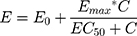Back to Journals » Journal of Pain Research » Volume 14
The Relationship Between Androgens and Days per Month of Period Pain, Pelvic Pain, Headache, and TLR4 Responsiveness of Peripheral Blood Mononuclear Cells in Young Women with Dysmenorrhoea
Authors Evans S , Kwok Y , Solterbeck A , Pyragius C , Hull ML , Hutchinson MR , Rolan P
Received 18 October 2020
Accepted for publication 7 January 2021
Published 3 March 2021 Volume 2021:14 Pages 585—599
DOI https://doi.org/10.2147/JPR.S279253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Susan F Evans,1 Yuen Kwok,1 Ann Solterbeck,2 Carmen Pyragius,3 Mary Louise Hull,4 Mark R Hutchinson,1,5 Paul Rolan1
1Adelaide Medical School, University of Adelaide, Adelaide, South Australia, Australia; 2Statistical Revelations, Melbourne, Victoria, Australia; 3School of Paediatrics & Reproductive Health, University of Adelaide, Adelaide, South Australia, Australia; 4Robinson Research Institute, School of Pediatrics and Reproductive Health, University of Adelaide, Adelaide, South Australia, Australia; 5ARC Centre of Excellence for Nanoscale Biophotonics, University of Adelaide, Adelaide, South Australia, Australia
Correspondence: Susan F Evans
Adelaide Medical School, University of Adelaide, PO Box 4025, Norwood South, Adelaide, 5067, South Australia, Australia
Tel +61 418 840 895
Fax +61 8 8363 2911
Email [email protected]
Purpose: Women bear a disproportionate burden of persistent pain conditions when compared to men. To determine whether the hormonal environment affects the clinical experience of pain, as measured by the days per month of pelvic pain (DPelvicPM), period pain (DPeriodPM), headache (DHeadachePM) or the in vitro EC50 for Interleukin-1β (IL-1β) release following TLR4 stimulation with Lipopolysaccharide from Peripheral Blood Mononuclear Cells (PBMCs). Findings were stratified according to use or non-use of the oral contraceptive pill.
Patients and Methods: Fifty-six women aged 16– 35 years, with minimal or severe dysmenorrhea, and use or non-use of the OC, were enrolled. Blood was collected on two occasions in a single menstrual cycle: Days 1– 2 and Days 7– 10. Hormonal analysis for testosterone, dihydrotestosterone, dehydroepiandrosterone, Androstenedione, 3α-Androstanediol, 3β-androstanediol, estradiol, estrone, 17α-hydroxyprogesterone, progesterone, cortisol and sex-hormone binding globulin was undertaken using ultra-sensitive Liquid Chromatography Mass–Spectrometry (LC-MS). PBMCs were exposed to lipopolysaccharide (LPS) and the resulting Interleukin-1β output was determined.
Results: Non-users of the OC showed a strongly inverse correlation between a reducing free androgen index (FAI) and increasing DPelvicPM (p=0.0032), DPeriodPM (p=0.013), DHeadachePM (p=0.041). Non-users of the OC showed a significant increase in DPelvicPM (p=0.049) on Days 7– 10. Modestly significant associations were found between reduced androgens and potentiated LPS-induced IL-1β (lower EC50).
Conclusion: This is the first study to investigate the relationship between the hormonal environment and activation of the immune system in young women with dysmenorrhoea-related pain conditions. Low androgen levels were consistently associated with increased pain. Translational implications for the findings are discussed.
Keywords: pain, testosterone, oral contraceptive pill, dysmenorrhoea, pelvic pain, IL-1β
Introduction
Women bear a disproportionate burden of pain compared with men across the majority of pain conditions,1 and ample evidence suggests a role for both the hormonal and immune environment in the development of female pain. Before puberty, the prevalence of chronic pain conditions is approximately equal in boys and girls.2 However, girls are over-represented by the age of 12–14 years, with the presence of persistent pain correlating more closely with the stage of pubertal development than with age.3 A major contributor to the disparity in chronic pain between girls and boys is the presence of dysmenorrhea and abdominal pain.4 Dysmenorrhea frequently predates,5 and is believed to be an etiological factor in, the development of chronic pelvic pain in women.6
Compelling epidemiological, clinical and experimental evidence in both human and animal studies demonstrates that increased peripheral and central nervous system immune system activity, via Toll-Like Receptors (TLRs), is involved in the initiation and maintenance of chronic pain conditions.7–9 Our group has already demonstrated that immune pathology, including an increase in TLR4 responsiveness, is present in women affected by dysmenorrhea-related pelvic pain.10
Toll-like receptors are pattern-recognition receptors on the surface of cells which recognise molecular patterns typically associated with microbial pathogens, and which respond with the release of cytokines including Interleukin-1β (IL-1β) that promote inflammation. Lipopolysaccharide (LPS) within the cell wall of gram-negative bacteria is a potent agonist of TLR4, an antigen on immune cells including mononuclear cells in the peripheral blood, and glial cells within the brain and spinal cord. Altered TLR inflammatory responses have already been demonstrated in visceral medical conditions characterized by persistent pain, such as inflammatory bowel disease and painful bladder syndrome.11,12
In our previously published work,10 peripheral blood from women with dysmenorrhea-related pelvic pain showed significantly enhanced IL-1β release from peripheral blood mononuclear cells (PBMCs) following in vitro TLR4 stimulation with lipopolysaccharide, when compared with pain-free controls. The present study further investigates this cohort of women to determine whether the hormonal environment affects the degree of TLR4-induced immune activation, or the experience of pelvic pain.
