Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 13
The Relationship Between Human Papillomavirus, OFD1 and Primary Ciliogenesis in the Progression of Oropharyngeal Cancer: A Retrospective Cohort Study
Authors Meng H , Yang X , Liu R, Bao J, Hou Y , Sun J, Miao S, Qu G
Received 11 July 2020
Accepted for publication 8 October 2020
Published 17 November 2020 Volume 2020:13 Pages 633—644
DOI https://doi.org/10.2147/PGPM.S271735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin Bluth
Hong-xue Meng,1,2,* Xin-xin Yang,1,* Rui-qi Liu,3,* Jun-jie Bao,4 Yun-jing Hou,1 Ji Sun,5 Su-sheng Miao,5 Guo-fan Qu4
1Department of Pathology, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 2Department of Pathology, Harbin Medical University, Harbin, People’s Republic of China; 3Department of Radiation Oncology, Sun Yat-Sen University Cancer Hospital, Guangzhou, People’s Republic of China; 4Department of Orthopedics, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 5Department of Otolaryngology, Head and Neck Surgery, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong-xue Meng
Department of Pathology, Harbin Medical University Cancer Hospital, 150 Haping Road, Harbin, People’s Republic of China
Tel +86-451-85718261
Email [email protected]
Guo-fan Qu
Department of Orthopedics, Harbin Medical University Cancer Hospital, 150 Haping Road, Harbin, People’s Republic of China
Tel +86-451-85718262
Email [email protected]
Purpose: Infection with human papillomavirus (HPV) has been indicated to be a important risk factor for oropharyngeal squamous cell carcinoma (OPSCC). Primary ciliogenesis defects contribute to tumorigenesis, and OFD1 at centriolar satellites is a crucial suppressor of primary ciliogenesis. To identify novel markers associated with HPV-induced carcinogenesis, the interactions between HPV infection and primary ciliogenesis in the tumorigenesis and progression of OPSCC were investigated in this study.
Patients and Methods: The 1530 OPSCC patients recruited in this research were treated from 2000 to 2017. Immunohistochemistry and RT-PCR were performed on tissue samples to compare the expression of p16, TSLP, TGFβ 1, IFNγ, OFD1, and their relationship with clinical characteristics of patients.
Results: We speculate that the positive expression of p16 is related to early primary OPSCC, and the survival rate of p16 positive patients after radiotherapy and surgery is higher. Expression of TSLP on dendritic cells in HPV-positive OPSCC correlated with the expression of OFD1. HPV-positive OPSCC showed increased expression of OFD1 combined with reduced ciliogenesis. Hence, TSLP induced by HPV infection may reduce the invasive potential of OPSCC cells by promoting OFD1 expression, thereby inhibiting primary ciliogenesis.
Conclusion: Our study demonstrated that HPV may be related to the progression of OPSCC by regulating OFD1 expression and primary ciliogenesis, making this protein a potential therapeutic target.
Keywords: HPV, p16, oropharyngeal cancer, pathogenesis, prognosis
Introduction
Head and neck cancer was the seventh most common cancer worldwide, and the proportion of Oropharyngeal squamous cell carcinoma (OPSCC) in head and neck cancer is increasing year by year., which is characterized by nodal metastases and high recurrence rates.1 OPSCC can usually be suppressed by surgery and adjuvant radiotherapy in the early stages of development, but the 5-year survival rate in locally advanced patients is only 48%, and only 26% in patients with metastatic disease.2,3
Studies have confirmed that high-risk factors for head and neck squamous cell carcinoma (HNSCC) include tobacco, alcohol, and poor oral hygiene. However, the incidence of these risk factors is declining in recent years.3 Human papillomavirus (HPV) infection has become a important high-risk factor for HNSCC. Including the United States, Australia and some European countries, the prevalence of HPV-related HNSCC, especially OPSCC has been rising.4,5 Nonetheless, in the Chinese patient cohort, the prevalence, prognosis and significance of OPSCC caused by high-risk HPV infection still remain underexplored.
