Back to Journals » International Journal of General Medicine » Volume 15
The Relationship Between Klotho and SIRT1 Expression in Renal Aging Related Disease
Authors Su H, Gao D, Chen Y, Zuo Z
Received 4 August 2022
Accepted for publication 29 September 2022
Published 21 October 2022 Volume 2022:15 Pages 7885—7893
DOI https://doi.org/10.2147/IJGM.S384119
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hong Su,1– 3 Diansa Gao,1 Yanlin Chen,1 Zhong Zuo1
1The First Affiliated Hospital of Chongqing Medical University, Chongqing, 400016, People’s Republic of China; 2The Chongqing Key Laboratory of Translational Medicine in Major Metabolic Diseases, Chongqing, 400016, People’s Republic of China; 3Department of Pathology, Shenyang KingMed Center for Clinical Laboratory Co., Ltd, Shenyang, 110164, People’s Republic of China
Correspondence: Zhong Zuo, Email [email protected]
Background: This study focused on renal arteriosclerosis and aimed to explore the relationship between Klotho and SIRT1 by morphological staining, which will help to provide new ideas for the treatment of renal-aging-related diseases and a theoretical basis for the development of new drugs.
Methods: Kidney tissue samples were collected from patients who underwent nephrectomy. HK-2 cells were cultured. The Hematoxylin-eosin (HE) staining, Periodic Acid-Schiff (PAS) staining, Masson’s Trichrome staining, Immunohistochemistry (IHC) staining, Immunofluorescence (ICC) and bioinformatics means were used for this study.
Results: HE staining showed that glomerulosclerosis was atrophic and cast was significantly increased luminal narrowing of renal arterioles in aging group. PAS staining showed that the number of podocytes was reduced, the mesangial matrix expansion and the intimal fibrosis of renal arterioles. Masson’s trichrome staining showed that there was massive collagen proliferation in the tubulointerstitial in aging group, as well as intimal thickening and fibrin deposition in the tubular walls of arterioles. IHC staining showed that the expression of Klotho and SIRT1 protein was downregulated in aging group and the trend of the two was positively correlated (P < 0.01). Klotho and SIRT1 co-localized in HK-2 cells and kidney tissue. The GEPIA database analysis showed a significant positive correlation between Klotho and SIRT1 in multiple human tissues and tumors.
Conclusion: Glomerulosclerosis in aging group is accompanied by low expression of Klotho and SIRT1 in renal tissue, and Klotho is positively correlated with SIRT1. Klotho-SIRT1 pathway may be involved in the occurrence and development of renal-aging-related diseases.
Keywords: renal arteriosclerosis, aging, Klotho, SIRT1
Introduction
Aging is an intrinsic pathogenic factor of arteriosclerosis. Aging-related arteriosclerosis (AS) can lead to kidney disease.1 Renal arteriosclerosis can involve pathological changes in blood vessels at all levels and lead to glomerular sclerosis and tubulointerstitial fibrosis. Renal arteriosclerosis also plays an important role in the development of renal aging and chronic kidney disease (CKD), especially in hypertensive nephropathy, which is one of the most common primary diseases leading to end-stage renal disease dialysis.2 Renal arteriosclerosis affects CKD patient prognosis, increases economic burden, and decreases quality of life. At present, there is no effective treatment for arteriosclerosis related to renal aging, so studying its pathogenesis will help to provide targeted treatment ideas and provide a theoretical basis for the development of specific drugs.
Klotho, an antiaging protein closely related to arteriosclerosis, is mainly expressed in the kidney,3 and its expression decreases with age.4 Klotho proteins are divided into membrane-bound, soluble and secreted forms, and circulating Klotho includes soluble and secreted forms.5 Studies have shown that the expression of circulating blood Klotho is significantly reduced in patients with arteriosclerosis and hypertension, and low levels of Klotho increase the incidence of coronary artery disease and cerebrovascular accidents in patients with type 2 diabetes.6 Low abundance Klotho is considered an early predictor of arteriosclerosis.7 Combating aging-related diseases may be responsible for the decrease in Klotho, and low levels of Klotho also aggravate disease progression.