In this study, immune system TLR4-dependent reactivity is measured by determining the dose of LPS required to achieve 50% of maximal IL-1β release response (EC50) from peripheral blood mononuclear cells (PBMCs). Correlations between the levels of individual steroid hormones and cortisol with the days of pelvic pain per month (DPelvicPM)l, the days of period pain per month (DPeriodPM)id, and the days of headache per month (DHeadachePM), allow an objective assessment of the relationship between hormone levels and the subjective experience of pain. Correlations between the levels of individual steroid hormones with the EC50 allow an assessment of the relationship between hormone levels and the degree of immune system activation. The self-reported number of days of period (DPeriodPM) or pelvic pain per month (DPelvicPM), is used as an indicator of pelvic pain severity.
We investigate the hypothesis that androgens may be protective against the development of chronic pain conditions in women, and discuss the potential role of androgens as a treatment option for women with chronic dysmenorrhea-related pelvic pain.
Methods
Study Design
This is an observational clinical and laboratory study further extending analysis of the patient cohort reported by Evans et al10, and approved by the Human Research Ethics Committee of the Royal Adelaide Hospital, Adelaide, South Australia. HREC/14/RAH/63. RAH Approval No. 140,217. The study was conducted in accordance with the Declaration of Helsinki.13 All participants below the age of 18 were interviewed in the presence of a parent or guardian.
Participants
The target population consisted of girls and women aged between 16 and 35 years with either minimal, or severe, dysmenorrhea. Participants with minimal dysmenorrhea had self-reported pain of between 0 and 3 on an 11-point numerical scale14 on the worst day of their menstrual period. At the screening visit, participants with severe dysmenorrhea had self-reported pain of between 7 and 10 on the 11-point numerical scale on the worst day of their menstrual period. Participants were further separated according to use or non-use of the oral contraceptive pill (OC). Potential participants were identified either when they responded to recruitment notices displayed to the general public, which provided a link to an anonymous Survey Monkey questionnaire, or through a private clinic, Pelvic Pain SA. Eligible participants from the general public were offered the opportunity of an initial phone interview to provide further information about the study, to obtain consent for further participation in the study, and to arrange a screening assessment visit with the Principal Investigator (PI). Figure 1 shows the Enrolment Flow Chart for study inclusion. Study exclusion criteria (Box 1) ensured that women possessing factors that might influence inflammation, TLR receptor response, the immune system, or the severity of dysmenorrhea were excluded from the study.
 |
Box 1 Study Exclusion Criteria |
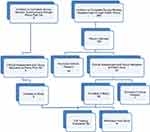 |
Figure 1 Enrolment flow chart for participant recruitment and study inclusion. |
Three hundred and sixty women completed the online questionnaire, with 105 women choosing to provide their contact details for consideration for study inclusion. Phone interview assessment by the Principal Investigator further excluded 44 women based on the presence of exclusion criteria. Sixty-one women recruited from the general public and 5 additional women recruited through Pelvic Pain SA, Adelaide, Australia proceeded to the Screening Visit, where they underwent a full clinical assessment, and the appropriate study group allocation was accurately determined. Participants below the age of 18 were interviewed in the presence of their parent or guardian, who co-signed the consent form with the participant. One participant was excluded due to previous pregnancy. Thus, a total of 65 women were enrolled in the study. Nine women were excluded during the study due to a high pre-test level of C-reactive protein (1), use of marijuana (1), irregular menstrual cycles (3), non-attendance for testing (2), insufficiently severe pain (1), and the development of an unrelated neurological illness (1). Fifty-six women satisfied all inclusion and exclusion criteria and completed the testing procedures.
Laboratory Methods
Study Visit Schedule and Specimen Collection
Participants were assessed on two occasions during a single menstrual cycle: Days 1–2 and Days 7–10. At each study visit, participants attended the Pain and Anaesthesia Research Centre (PARC) of the Royal Adelaide Hospital, Adelaide, Australia. At their visit, participants were asked to confirm the absence of exclusion criteria, report the number of days of period pain and pelvic pain per month, and provide a blood sample for analysis. The use of anti-inflammatory medications was avoided for 5 drug half-lives prior to blood sampling.