The current rising in OPSCC is mainly related to high-risk HPV infection.2 90% of HPV-positive OP cancers are infected with high-risk HPV16 (the dominant subtype).6 In cancers associated with HPV infection, the viral oncoproteins E6 and E7 are always overexpressed. E7 can bind to the retinoblastoma (Rb) protein and destroy the E2F/Rb complex. After Rb protein is degraded, cell cycle limitation is eliminated and transcription factor E2F is associated with increased p16 (INK4) protein expression.7
HPV infection is closely related to p16 protein expression in OPSCC. Therefore, in these high-risk tumors, p16 can be used as a surrogate biomarker to detect the presence of HPV infection.9,10 HPV is transcribed before p16 expression. Therefore, p16 expression can be used as an indicator to identify tumors with HPV transcription activity.11 Moreover, compared with the use of histochemical methods to detect HPV DNA, p16 expression can be used as an independent prognostic factor for overall survival and progression-free survival.12 Then, p16 immunohistochemistry (IHC) in combination with HPV-DNA detection by PCR has been found to be a powerful indicator of clinical outcome in patients with OPSCC using univariate analysis.7,13 And previous studies have indicated that p16 may enhance the immunogenicity of dendritic cells (DC) by means of Th1 cytokine secretion and cyclin-dependent pathways.8
HPV infection can give rise to a very extensive of epithelial lesions. However, only patients with high-risk types of HPV is possible to transform cancer.14,15 The activated thymic stromal lymphopoietin (TSLP) secreted by the head and neck epithelium can bind to the heterodimeric receptor composed of IL-7 receptor alpha chain (IL-7Rα) and TSLP receptor (TSLPR) chain.16,17 Exogenous stimulation (trauma, infection, etc.), Toll-like receptor signal transduction, and host-derived pro-inflammatory and Th2 cytokines can all induce TSLP activation. Because TSLP, IFNγ and TGFβ1 can represent the levels of the various components of the immune response, so in our research we tested their content to understand how they are related in tumor immune response.
The proportion of HPV DNA-positive head and neck cancer (HNC) caused by HPV infection in the overall HNC is indetermination, and its estimation remains a formidable challenge. Therefore, it is very significance to explore the carcinogenesis induced by HPV infection and its related expression patterns of individual and combined markers, which can be used to evaluate the biological and carcinogenic activity of HPV in OPSCC. For example, the involvement of TSLP in OPSCC with HPV infection is unknown.
The primary cilium is a organelle based on microtubule structures and play an important role in sensory and signaling pathways. The maintenance of the normal function of many signaling pathways is inseparable from the cilium structure. These signaling pathways play a vital role in the development of many types of cancer.There are abnormalities and defects in the function of primary cilia in human cancer cells, which promotes the occurrence and development of tumors.18,19 OFD1 on the centromeric satellite is a key factor used to inhibit the growth of primary cilia in human cancer cells. The growth of mammalian primary cilia is generally performed by removing OFD1 by autophagy.20 OFD1 expression could be regulated by DCs. However, it is unknown whether TSLP regulates OFD1 expression and primary ciliogenesis.
This study aims to evaluate the prevalence, prognosis, and clinicopathologic characteristics of HPV-positive oropharyngeal cancer in Northeast China, and to clarify the role of TSLP, p16 and OFD1 in the occurrence and development of OPSCC.
Patients and Methods
Patients
One thousand five hundred and thirty patients with pathology-proven oropharyngeal cancer (January 2000 to January 2017) were enrolled from Harbin Medical University Cancer Hospital, which was the cancer center for northeast China. The acquired tissue was taken during the operation of the patient.
Ethics Statement
This research strictly follows the Helsinki Declaration. This research was approved by the institutional ethics committee of the Cancer Hospital Affiliated to Harbin Medical University.
Clinical Parameters
The clinical data of patients include data on gender, age, history of smoking, history of alcohol, treatment.