Another anti-arteriosclerosis factor, SIRT1, which is encoded by the SIRT1 gene for the nicotinamide adenine dinucleotide dependent protein deacetylase sirtuin-1 protein, belongs to class III histone deacetylases and is expressed in multiple organs.8 Certain drugs can be used to treat arteriosclerosis through SIRT1, such as Araloside C and rapamycin promote the expression of SIRT1 in ApoE−/− mice, mice, and improve arteriosclerosis and inhibit atherosclerotic plaque growth in mice through SIRT1 mediated regulation of autophagy.9,10 It has been shown that high-risk plaque (HRP) patients have decreased serum levels of SIRT1,11 and such results are similar to arteriosclerosis-related decreases in circulating blood Klotho. Based on the above studies, both Klotho and SIRT1 are involved in the occurrence and development of arteriosclerosis, and the relationship between the two is the focus of this study. We investigated the relationship between Klotho and SIRT1 using renal-aging-related arteriosclerosis as an entry point.
Materials and Methods
Kidney Tissue Samples
Renal tissue samples (n = 80) from patients who underwent nephrectomy with complete clinical data were collected from January to December 2019 at the Department of Urology, the First Affiliated Hospital of Chongqing Medical University. These samples were preserved in 4% formalin.
Renal Histopathological Staining
After fixation of kidney tissue, routine paraffin embedded sections were used, one of which was subjected to HE staining. Special staining was performed according to the instructions of PAS Staining Kit (G1281, Solarbio, China) and Masson’s trichrome staining kit (G1340, Solarbio, China). Klotho antibody (SAB3500604, Sigma, USA, 1:100) and SIRT1 antibody (8469, Cell Signaling Technology, USA, 1:200), the secondary antibody was MaxVisionTM3HRP (KIT5220, MXB, China), and EDTA (pH = 9.0) was used for high temperature and high pressure heat repair, reference instructions for IHC staining.
Histopathologic Evaluations
Kidney tissue samples were evaluated by light microscopy and diagnosed by a renal pathologist. The percentage of the number of glomeruli with sclerotic atrophy compared with the total glomerular number was used to assess the degree of glomerulosclerosis. Tissue specimens with glomerulosclerosis <10% were contrasted with those with glomerulosclerosis ≥10%.12 Lesions of both the tubules and the interstitium often occur concomitantly, not one normal and the other severe. The degree of tubulo-interstitial injury (TI) was assessed using the percentage of the area occupied by interstitial fibrosis of the renal cortex and the presence of dilation or atrophy of the tubules, with <25% versus ≥25% of the area damaged.13 According to the above standard scores, those with glomerulosclerosis <10% and TI <25% were the control group (CON), those with glomerulosclerosis ≥10% and TI ≥ 25% were the aging group (Aging), and those who did not meet this classification criteria were not included in this study.
Cell Culture
HK-2 cells were kindly provided by Stem Cell Bank, Chinese Academy of Sciences, which were cultured in Dulbecco’s modified Eagle’s and Ham’s F-12 medium (DMEM/F12, GIBCO) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin.
Immunofluorescence
Cell slides were prepared, fixed with 4% paraformaldehyde and blocked with 3% bovine serum albumin (BSA). Immunofluorescence pretreatment of kidney tissue is the same as immunohistochemistry. Primary antibodies were incubated overnight and fluorescent secondary antibodies (8889 and 4412s, Cell signaling technology, USA, 1:1000) were incubated for 1.5h in the dark. Excess antibody was washed away with PBST. DAPI was added dropwise for nuclear staining of cells (Beyotime, China). Anti-fluorescence quenchers were added dropwise and the plates were blocked for observation.
GEPIA Database
GEPIA (http://gepia.cancer-pku.cn/) is a tool for analyzing the RNA sequencing expression data of 9,736 tumors and 8,587 normal samples from the TCGA and the GTEx projects, using a standard processing pipeline. The RNA-Seq datasets GEPIA2 used is based on the UCSC Xena project (http://xena.ucsc.edu), which are computed by a standard pipeline. Use the non-log scale for calculation and use the log-scale axis for visualization.14
Statistical Analysis
Statistical analysis of the data was performed using GraphPad Prism 8.3 and SPSS19.0. Normality test and homogeneity of variance test were performed for each data. One-way ANOVA was used to compare measurement data between multiple groups, and unpaired t-test was used to compare differences between two groups. Correlation between Klotho and SIRT1 protein expression was analyzed by Pearson’s correlation. Differences were considered statistically significant when P < 0.05.