Analysis of blood samples included: a measurement of high-sensitivity C-reactive protein (CRP) to exclude the presence of un-recognised pre-test inflammation; a quantitative assessment of luteinising hormone (LH) and follicle-stimulating hormone (FSH) to confirm baseline hormonal status on Days 1–2; a quantitative assessment of Sex Hormone Binding Globulin (SHBG) (Healthscope Laboratories, Adelaide, Australia) to allow later calculation of the Free Androgen Index (FAI) and Free Estrogen Index (FEI); and an extended hormonal profile analysed as a single batch, using ultra-sensitive Liquid Chromatography–Mass Spectrometry (LC-MS) (Anzac Research Institute, Sydney, Australia). The hormonal analysis included measurement of testosterone (T), dihydrotestosterone (DHT), dihydroepiandrosterone (DHEA), androstenedione (adione), 3α-androstanediol (3αdiol), 3β-androstanediol (3βdiol), estradiol (E2), estrone (E1), 17α-hydroxyprogesterone (17OHP4), progesterone (P4) and cortisol (C). Measurements for T, DHT, DHEA, adione, 3αdiol, 3βdiol, 17OHP4, P4 and C are measured in ng/mL. Measurements for E2 and E1 are in pg/mL. Our use of high-sensitivity liquid chromatography–mass spectrometry (LCMS) steroid hormonal assays overcomes difficulties in androgen research in women associated with the low levels of androgens present in women compared to men. Hormonal analysis on both Days 1–2 (menstrual phase) and Days 7–10 (mid follicular phase) of a single menstrual cycle allows estimates of within-subject variability to be distinguished from between-subject and assay variability.
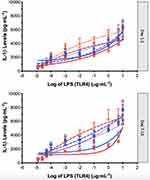
The IL-1β release from PMBCs across a range of concentrations of lipopolysaccharide stimulation of TLR4 receptors was used as a measure of TLR4 immune activation (Figure 2) and was determined using the technique developed by Kwok15,16 and published in detail by Evans et al.10
All blood samples were taken between the hours of 0830 and 1200.
Statistical Analysis
Determination of EC50
Immune system TLR4-dependent reactivity is measured by determining the dose of LPS required to achieve a 50% of maximal IL-1β release response (EC50) from peripheral blood mononuclear cells (PBMCs). Statistical modelling and a non-linear mixed-effects model approach was used to estimate the EC50 for each subject, at each testing timepoint.10 Concentration–response curves to LPS stimulation were fitted to an Emax model using the Hill equation,17 with the slope parameter fixed to 1 to reduce the number of parameters to be estimated. The model used was of the form:
where E0 is the response Y at baseline (absence of dose), Emax is the asymptotic maximum dose effect (maximum effect attributable to the drug) and EC50 is the concentration which produces 50% of the maximal effect. Individual Emax models were fitted for each subject at each timepoint (Days 1–2 or Days 7–10).
All model fitting and all analyses were performed using Statistical Analysis Software (SAS v 9.4). Starting values were set with Max representing the maximum response observed for that participant at that timepoint, and Min representing the minimum response for that participant at that timepoint. From the model, the differences in LS means for contrasts of interest were obtained with 95% confidence limits and relevant P-values by factor. The statistical analysis used is further described in our previous paper by Evans et al.10
 |
Figure 3 Comparison between DPelvicPM and levels of Free Androgen Index according to day of testing (Days 1–2 or Days 7–10) and OC use (No OC, OC, or Combined). |
A lower EC50 represents increased responsiveness of PBMCs to stimulation with LPS, a proxy measurement for increased immune system activation. A higher EC50 represents reduced responsiveness of PBMCs and reduced negative regulatory pressure and/or controls.
Determination of Free Androgen Index and Free Estrogen Index
The FAI was determined according to the formula: FAI = 100*testosterone/SHBG.
The FEI was determined according to the formula: FEI = 100*estradiol/SHBG.
Statistical Comparisons
Two-tailed non-parametric Spearman correlations were undertaken to determine the p-value and Spearman’s Rank Correlation Coefficient (r). Correlations were made between the levels of individual hormones, cortisol, the FAI, and the FEI, with the days per month of pelvic pain (DPelvicPM), period pain (DPeriodPM), headache (DHeadachePM), and the EC50 for IL-1β release from PBMCs (Tables 1–4). Results were reported according to the use or non-use of the OC, and testing on Days 1–2 or Days 7–10 of the menstrual cycle.
Results
Participant Recruitment
Three hundred and sixty women completed the online Survey Monkey questionnaire. Following application of the exclusion criteria, 65 women were enrolled in the study. Nine women were excluded during the study due to a high pre-test level of C-reactive protein (1), use of marijuana (1), irregular menstrual cycles (3), non-attendance for testing (2), insufficiently severe pain (1), and development of an unrelated neurological illness (1). Fifty-six women aged between 16 and 35 years, with regular menstrual cycles, no previous pregnancy, normal body mass index (BMI) and good general health, apart from the presence of dysmenorrhea-related pelvic pain, completed the study. There were no differences in age (p=0.52), height (p=0.52) or weight (p=0.68) of the women in each group (one-way ANOVA and Kruskal–Wallis analysis).