Histopathological Diagnosis
The diagnosis and categorize of all cases strictly follow the relevant WHO principles. Two pathologists reviewed all slides and scored the pathological variables. The TNM classification of HPV-positive oropharyngeal cancer was developed by the International Oropharyngeal Cancer Staging Network Cooperation Organization (ICON-S).21,22
Antibodies and Immunohistochemistry (IHC)
For IHC, formalin-fixed, the 4 µm thick FFPE was blocked with 1% H2O2, and then incubated with trypsin at 37 °C for 30 minutes for antigen retrieval. IHC was performed according to the manufacturer’s instructions. Sections were incubated with the following primary antibodies overnight at 4 °C: rabbit anti-human P16 (1:100, INK4a, IgG, Zhongshan Tech, China), rabbit anti-human TSLP (10 µg/mL, ab47943, IgG, Abcam, USA), goat anti-human TSLPR (10 µg/mL, IgG, R&D, USA), rabbit anti-human OFD1 (1:100, IgG, Abcam, USA), goat anti-human TGF-β1 (10 µg/mL, IgG, R&D, USA), rabbit anti-human IFNγ (1:100, IgG, Abcam, USA), mouse anti-human DC-SIGN (1:100, IgG, Abcam, USA). To demonstrate the structures of cilia, we stained them with ARL13B (1:100, IgG, Abcam, USA), a ciliary membrane marker. Sections were then incubated with Streptavidin-Biotin Universal Detection System (Beckman Coulter, USA), and visualized using DAB.
For IHC analysis of p16 expression, cells presenting nuclear and cytoplasmic staining were classified as positive, and were scored semi-quantitatively according to a previous study,23 as follows: negative (no cells were positive); sporadic (isolated cells were positive, but < 5%); focal (small cell clusters, but < 80% of the cells were positive); and diffuse (> 80% of the cells were stained). Strong and diffuse nuclear and cytoplasmic staining in ≥ 80% of tumor cells was defined as p16 positive.
In situ Hybridization (ISH)
For IHC, fix it with formalin, FFPE 4 µm thick and seal it, then incubate with HPV probe (1: 100, Abcam).
DNA Extraction and PCR Analysis
We used the DNeasy Micro kit (Qiagen, Hilden, Germany) to extract and purify total DNA from formalin-fixed paraffin-embedded tissue according to the manufacturer’s instructions. The DNA was amplified after 35 cycles for PCR analysis. The forward and reverse primers are: β-globin 5′- GAA GAG CCA AGG ACA GGT AC −3′ (forward) and 5′- CAA CTT CAT CCA CGT TCA CC −3′ (reverse); HPV 5′- CGT CCM ARR GGA WAC TGA TC 3′ (forward) and 5′- GCM CAG GGW CAT AAY AAT GG −3′ (reverse). The cycling conditions of HPV and β-globin are: the denaturation temperature is at 94 °C for 1 minute, the annealing temperature is 56 °C for 1 minute, the extension temperature is 72 °C for 1 minute, and finally Extend for 10 minutes at 72 °C. The PCR products were analyzed by 4% agarose gel electrophoresis, and observed on the imager using ethidium bromide.
RNA Extraction and RT-PCR Analysis
Total RNA was extracted and purified from LCM-captured cells from formalin-fixed, paraffin-embedded tissues using the RNeasy Micro kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. We used the QuantiTect reverse transcription kit (Qiagen, Hilden, Germany) for the synthesis of complementary DNA (cDNA). The resulting cDNA was used as a template for PCR analysis. Forward and reverse specific primers, amplicon size and annealing temperature are: β-actin 5′-CAGAGCAAGAGAGGCATCCT-3′ (forward) and 5′-ACGTACATGGCTGGGGTG-3′ (reverse), 227 bp, 55 °C; TSLP 5′-TATGAGTGGGACCAAAAGTACCG-3′ (forward) and 5′-GGGATTGAAGGTTAGGCTCTGG-3′ (reverse), 97 bp, 55 °C; TSLPR 5′-GAGTGGCAGTCCAAACAGGAA-3′ (forward) and 5′-ACATCCTCCATAGCCTTCACC-3′ (reverse), 103 bp, 62 °C; TGF-β1 5′-ACCAACTATTGCTTCAGCTC-3′ (forward) and 5′-TTATGCTGGTTGTACAGGG-3′ (reverse), 197 bp, 50 °C. The PCR products were analyzed by 4% agarose gel electrophoresis, and observed on the imager using ethidium bromide.
Quantitative Real-Time PCR Analysis
We used QuantiTect RT kit (Qiagen, Hilden, Germany) for reverse transcription of the same amount of RNA (50 ng) from the sample. In addition, we use the Fast SYBR Green Master Mix (Applied Biosystems, Germany) for the amplified cDNA, and the 7500 Fast Real-Time PCR System (Applied Biosystems) for PCR analysis samples. Primers for β-actin, TSLP, TSLPR, and TGF-β1 were described in the RT-PCR section. We applied the relative standard curve method to clarify its relative expression. And the data was normalized to β-actin expression.