Results
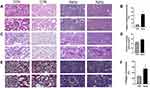
Kidneys of the Aging Group Were Morphologically Distinct from Those of the Control Group
Based on the scores, we divided the sample into control (n = 28) and senescence (n = 30) groups. Another 22 patients did not meet the inclusion criteria and were not included in this study. Glomerulosclerosis is the terminal lesion of various glomerular injuries. HE staining showed decreased glomerular vascularity, ischemic constriction, and thicker staining in the aging group (Figure 1A left), as well as marked intimal thickening and luminal narrowing of renal arterioles (Figure 1A right) and more glomerulosclerosis than in the control group (Figure 1B) (P < 0.01). PAS staining showed that the number of podocytes was decreased, and the mesangial matrix proliferation (Figure 1C left), and the intimal fibrosis of renal arterioles (Figure 1C right), and the relative value of mesangial matrix area was higher in aging group than in control group (P < 0.05)(Figure 1D). Masson’s trichrome staining showed massive collagen hyperplasia and fibrin deposition in the tubulointerstitium of aging group (Figure 1E left), and the intima of arterioles was thickened with onion skin like changes, the lumen was significantly narrowed (Figure 1E right), and the area fraction of collagen was higher than that of control group (P < 0.01) (Figure 1F).
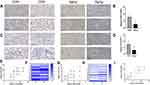
Decreased Expression of Klotho and SIRT1 in Aging Group
The localization and expression intensity of Klotho and SIRT1 in kidney tissue were detected by immunohistochemistry. In terms of localization, Klotho is mainly distributed in the cell membrane, and SIRT1 is mainly distributed in the nucleus. In terms of the intensity of protein expression, the positive staining of Klotho and SIRT1 was significantly attenuated in the kidney tissue of the aging group compared with that of the control group, suggesting that the amounts of both proteins were significantly reduced (P < 0.01). The results of IHC showed that the expression of Klotho and SIRT1 was significantly decreased in renal tissues with the presence of significant renal arteriosclerosis (Figure 2A and Figure 2). Compared with the control group, the expression level of Klotho protein in the kidney tissue of the aging group was significantly decreased (P < 0.01, Figure 2B), and the expression level of SIRT1 protein was also significantly decreased (P < 0.01) (Figure 2D). In the aging group, there was a significant positive correlation between Klotho protein and SIRT1 protein expression levels (P < 0.01) (Figure 2G and Figure 2), and the same trend was observed in the control group (P < 0.01) (Figure 2E and Figure 2). In addition, grouped by age 60 years old, the expression relationship between Klotho and SIRT1 was similar to the above (Supplementary Figure 1). Klotho was positively correlated with SIRT1 expression in all samples (P < 0.01) (Figure 2I).
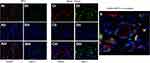
Klotho Co-Expressed with SIRT1 in HK-2 Cells and Kidney Tissue
Immunofluorescence observed that under normal physiological conditions, HK-2 cells and kidney tissue co-expressed Klotho and SIRT1, providing a structural basis for the study of molecular mechanisms study (Figure 3).
Klotho is Strongly Correlated with SIRT1 Expression in Multiple Human Tissues and Tumors
GEPIA database analyzed multiple human tissues, the correlation between Klotho and SIRT1 expression (Figure 4). We found a significant positive correlation between Klotho and SIRT1 expression in normal human tissues (endoderm-derived brain tissue, mesoderm derived cardiac vasculature tissue and ectoderm derived gastrointestinal epithelial cells) as well as in blood whole blood (P < 0.01). In addition, Klotho expression was consistently highly positively correlated with SIRT1 expression in Pan cancer tissues of multiple tumors and their adjacent noncancerous tissues (P < 0.01).