Multiple correlations were made between hormone levels, pain indices (DPelvicPM, DPeriodPM, DHeadachePM) and the EC50 for LPS-induced IL-1β release (EC50)
Comparison Between Hormonal Levels and Days of Pelvic Pain per Month (DPelvicPM)
Days 1–2: Women who were non-users of the OC showed a significant inverse correlation between the DPelvicPM and levels of androstenedione (p=0.036), DHEA (p=0.0018) and FAI (p=0.0032) (Table 1, Figure 3). The combined cohort of all participants showed a significant inverse correlation between the DPelvicPM and levels of testosterone (p=0.021), DHEA (p=0.019) and FAI (p=0.0026).
 |
Figure 4 Comparison between DPelvicPM and the Estradiol according to day of testing (Days 1–2 or Days 7–10) and OC use (No OC, OC, or Combined). |
Days 7–10: Women who were non-users of the OC showed a significant inverse correlation between DPelvicPM and the FAI (p=0.0058). Women who were users of the OC showed a significant positive correlation between DPelvicPM and levels of estradiol (p=0.049) or the FEI (p=0.049) (Table 1, Figure 4). There was no effect of cortisol on DPelvicPM.
Comparison Between Hormonal Levels and Days of Period Pain per Month (DPeriodPM)
Days 1–2: Women who were non-users of the OC showed a significant inverse correlation between DPeriodPM and levels of androstenedione (p=0.035), DHEA (p=0.013) and FAI (p=0.013) (Table 2, Figure 5). The combined cohort of all participants showed a significant inverse correlation between DPeriodPM and levels of DHEA (p=0.060) and FAI (p=0.013).
 |
Figure 5 Comparison between DPeriodPM and the Free Androgen Index according to day of testing (Days 1–2 or Days 7–10) and OC use (No OC, OC, or Combined). |
Days 7–10: Women who were non-users of the OC showed a significant inverse correlation between DPeriodPM and FAI (p=0.029).
There was no effect of estradiol, FEI or Cortisol on DPeriodPM.
Comparison Between Hormonal Levels and Days of Headache per Month (DHeadachePM)
Days 1–2: Women who were users of the OC showed a significant inverse correlation between DHeadachePM and levels of testosterone (p=0.049) (Table 3). The combined cohort of all participants showed a significant inverse correlation between DHeadachePM and levels of testosterone (p=0.010), DHEA (p=0.035), and FAI (p=0.041).
Days 7–10: The combined cohort showed a significant inverse correlation between DHeadachePM and DHEA (p=0.047).
There was no effect of estradiol, FEI or Cortisol on DPeriodPM.
Comparison Between Hormonal Levels, Cortisol and EC50 for LPS-Induced IL-1β Release (EC50)
Days 1–2: The combined cohort of all participants showed a significant positive correlation between the EC50 of LPS-induced in vitro IL-1β release (increased responsiveness of PBMCs) and androstenedione levels (Table 4).
Days 7–10: Users of the OC showed a significant positive correlation between the EC50 of LPS-induced in vitro IL-1β release and levels of testosterone (p=0.042). Non-users of the OC showed a highly significant positive correlation between levels of cortisol and the EC50 of LPS-induced in vitro IL-1β release increased (p=0.0011, Spearman’s coefficient r=+0.63). Users of the OC showed a highly significant inverse correlation between cortisol and the EC50 of LPS-induced in vitro IL-1β release (p=0.0080, Spearman coefficient r=−0.46) (Table 4, Figure 6).
 |
Figure 6 Comparison between EC50 and levels of cortisol according to day of testing (Days 1–2 or Days 7–10) and OC use (No OC, OC, or Combined). |
There was no significant effect of DHEA, estradiol, FAI or FEI on EC50.
Comparison of Hormone and Cortisol Levels Between Users or Non-Users of the OC
A one-way ANOVA with Tukey’s post hoc test was used to compare hormone levels in women who were users or non-users of the OC on both Days 1–2 and Days 7–10 (Table 5). In women who use the OC, the pill taken on Days 1–2 contains lactose only (lactose pill days). The pill taken on Days 7–10 contains ethinylestradiol in combination with a synthetic progestogen (hormone pill days).
 |
Table 5 Comparison of Means for SHBG, Androgen, FAI, Estrogen, FEI and Cortisol Levels in Users and Non-Users of the OC According to Day of Testing (Days 1–2 or Days 7–10 of Menstrual Cycle) |
Days 1–2: (lactose pill days) Use of the OC was associated with a significantly higher level of SHBG (p=0.0001) and cortisol (p=0.0001). Use of the OC was associated with a significantly lower level of DHEA (p=0.0043) and FAI (p=0.0013). There was no significant difference in testosterone, estradiol, or FEI.
Days 7–10: (hormone pill days) Use of the OC was associated with a significantly higher level of SHBG (p=0.0001) and cortisol (p=0.0001). Use of the OC was associated with a significantly lower level of androstenedione (p=0.0019), DHEA (0.0281) and FAI (p=0.0001).
There was no significant difference in testosterone, estradiol or FEI.
The following hormones were present at levels too low for statistical analysis: estrone, progesterone, 17-hydroxyprogesterone, 3bdiol and estrone. Levels of DHT and 3alpha-androstanediol were not significantly associated with alteration in either symptoms or the EC50.