Statistical Analysis
In our research, Mann–Whitney U-test was performed to compare data between P16 positive and negative patients. We speculated the relationships among clinical characteristics, HPV status, and expression of p16, TSLP, TGFβ1, IFNγ, and OFD1 using Spearman correlation analysis and Pearson’s correlation analysis (SAS Institute Inc., Cary, NC, USA). Differences with p-values of less than 0.05 were considered significant.
Results
Clinical and Pathological Parameters
Table 1 shows the clinicopathological characteristics of OPSCC in our cohort (1530 cases). The patients were predominantly male (n = 1197; 78.23%) and smokers (n = 1326; 86.67%). 920 had a history of drinking (60.13%). Treatment consisted in surgery only (n = 1287), postoperative radiotherapy (n = 235), postoperative chemotherapy (n = 5), or surgery followed by radiotherapy and chemotherapy (n = 3). We have follow-up information for all 1530 patients. After completing initial treatment, there are residual diseases in 286 persons (18.69%). A complete response (CR) was achieved in 1244 patients (81.31%), of which 773 (50.52%) maintained CR during the follow-up and the other 471 (30.78%) subsequently showed recurrence or metastasis.
 |
Table 1 The Relationship Between P16, HPV Status, and Clinical Parameters of Patients |
According to the histological type, there were 204 cases (13.33%) in 1530 cases of SCC NOS/conventional non-keratinization treatment, and 1301 cases (85.03%) in conventional keratinization (Table 2). Regarding the differentiation, 497 (32.48%), 848 (55.42%), and 185 (12.09%) cases showed good, moderate, and poor differentiation, respectively. Most patients have lymphatic infiltration (645, 42.16%).
 |
Table 2 The Relationship Between P16, HPV Status, and Histological Diagnosis |
In the TNM staging, 1455 patients (95.1%) had low T stage OPSCC tumors (T1/T2), and other 75 patients (4.9%) had high T stage OPSCC tumors (T3/T4); In addition, there were 645 patients (42.16%) emerged clinically positive lymph node metastasis (N +). Finally, 1368 patients (89.41%) had a high clinical stage (III/IV), and the remaining 162 patients (10.59%) had a low clinical stage (I/II).
P16 Protein Overexpression, HPV DNA-PCR, and HPV-ISH
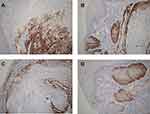
IHC showed that p16 was overexpressed in 81 (5.29%) of these 1530 cases. In addition over 80% of carcinoma cells and in situ carcinoma cells have strong and diffuse staining in the nucleus and cytoplasm in these p16 positive cases (Figure 1). Among all cases, 78 (5.10%) were shown to be HPV-positive by PCR (Figure 2). ISH results showed that 38 patients were positive for HPV. Of these, 5 cases were positive for HPV6, 3 positive for HPV11, 2 for HPV13, 25 for HPV16, and 3 for HPV18.
 |
Figure 2 PCR analysis of HPV DNA in patients with oropharyngeal cancer. Using PCR, HPV status was determined in patients with oropharyngeal cancer. |
Consistently, there is a good correlation between HPV positive and p16 overexpression. Overexpression of p16 as a predictor of HPV infection showed high sensitivity (100%) and high specificity (96%). Table 1 provides a detailed description of the relationship between HPV infection and clinicopathological variables. HPV infection was significantly more frequent in patients who were older (P < 0.01) or male (P < 0.05), had moderate and poor differentiation (P < 0.05), conventional keratinizing type (P < 0.01), lymph node metastasis (P < 0.05), low T stage (P < 0.05), high clinical stage (P < 0.05), and p16 overexpression (P < 0.01). Specifically, HPV and p16-positive patients were more likely to maintain the CR during the follow-up period.