Discussion
Aging is divided into physiological aging and pathological aging: physiological aging, ie, natural aging, refers to the degenerative changes that occur in both the structure and physiological functions of human tissues; Pathological aging refers to a state in which a series of physiological and pathological processes comprehensively act on an organism and is classified as a chronic disease.15 Aging-related arteriosclerosis can lead to cardiovascular and kidney disease.1 CKD is one of the important diseases affecting the quality of survival of elderly patients, and the structural changes mainly include glomerulosclerosis, thickening of the basement membrane, tubular atrophy, interstitial fibrosis and so on.16 The 2019 report of the China kidney disease network (CK-NET) shows that nearly half of the patients with CKD are aged 60 years or older, and the rate of hospitalization and mortality increases with age, and heart failure and coronary heart disease are the major life-threatening factors for CKD patients, especially those on dialysis.17 Patients with CKD stage 5 have a more than 2-fold increased cardiovascular risk independent of traditional risk factors.18 Studies have shown that arteriosclerosis is closely associated with multiple aging-related diseases, and the incidence of arteriosclerosis in patients with CKD is higher than that in the general population, and the age of onset is also earlier.19 The currently known reason is mainly that CKD patients share common risk factors with atherosclerosis, such as age, hypertension, and diabetes.19 It can be seen that cardiovascular disease and CKD are closely associated, while arteriosclerosis is involved in the formation and development of them.
At present, the commonly used clinical evaluation indicators of renal function mainly include creatinine, cystatin C, and eGFR. However, the above indicators all reflect the total renal function and cannot achieve unilateral renal function evaluation. SPECT renal dynamic imaging allows the assessment of unilateral renal function, however it is not widely used clinically. In our collected cases, only 15.6% of patients underwent SPECT-renal dynamic imaging before surgery. Special histological staining in this study revealed that in the aging group, glomerulosclerotic atrophy, a significant increase in tubular type, a decrease in the number of podocytes, mesangial matrix proliferation, a large amount of collagen proliferation in the tubulointerstitium, arteriolar intimal thickening, and fibrin deposition in the tubular walls were observed. The immunohistochemistry results showed that the aging group with severe renal structural damage had significantly lower Klotho expression intensity in their renal tissues than the control group. At the same time, the serum eGFR, creatinine, and cystatin C of some patients who underwent nephrectomy were in the normal range, while SPECT-renal dynamic imaging showed unilateral renal function impairment, and the renal Klotho expression was significantly decreased, suggesting that the renal expression of Klotho was positively correlated with unilateral renal function, which was consistent with the findings of Ming-Chang Hu et al.20 Therefore, conventional serum renal function indicators cannot comprehensively assess unilateral renal function. In patients with renal arteriosclerosis who must undergo renal biopsy, if combined with IHC to detect Klotho protein expression, it can play an important role in the evaluation of unilateral renal function to a certain extent.
Kidney is the major site of Klotho expression.5 Circulating blood Klotho levels are positively correlated with renal function, and high levels of Klotho contribute to the protection of renal function.21 Clinical studies have found that soluble Klotho levels in blood and urine can be reduced in the early stages of CKD,22 which may be due to the decrease in the expression of membrane-bound type or the decrease in shedding ability.23 In aged mice, DNA methylation of renal Klotho, which leads to a decrease in circulating Klotho, can cause reduced renal blood flow and aging-related salt-sensitive hypertension.24 SIRT1 is expressed in both cardiovascular and renal compartments and exerts protective functions. In the cardiovascular system, activation of the SIRT1/FOXO1 pathway reduces the release of prothrombotic factors vWF and P-selectin and reduces arterial thrombosis,25 both overexpression and activation of SIRT1 can inhibit TGF- β/Smad pathway thereby attenuating isoproterenol induced myocardial fibrosis.26 In the kidney, SIRT1 participates in renal energy metabolism response, counteracts mitochondrial dysfunction, attenuates acute kidney injury, and delays renal aging.27 SIRT1 is involved in blood pressure regulation by regulating the RAAS system, sodium reabsorption in the collecting duct, and regulation of vascular tone,18 and regulates water sodium balance through the renal Ghrelin/SIRT1 pathway.22
The previous study of our group found that the expression and activity of SIRT1 decreased in the aorta of Klotho gene-deficient mice, which induced the formation of arteriosclerosis in mice. Exogenous activation of SIRT1 did not affect the expression level of Klotho in tissues28, suggesting that Klotho may be an upstream regulator of SIRT1. In this study, compared with the control group, the expressions of Klotho and SIRT1 in the renal tissue of the aging group were significantly decreased, indicating that both play a negative role in renal arteriosclerosis, and the expressions of the two were significantly positively correlated. We speculate that there is a Klotho-SIRT1 regulatory pathway in renal arteriosclerosis, and the specific mechanism needs to be further studied.
