, Jungner I, Hammar N. Fructosamine is a risk factor for myocardial infarction and all-cause mortality – longitudinal experience from the AMORIS cohort. Nutr Metab Cardiovasc Dis. 2015;25(10):943–950. doi:10.1016/j.numecd.2015.07.002
54. Mazidi M, Mikhailidis DP, Banach M. Higher dietary acid load is associated with higher likelihood of peripheral arterial disease among American adults. J Diabetes Complications. 2018. doi:10.1016/j.jdiacomp.2018.03.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