Relationship Between HPV and Expression of TSLP, TGFβ1, IFNγ, and OFD1
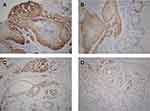
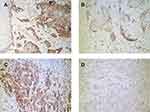
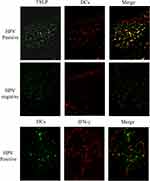
Expression of TSLP, TGFβ1, and IFNγ was higher in OPSCC tissues from HPV-positive than from HPV-negative patients (Figure 3). Among them, their statistical values are as follows: TSLP (p<0.05, r=0.62), TGFβ1 (p<0.05, r=0.65), IFNγ (p<0.05, r=0.73). Furthermore, higher expression of OFD1 combined with less ciliogenesis was observed in HPV-positive OPSCC tissues (Figure 4), which may explain why HPV-positive OPSCC cases showed more frequently moderate and poor differentiation and lymph node metastasis. Correlation analysis by Spearman showed that the expression of TSLP was related to the expression of TGFβ1, IFNγ and OFD1. Using double immunofluorescence, we found that TSLP was overexpressed on DCs in HPV-positive OPSCC, and that TSLP expression on DCs correlated with OFD1 expression (Figure 5). Moreover, the mRNA levels of TSLP, TGFβ1, IFNγ, and OFD1 were higher in the HPV-positive than in the HPV-negative group (P < 0.05, Figure 6).
Prognostic Analysis of HPV and P16 Status
The positive expression of p16 in patient cells is associated with a significantly improved overall survival rate (OS, P = 0.05). However, the results obtained through multivariate analysis are not clearly consistent. Our univariate analysis demonstrated that high clinical stage (P = 0.0475), high T stage (P = 0.0002), age (P = 0.0058), HPV negativity (P = 0.0029), p16 negativity (P = 0.003), and TSLP positivity (P = 0.0001) were all significantly related to OS reduction. Multivariate analysis confirmed that only TSLP positivity (P = 0.0004) was consistent with OS reduction and could be used as an independent risk factor.
Discussion
Previous studies suggested that patients with HPV-positive HNSCC had higher survival rates than HPV-negative patients.2 In addition, scientific research indicated that HPV infection status can be used as a predictor of response to treatment, and is related to improved response to radiation therapy and chemotherapy. The study included 1530 patients in 16 years, and there are some inherent biases typical of retrospective cohorts. Although the use of adjuvant chemotherapy and high-conformal radiation technology has been increased clinically, the principle for patients to choose treatment methods is still mainly focused on surgical treatment and radiotherapy. Therefore, we use HPV-DNA testing as a gold standard and apply it clinically to evaluate the potential value of novel surrogate markers for HPV-induced cellular transformation. It is crucial to notice that our estimate of the HPV attribution score is much lower than the results of a recent meta-analysis of HPV in HNC: the oropharynx was 33.6%, the OC was 22.2% and the larynx was 20.2% worldwide.24 There are many reasons for this discrepancy, which may be due to the sample originating from different geographical locations, or it may be caused by the high heterogeneity of the procedures and steps used in the laboratory measurement and the measurement method.
HPV-AF estimates were also heterogeneous of in terms of sex and age at diagnosis. In our cohort, the estimated value of HPV-AF in men is significantly higher than that of women. This result is substantially different from the European population in other studies.Additionally, we also discover that the estimated value of HPV-AF is much higher in the age range of 46–55 years. Moreover, nearly 90% (86.67%) of the 1336 patients had a history of smoking, and nearly 90% (89%) of patients with positive expression of p16 protein were used to smoking. In our cohort, there was a 60.13% (n = 920) drinking rate, and nearly 70% of p16-positive patients drank alcohol.
It can be hypothesized that this huge heterogeneity of HPV-AFs reflects the obvious trend of geographical, temporal and demographic changes in people with smoking and oral HPV infection, leading to the rapid development of the epidemiology of HPV positive HNCs. We speculate that in our study cohort, tobacco smoking had a greater effect on oropharyngeal carcinogenesis than those that were almost universally caused by oral HPV infections in the 1960s and early 1980s, in which patients were approximately 46–55 years old. But the situation has changed since the 1980s. Although the number of tobacco smokers has decreased, the number of people who have been exposed to HPV in the oral cavity due to the prevalence of oral sex practices has increased. Therefore, the current prevalence and trend of HNC driven by HPV infection may virtually depend on these risk factors exposed 20–30 years earlier.