Klotho and SIRT1 have been widely studied, but the correlation and regulatory mechanism between the two have been rarely reported. Through the GEPIA database, we found that Klotho and SIRT1 were highly positively correlated in cells derived from the outer, middle, and inner germ layers of normal human tissues and in blood. We speculate that the Klotho-SIRT1 regulatory pathway is widespread in human tissues, which may play an important biological regulatory function. In addition, both were also found to be highly correlated in cancerous and paracancerous tissues of multiple tumors, indicating that in pathological conditions, the pathway is still widespread. Combined with the conclusion observed in this experiment that Klotho is mainly localized at the cell membrane, SIRT1 is mainly localized in the nucleus, and the colocalization of the two on the same cell, various lines of evidence give us reason to believe that there is a Klotho-SIRT1 signaling pathway involved in the occurrence and development of aging related diseases. In-depth study of the up and downstream regulatory mechanisms of this pathway may open new ideas for the prevention and treatment of multiple diseases.
Conclusions
In conclusion, the renal structure of aging patients was significantly damaged compared with the control group, and this was accompanied by down-regulation of Klotho and SIRT1 protein expression. HK-2 cells and kidney tissues have co-localized expression of Klotho and SIRT1, which can be a cell model for the study of the molecular relationship between the two. GEPIA database shows a significant positive correlation between Klotho and SIRT1 in multiple human tissues and tumors. Klotho may be an upstream regulator of SIRT1, and exploration of its signaling pathway will be the direction of future research.
Abbreviations
ApoE, Apolipoprotein E; AS, Arteriosclerosis; BSA, Bovine serum albumin; CKD, Chronic Kidney Disease; CK-NET, China Kidney Disease Network; DNA, Deoxyribonucleic acid; eGFR, Estimated glomerular filtration rate; HE, Hematoxylin-eosin; HRP, High-risk plaque; ICC, Immunofluorescence; IHC, Immunohistochemistry; PAS, Periodic Acid-Schiff; RAAS, Renin angiotensin aldosterone system; SPECT, Single photon emission computed tomography; TI, Tubulo-interstitial injury.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study was carried out following the rules of the Declaration of Helsinki of 1964. The methods employed in this study were in accordance with the requirements of medical ethics. This study was approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University. Informed consent was exempted since this study used tissue samples discarded after pathological diagnosis was completed, without any adverse effects on the diagnostic treatment of patients, and with the patient personal information implicit throughout this study.
Funding
This work was supported by the National Nature Science Foundation of China (No. 81701384) and the Fundamental Science and Advanced Technology Research of Chongqing (CSTC2019jcyjmsxm0425).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Carracedo J, Alique M, Vida C, et al. Mechanisms of cardiovascular disorders in patients with chronic kidney disease: a process related to accelerated senescence. Front Cell Dev Biol. 2020;8:185. doi:10.3389/fcell.2020.00185
2. Hart PD, Bakris GL. Hypertensive nephropathy: prevention and treatment recommendations. Expert Opin Pharmacother. 2010;11(16):2675–2686. doi:10.1517/14656566.2010.485612
3. Zhou H, Pu S, Zhou H, et al. Klotho as potential autophagy regulator and therapeutic target. Front Pharmacol. 2021;12:755366. doi:10.3389/fphar.2021.755366
4. Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36(2):174–193. doi:10.1210/er.2013-1079
5. Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi:10.1038/36285
6. Pan HC, Chou KM, Lee CC, et al. Circulating Klotho levels can predict long-term macrovascular outcomes in type 2 diabetic patients. Atherosclerosis. 2018;276:83–90. doi:10.1016/j.atherosclerosis.2018.07.006