When considering these results overall, lower levels of androgenic hormones, and particularly the calculated Free Androgen Index, were inversely associated with increasing DPelvicPM, DPeriodPM, and DHeadachePM. Higher estradiol levels on Days 7–10 (pill days), particularly in OC users, were associated with an increase in the DPelvicPM, but not the DPeriodPM, DHeadachePM or EC50. Weak associations were found between lower levels of androgen activity, and a lower EC50 consistent with a mildly enhanced immune responsiveness. Strong associations were found between cortisol levels and EC50 on Days 7–10 (pill days), with divergent direction of action according to use or non-use of the OC.
Discussion
The Association Between Hormone Levels and Pain Symptoms
Multiple lines of evidence18,19 describe the excess of chronic pain conditions in females when compared to males. Pain research to date has predominantly considered ways in which the relative estrogen dominance in females may predispose females to chronic pain, as opposed to the ways in which the relative androgen dominance in males may protect males from these conditions. The physiological and potentially therapeutic roles of androgens in females has been under-researched. Our research found a highly significant inverse relationship between androgen levels, particularly a low FAI, and increasing pain symptoms, particularly in non-users of the OC. Oral contraceptive use results in both the suppression of testosterone production by the ovaries, and the induction of SHBG by the liver, with a consequently lower FAI (Figure 3).
Multiple mechanisms of action in both the periphery and the central nervous system support an inverse relationship between testosterone levels and pain. Studies of male-to-female transgender patients show an increase in chronic pain conditions following hormonal transition from an androgenic to an estrogenic hormonal environment,20,21 while female-to-male transgender patients show an improvement in pre-existing pain conditions following the administration of testosterone. Our research found a highly significant association between androgen levels and pain symptoms, particularly in women who were non-users of the OC, where the ovarian production of testosterone is unsuppressed and the production of SHBG within the liver is not enhanced.
In patients with Rheumatoid Arthritis, an inflammatory condition associated with reduced androgen levels in both males and females, there is increased production of proinflammatory cytokines within synovial cells.22 Within these cells, increased levels of IL-1β, tumour necrosis factor (TNF) and Interleukin-6 (IL-6) enhance the activity of aromatase,23 resulting in the increased conversion of testosterone to estradiol, increased levels of estrogen, and reduced levels of androgens.22,24–26 The disease-modifying effects of anti-TNF medications in patients with RA may act through the local enhancement of androgen activity.27
Previous human studies have demonstrated the central nature of dysmenorrhea-related pelvic pain, its relationship to viscero-visceral hyperalgesia, and its association with pain co-morbidities including migraine.28–31 Within the dorsal horn of the spinal cord, it has been proposed that androgens act in concert with estrogen to increase endogenous opioids following a nociceptive stimulus.32 White and Robinson proposed that a low testosterone level fails to induce sufficient endogenous opioids to dampen pain signals.33 Fibromyalgia, a centrally mediated pain condition, is associated with reduced levels of androgens, and a strong association has been found between the days where symptoms were severe, and the days where testosterone levels were low.34 At the neuronal level, dihydrotestosterone, a metabolite of testosterone, provides neuroprotection against microglial inflammatory responses via suppression of TLR4, and inhibition of TNF-alpha and IL-1β production.35 Within the brain, Vincent et al36 found reduced activation of brain centres associated with descending pain inhibition, including the ventral rostral medulla, in healthy women with low androgen levels undergoing a cold thermal pain stimulus, despite no variation in the temperature required to induce pain.
In contrast with the robust association with androgenic activity, our research found a modest correlation between estradiol levels on Days 7–10 and pain symptoms. Our findings are consistent with established clinical practice, where lower estrogen OCs or estrogen suppression is prescribed to reduce the frequency or volume of menstrual bleeding.37 The oral contraceptive pill has known effectiveness at the level of the uterus: reducing menstrual flow,38 and reducing prostaglandin release.39,40 It is yet to be determined whether the OC-induced androgen suppression and SHBG induction may have unintended negative effects on the development of central pain sensitization.
The Association Between Hormone Levels and EC50 of LPS-Induced IL-1β Release from PBMCs
Activation of the innate immune system via TLR4 is known to be involved in the initiation and maintenance of chronic pain,8,9,41,42 with activation showing as increased responsiveness of immune cells to TLR stimulation. Our group has already demonstrated an increased responsiveness of PBMCs to TLR4 activation with LPS within this group of women with dysmenorrhea-related pelvic pain, compared to pain-free controls.10 Our finding of a modest association between reduced androgen activity and a lower LPS-induced IL-1β EC50 (increased immune activation) suggests that activation of the immune system via TLR4 comprises one factor affecting pain symptoms, but that immune mechanisms outside the hormonal environment are also involved. It also suggests that the ability of hormonal manipulation to manage chronic pelvic pain is limited. Our study found no significant effect of estrogens on EC50. This is consistent with the research of Bouman et al43 who found that neither estradiol nor progesterone influenced the release of cytokines from monocytes in humans. However, they did not assess the effect of androgens.