Previous studies have indicated that regardless of the HPV infection status in the oropharynx, p16 protein can independently serve as a predictor of radiotherapy response.6,7 Among patients undergoing surgery and postoperative adjuvant radiotherapy, the overall survival and disease-specific survival of patients with positive p16 protein were significantly longer than those with negative p16.8 The results shown in our univariate analysis are consistent with it. However, our multivariate analysis showed that p16 expression cannot be used as an independent predictor of survival. It can be reasonably assumed that p16 affects the survival of patients by affecting the invasive potential and proliferative capacity of the primary tumor. Nonetheless, scientists have fully affirmed the role of HPV and p16 as therapeutic targets and biomarkers in HNSCC.
In addition, we found that the protein and mRNA expression of TSLP in DCs was higher in HPV-positive than in negative patients, consistent with the fact that exogenous stimulation (including infection) can induce TSLP. Furthermore, the expression of OFD1 was markedly correlated with increased expression of TGFβ1 and IFNγ in HPV-positive patients. Primary ciliogenesis was also reduced in HPV-positive patients. These results are consistent with former studies, that OFD1 is a key factor in the expression of the primary ciliogenesis in human cancer cells. We can speculate that HPV has caused changes in the immune system by affecting TSLP and dendritic cells, leading to changes in OFD1 and other indicators that affect the growth of ciliogenesis and affect the development of tumors.Additionally, TSLP protein expression correlates with that of TGFβ1, IFNγ, and OFD1 in HPV-positive OPSCCs, indicating that TSLP and primary ciliogenesis are closely related with the role of OFD1 in HPV-positive OPSCCs.
Conclusion
Our research indicates that the expression of p16 protein is related with early primary OPSCC and patients with P16 positive have longer survival after radiotherapy or surgery. In addition, the expression of p16 cannot be used as an independent predictor of survival. HPV infection can affect its invasive potential by promoting the expression of OFD1, whose expression affects the growth of primary ciliogenesis. TSLP induced by HPV infection is also possible to play a part in regulating primary ciliogenesis, which may modulate tumorigenesis and tumor invasion. Further research is required to clarify the role of HPV and TSLP in OPSCCs at the molecular level.
Abbreviations
FDC, follicular dendritic cell; FDC-SP, follicular dendritic cell secreted protein; IHC, immunohistochemistry; OPSCC, Oropharyngeal squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; HPV-AF, HPV-attributable fraction; TSLP, thymic stromal lymphopoietin.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee of the Cancer Hospital Affiliated to Harbin Medical University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.(2019-112)
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (82072985), the National Nature Science Foundation of Heilongjiang Province (YQ2020H036), the Postdoctoral Scientific Research Developmental Fund of Heilongjiang Province (LBH-Q18076), the N10 Found project of Harbin Medical University Cancer Hospital (2017-03), the Youth Elite Training Foundation of Harbin Medical University Cancer Hospital (JY2016-06), and the Outstanding Youth Foundation of Harbin Medical University Cancer Hospital (JCQN-2018-05), Special funds of central finance to support the development of local University (2019), Wu-Jieping Medical Foundation (320.6750.19089-22, 320.6750.19089-48).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Chow LQM. Head and neck cancer. N Engl J Med. 2020;382(1):60–72. doi:10.1056/NEJMra1715715
2. Cramer JD, Burtness B, Le QT, Ferris RL. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16:669–683.
3. Lechner M, Jones OS, Breeze CE, Gilson R. Gender-neutral HPV vaccination in the UK, rising male oropharyngeal cancer rates, and lack of HPV awareness. Lancet Infect Dis. 2019;19(2):131–132. doi:10.1016/S1473-3099(18)30802-8
4. Drolet M, Bénard E, Pérez N, Brisson M. HPV vaccination impact study group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497–509. doi:10.1016/S0140-6736(19)30298-3
5. Worsham MJ, Chen KM, Datta I, et al. The biological significance of methylome differences in human papilloma virus associated head and neck cancer. Oncol Lett. 2016;12(6):4949–4956. doi:10.3892/ol.2016.5303
6. Shaikh MH, Bortnik V, McMillan NA, Idris A. cGAS-STING responses are dampened in high-risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb Pathog. 2019;132:162–165. doi:10.1016/j.micpath.2019.05.004
7. Jalaly JB, Hosseini SM, Shafique K, Baloch ZW. Current status of p16 immunohistochemistry and HPV testing in fine needle aspiration specimens of the head and neck. Acta Cytol. 2020;64(1–2):30–39. doi:10.1159/000496158
8. Lechner M, Chakravarthy AR, Walter V, et al. Frequent HPV-independent p16/INK4A overexpression in head and neck cancer. Oral Oncol. 2018;83:32–37. doi:10.1016/j.oraloncology.2018.06.006
9. Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol. 2013;2:51–61.