7. Docherty CK, Strembitska A, Baker CP, et al. Inducing energetic switching using Klotho improves vascular smooth muscle cell phenotype. Int J Mol Sci. 2021;23(1):1. doi:10.3390/ijms23010217
8. Guan Y, Hao CM. SIRT1 and kidney function. Kidney Dis. 2016;1(4):258–265. doi:10.1159/000440967
9. Luo Y, Lu S, Gao Y, et al. Araloside C attenuates atherosclerosis by modulating macrophage polarization via Sirt1-mediated autophagy. Aging. 2020;12(2):1704–1724. doi:10.18632/aging.102708
10. Yuan P, Hu Q, He X, et al. Laminar flow inhibits the Hippo/YAP pathway via autophagy and SIRT1-mediated deacetylation against atherosclerosis. Cell Death Dis. 2020;11(2):141. doi:10.1038/s41419-020-2343-1
11. He X, Zheng J, Liu C. Low serum level of sirtuin 1 predicts coronary atherosclerosis plaques during computed tomography angiography among an asymptomatic cohort. Coron Artery Dis. 2019;30(8):621–625. doi:10.1097/mca.0000000000000804
12. Bazzi C, Petrini C, Rizza V, et al. A modern approach to selectivity of proteinuria and tubulointerstitial damage in nephrotic syndrome. Kidney Int. 2000;58(4):1732–1741. doi:10.1046/j.1523-1755.2000.00334.x
13. Lee J, Lee Y, Kim KH, et al. Chemokine (C-C Motif) ligand 8 and tubulo-interstitial injury in chronic kidney disease. Cells. 2022;11(4). doi:10.3390/cells11040658
14. Tang Z, Kang B, Li C, et al. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–w560. doi:10.1093/nar/gkz430
15. De Winter G. Aging as disease. Med Health Care Philos. 2015;18(2):237–243. doi:10.1007/s11019-014-9600-y
16. Karam Z, Tuazon J. Anatomic and physiologic changes of the aging kidney. Clin Geriatr Med. 2013;29(3):555–564. doi:10.1016/j.cger.2013.05.006
17. Zhang L, Zhao MH, Zuo L, et al. China kidney disease network (CK-NET) 2016 annual data report. Kidney Int Suppl. 2020;10(2):e97–e185. doi:10.1016/j.kisu.2020.09.001
18. Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi:10.1016/s0140-6736(10)60674-5
19. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney. 1998;32(5 Suppl 3):S112–119. doi:10.1053/ajkd.1998.v32.pm9820470
20. Hu MC, Shi M, Zhang J, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78(12):1240–1251. doi:10.1038/ki.2010.328
21. Drew DA, Katz R, Kritchevsky S, et al. Association between Soluble Klotho and change in kidney function: the health aging and body composition study. JASN. 2017;28(6):1859–1866. doi:10.1681/asn.2016080828
22. Barker SL, Pastor J, Carranza D, et al. The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015;30(2):223–233. doi:10.1093/ndt/gfu291
23. Saar-Kovrov V, Donners M, van der Vorst EPC. Shedding of Klotho: functional implications in chronic kidney disease and associated vascular disease. Front Cardiovasc Med. 2020;7:617842. doi:10.3389/fcvm.2020.617842
24. Kawarazaki W, Fujita T. Kidney and epigenetic mechanisms of salt-sensitive hypertension. Nat Rev Nephrol. 2021;17(5):350–363. doi:10.1038/s41581-021-00399-2
25. Wu Q, Hu Y, Jiang M, et al. Effect of autophagy regulated by Sirt1/FoxO1 pathway on the release of factors promoting thrombosis from vascular endothelial cells. Int J Mol Sci. 2019;20(17):17. doi:10.3390/ijms20174132
26. Ren H, Shao Y, Wu C, et al. VASH-1 regulates oxidative stress and fibrosis in diabetic kidney disease via SIRT1/HIF1α and TGFβ1/Smad3 signaling pathways. Front Mol Biosci. 2020;7:137. doi:10.3389/fmolb.2020.00137
27. Valdivielso JM, Rodríguez-Puyol D, Pascual J, et al. Atherosclerosis in chronic kidney disease: more, less, or just different? Arterioscler Thromb Vasc Biol. 2019;39(10):1938–1966. doi:10.1161/atvbaha.119.312705
28. Gao D, Zuo Z, Tian J, et al. Activation of SIRT1 attenuates Klotho deficiency-induced arterial stiffness and hypertension by enhancing AMP-activated protein kinase activity. Hypertension. 2016;68(5):1191–1199. doi:10.1161/hypertensionaha.116.07709
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.