The Association Between Cortisol and EC50 of LPS-Induced IL-1β Release from PBMCs
Our finding of a highly significant association between increased levels of cortisol (a suppressor of inflammation) and an increased EC50 of LPS-induced IL-1β release (reduced activation of the innate immune system) in non-users of the OC is consistent with an inflammation-based model of chronic pain. The divergent effect in OC users, where higher levels of cortisol were associated with a lower EC50 of LPS-induced IL-1β release (increased activation of the innate immune system) is a potential cause for concern to health care providers, owing to the known association between immune activation and chronic pain. Our findings are consistent with those of Vincent et al44 who described a significantly lower mean level of cortisol in women with dysmenorrhea where all participants were non-users of the OC, suggesting an impairment of the immune response. Use of the OC has already been associated with a reduced cortisol response to cold-pressor testing and emotional memory responses.45–47
Measurement of the FAI Within Clinical Practice
The majority of testosterone within the circulation is protein-bound to either SHBG or albumin, leaving a relatively small percentage of testosterone in its unbound, bioavailable state. While there are multiple cellular mechanisms involved, it is the unbound testosterone fraction which contributes maximally to its clinical effects. The Free Androgen Index (FAI) provides a simple and easily calculated estimate of androgen activity, requiring only a measurement of total testosterone and SHBG. FAI results are less reliable as an indicator of testosterone activity in populations where SHBG levels are below 30nmol/l, such as in males or women with Polycystic Ovarian Syndrome, obesity, or insulin resistance.48 For these reasons, the Calculated Free Testosterone (cFT) is preferred in some laboratories. The cFT incorporates albumin levels, and utilises the more complex Vermeulen Equation,49 to provide an estimate which more closely matches the results obtained by dynamic equilibrium testing in these groups. Our research excluded women with a BMI > 30, and women with irregular menstrual cycles. The FAI is therefore an appropriate test within our study population.
Translational Potential
Our key research finding of an inverse association between androgen levels and pain symptoms, both within and outside the pelvis, offers a new approach to the management of pain in women. Multiple androgen-enhancing options are already available. Progestogen-only contraceptive users avoid the estrogen-induced rise in SHBG associated with combined OCs, and Máximo et al50 demonstrated that women using progestogen-only contraception have a higher pain threshold than women using the combined OC. Intrauterine contraceptive devices maintain natural ovarian testosterone production by avoiding ovarian suppression, and their estrogen-induced rise in SHBG. When considering the further development of OC formulations, an optimal OC for a woman with pain might comprise an estrogen with minimal induction of SHBG, and a progestogen with minimal anti-androgen activity. Conservation of the ovaries at the time of hysterectomy maintains gonadal production of testosterone in women where post-surgical testosterone replacement is not anticipated.51
Testosterone therapy is recommended to reduce pain perception and fatigue in women with opioid-induced androgen deficiency (OPIAD),52 and White et al reduced tender points and stiffness in women with fibromyalgia by using testosterone gel.33 However, the role of androgen replacement in the absence of opioid use in women with low androgen levels and chronic pain is less established. Where levels of estrogen or progestogen are low, these are replaced to conserve bone density and prevent endometrial hyperplasia. The supplementation of testosterone to prevent fatigue, improve sexual function, or reduce chronic pain, anxiety and low mood, has been under-utilised. In women using hormonal replacement, the estrogen-induced rise in SHBG may further reduce androgen activity through increased testosterone binding.
Optimal testosterone levels for the reduction of pain have yet to be determined. Huang et al53 researched the effect of increasing doses of testosterone over 24 weeks in hysterectomized and oophorectomized women compared to placebo. They observed a significant dose–response relationship between free testosterone levels and the Psychological General Well-Being Index (PGWBI) score, lean body mass, and stair climbing power when compared to placebo, but only where supra-physiological levels were achieved. Fasting glucose, lipid profile and liver function tests remained unchanged from baseline following testosterone administration, and did not differ according to dose. Their study considers the use of testosterone as a pharmacological treatment, rather than a physiological replacement. The future development of selective androgen receptor modulators (SARMs) offers the potential of androgen effect without the potential side effect of virilization.
Our study raises substantial further research questions relating to the use of testosterone therapy to reduce pain or enhance quality-of-life in women with current endometriosis lesions. The aromatase-mediated conversion of testosterone to estradiol within endometriosis lesions has the potential to promote lesion growth. The optimal testosterone regime for these women, and the potential for testosterone to be used in combination with an aromatase inhibitor medication to prevent the conversion of testosterone to estrogen,54 has yet to be fully explored.
Our study has notable strengths, including the stringent exclusion criteria used, the lack of clinical co-morbidities or confounding medications in this young, healthy patient group, and the accurate measurement of steroid hormones at low levels, using liquid chromatography–mass spectrometry methods in a single batch. Another strength is the sampling at 2 stages of a single menstrual cycle in each participant. A potential study weakness is the use of blood hormonal assays rather than tissue hormonal assays. As shown in the synovial fluid of patients with rheumatoid arthritis,55 or the intralesional fluid in women with endometriosis lesions, the enzyme aromatase allows a local hormonal concentration of estradiol that may be many times greater than blood levels.56 Another weakness is the heterogeneity of participants within the oral contraceptive group. This group includes participants using a range of different oral contraceptive formulations for a range of clinical indications.