10. Emmett S, Boros S, Whiteman DC, Porceddu SV, Panizza BJ, Antonsson A. Sexual behaviour, HPV status and p16INK4a expression in oropharyngeal and oral cavity squamous cell carcinomas: a case–case comparison study. J Gen Virol. 2018;99(6):783–789. doi:10.1099/jgv.0.001069
11. Albers AE, Qian X, Kaufmann AM, Coordes A. Meta analysis: HPV and p16 pattern determines survival in patients with HNSCC and identifies potential new biologic subtype. Sci Rep. 2017;7(1):16715. doi:10.1038/s41598-017-16918-w
12. Gurín D, Slávik M, Shatokhina T, et al. Current perspective on HPV-associated oropharyngeal carcinomas and the role of p16 as a surrogate marker of high-risk HPV. Klin Onkol. 2019;32(4):252–260. doi:10.14735/amko2019252
13. Li P, Zhang X, Gu L, Zhou J, Deng D, Chen Y-J. P16 methylation increases the sensitivity of cancer cells to the CDK4/6 inhibitor palbociclib. PLoS One. 2019;14(10):e0223084. doi:10.1371/journal.pone.0223084
14. Munday JS, He YY, Aberdein D, Klobukowska HJ. Increased p16CDKN2A, but not p53, immunostaining is predictive of longer survival time in cats with oral squamous cell carcinomas. Vet J. 2019;248:64–70. doi:10.1016/j.tvjl.2019.04.007
15. Ni Y, Zhang X, Wan Y, et al. Relationship between p16 expression and prognosis in different anatomic subsites of OSCC. Cancer Biomark. 2019;26(3):375–383. doi:10.3233/CBM-192402
16. Meng H, Li H, Ohe R, et al. Thymic stromal lymphopoietin in tonsillar follicular dendritic cells correlates with elevated serum immunoglobulin A titer by promoting tonsillar immunoglobulin A class switching in immunoglobulin A nephropathy. Transl Res. 2016;176:1–17. doi:10.1016/j.trsl.2016.04.008
17. Borowski A, Vetter T, Kuepper M, et al. Expression analysis and specific blockade of the receptor for human thymic stromal lymphopoietin (TSLP) by novel antibodies to the human TSLPRα chain. Cytokine. 2013;61(2):546–555. doi:10.1016/j.cyto.2012.10.025
18. Fabbri L, Bost F, Mazure NM. Primary cilium in cancer hallmarks. Int J Mol Sci. 2019;20(6):1336. doi:10.3390/ijms20061336
19. May-Simera HL, Wan Q, Jha BS, et al. Primary cilium-mediated retinal pigment epithelium maturation is disrupted in ciliopathy patient cells. Cell Rep. 2018;22(1):189–205. doi:10.1016/j.celrep.2017.12.038
20. Tang Z, Lin MG, Stowe TR, et al. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature. 2013;502(7470):254–257. doi:10.1038/nature12606
21. Huang SH, Xu W, Waldron J, et al. Refining American joint committee on cancer/union for international cancer control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol. 2015;33(8):836–845. doi:10.1200/JCO.2014.58.6412
22. Dahlstrom KR, Garden AS, William WN, Lim JMY, Sturgis EM. Proposed staging system for patients with HPV-related oropharyngeal cancer based on nasopharyngeal cancer N categories. J Clin Oncol. 2016;34(16):1848–1854. doi:10.1200/JCO.2015.64.6448
23. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi:10.1056/NEJMoa0912217
24. Tumban E. A current update on human papillomavirus-associated head and neck cancers. Viruses. 2019;11(10):922. doi:10.3390/v11100922
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.