Conclusion
Our study provides new insights into the way the hormonal environment affects the experience of pain in women, using clinical variables that are readily determined in a primary care setting: the DPelvicPM, the DPeriodPM, and the DHeadachePM. Of the tests undertaken in our cohort of young women with normal BMI and regular menstrual cycles, the FAI proved to be a consistent indicator of difference.
Our findings support the concept that the female predominance of chronic pain relates more to the protection men receive from higher testosterone levels, than the risk women incur from higher estradiol levels. The potential for use of the OC to reduce menstrual symptoms, yet increase immune activation, with increased potential for the development of chronic pain, requires further research. If confirmed, this has widespread implications for the management of dysmenorrhea-related pelvic pain in young women.
Acknowledgments
Advice on editorial matters, Professor R Sussex. Statistical analysis for the determination of EC50, Dr Annie Solterbeck of Statistical Revelations. Statistical analysis for hormone comparisons, Dr Carmen Pyragius and Dr Matilda Darling.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The study was part funded by the Australia New Zealand College of Anaesthetists (ANZCA) Research Foundation and the Australian Research Council (FT180100565).
Disclosure
Susan F. Evans receives royalties from book authorship (Endometriosis and Pelvic Pain), has received payment from Pfizer and Bayer for educational presentations, is a shareholder in Alyra Biotech Pty Ltd (a company developing non-hormonal immune therapies for the management of pelvic pain) and Havah Therapeutics Pty Ltd (a company developing testosterone therapies for women with breast cancer), is involved in the development of novel treatments for pelvic pain; and has patents pending: PCT/AU2018/051383 and PCT/AU2020/050551, Alyra Biotech Pty Ltd. Paul E. Rolan is a shareholder in Havah Therapeutics, Alyra Biotech, Lipotek and iX Biopharma, a consultant to Bionomics and Novartis, and has received payment for educational presentations from Novartis and Seqirus. Ann Solterbeck is an employee of Statistical Revelations and reports personal fees from the University of Adelaide, during the conduct of the study. Mark R. Hutchinson is director of the Australian Research Council Centre of Excellence for Nanoscale BioPhotonics (CE140100003) and the recipient of an ARC Future Fellowship (FT180100565) and reports grants from Australian Research Council. His research program is supported by Novartis, Abbott, Pfizer and Regeneus, but these activities fall outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132:S26–S45. doi:10.1016/j.pain.2007.10.014
2. LeResche L. Gender differences in pain. Pain Forum. 1995;4(4):228–230. doi:10.1016/S1082-3174(11)80025-5
3. LeResche L, Mancl LA, Drangsholt MT, Saunders K, Korff MV. Relationship of pain and symptoms to pubertal development in adolescents. Pain. 2005;118(1):201–209. doi:10.1016/j.pain.2005.08.011
4. Perquin CW, Hazebroek-Kampschreur AAJM, Hunfeld JAM, et al. Pain in children and adolescents: a common experience. Pain. 2000;87(1):51–58. doi:10.1016/S0304-3959(00)00269-4
5. Hardi G, Evans S, Craigie M. A possible link between dysmenorrhoea and the development of chronic pelvic pain. Aust N Z J Obstet Gynaecol. 2014;54(6):593–596. doi:10.1111/ajo.12274
6. Westling AM, Tu FF, Griffith JW, Hellman KM. The association of dysmenorrhea with noncyclic pelvic pain accounting for psychological factors. Am J Obstet Gynecol. 2013;209(5):
7. Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23–36. doi:10.1038/nrn2533
8. Dodds KN, Beckett EAH, Evans SF, Grace PM, Watkins LR, Hutchinson MR. Glial contributions to visceral pain: implications for disease etiology and the female predominance of persistent pain. Transl Psychiatry. 2016;6(9).
9. Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2012;234(2).
10. Evans SF, Kwok YH, Solterbeck A, et al. Toll-Like Receptor responsiveness of peripheral blood mononuclear cells in young women with dysmenorrhea. J Pain Res. 2020;13:503–516. doi:10.2147/JPR.S219684
11. Belmonte L, Youmba SB, Bertiaux-Vandaële N, et al. Role of toll like receptors in irritable bowel syndrome: differential mucosal immune activation according to the disease subtype. PLoS One. 2012;7(8):e42777. doi:10.1371/journal.pone.0042777
12. Schrepf A, Bradley CS, O’Donnell M, et al. Toll-like receptor 4 and comorbid pain in interstitial cystitis/bladder pain syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav Immun. 2015;49:66–74. doi:10.1016/j.bbi.2015.03.003
13. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA. 2013;310(20).
14. Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073–1093. doi:10.1016/j.jpainsymman.2010.08.016
15. Kwok YH, Tuke J, Nicotra LL, Grace PM, Rolan PE, Hutchinson MR. TLR 2 and 4 responsiveness from isolated peripheral blood mononuclear cells from rats and humans as potential chronic pain biomarkers. PLoS One. 2014;8(10).
16. Kwok YH, Hutchinson MR, Gentgall MG, Rolan PE. Increased responsiveness of peripheral blood mononuclear cells to in vitro TLR 2, 4 and 7 ligand stimulation in chronic pain patients. PLoS One. 2012;7(8):44232. doi:10.1371/journal.pone.0044232
17. Goutelle S, Maurin M, Rougier F, et al. The Hill equation: a review of its capabilities in pharmacological modelling. Fundam Clin Pharmacol. 2008;22(6):633–648. doi:10.1111/j.1472-8206.2008.00633.x
18. Rosen S, Ham B, Mogil JS. Sex differences in neuroimmunity and pain. J Neurosci Res. 2017;95(1–2):500–508. doi:10.1002/jnr.23831
19. Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–866. doi:10.1038/nrn3360
20. Aloisi AM. Why we still need to speak about sex differences and sex hormones in pain. Pain Ther. 2017;6(2):111–114. doi:10.1007/s40122-017-0084-3
21. Aloisi AM, Bachiocco V, Costantino A, et al. Cross-sex hormone administration changes pain in transsexual women and men. Pain. 2007;132(Suppl 1):S60–S67. doi:10.1016/j.pain.2007.02.006
22. Cutolo M, Sulli A, Capellino S, et al. Anti-TNF and sex hormones. Ann N Y Acad Sci. 2006;1069:391–400. doi:10.1196/annals.1351.037
23. Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2(3):119–125. doi:10.1016/S1568-9972(03)00006-5
24. Castagnetta LA, Carruba G, Granata OM, et al. Increased estrogen formation and estrogen to androgen ratio in the synovial fluid of patients with rheumatoid arthritis. J Rheumatol. 2003;30(12):2597–2605.
25. Cutolo M, Villaggio B, Seriolo B, et al. Synovial fluid estrogens in rheumatoid arthritis. Autoimmun Rev. 2004;3(3):193–198. doi:10.1016/j.autrev.2003.08.003
26. Capellino S, Straub RH, Cutolo M. Aromatase and regulation of the estrogen-to-androgen ratio in synovial tissue inflammation: common pathway in both sexes. Ann N Y Acad Sci. 2014;1317:24–31. doi:10.1111/nyas.12398
27. Weidler C, Struharova S, Schmidt M, Ugele B, Schölmerich J, Straub RH. Tumor necrosis factor inhibits conversion of dehydroepiandrosterone sulfate (DHEAS) to DHEA in rheumatoid arthritis synovial cells: a prerequisite for local androgen deficiency. Arthritis Rheum. 2005;52(6):1721–1729. doi:10.1002/art.21112
28. Brinkert W, Dimcevski G, Arendt-Nielsen L, Drewes AM, Wilder-Smith OHG. Dysmenorrhoea is associated with hypersensitivity in the sigmoid colon and rectum. Pain. 2007;132:46–51. doi:10.1016/j.pain.2006.12.011
29. Evans SF, Brooks TA, Esterman AJ, Ml H, Rolan PE. The comorbidities of dysmenorrhea: a clinical survey comparing symptom profile in women with and without endometriosis. J Pain Res. 2018;11:3181–3194. doi:10.2147/JPR.S179409
30. Harel Z. Dysmenorrhea in adolescents. Ann N Y Acad Sci. 2008;1135(1):185–195. doi:10.1196/annals.1429.007
31. Smorgick N, Marsh CA, As-Sanie S, Smith YR, Quint EH. Prevalence of pain syndromes, mood conditions, and asthma in adolescents and young women with endometriosis. J Pediatr Adolesc Gynecol. 2013;26(3):171–175. doi:10.1016/j.jpag.2012.12.006
32. White HD, Robinson TD. A novel use for testosterone to treat central sensitization of chronic pain in fibromyalgia patients. Int Immunopharmacol. 2015;27(2):244–248. doi:10.1016/j.intimp.2015.05.020
33. White HD, Brown LAJ, Gyurik RJ, et al. Treatment of pain in fibromyalgia patients with testosterone gel: pharmacokinetics and clinical response. Int Immunopharmacol. 2015;27(2):249–256. doi:10.1016/j.intimp.2015.05.016
34. Schertzinger M, Wesson-Sides K, Parkitny L, Younger J. Daily fluctuations of progesterone and testosterone are associated with fibromyalgia pain severity. J Pain off J Am Pain Soc. 2018;19(4):410–417. doi:10.1016/j.jpain.2017.11.013
35. Yang L, Tong Y, Chen P-F, Miao S, Zhou R. Neuroprotection of dihydrotestosterone via suppression of the toll-like receptor 4/nuclear factor-kappa B signaling pathway in high glucose-induced BV-2 microglia inflammatory responses. NeuroReport. 2020;31(2):139–147. doi:10.1097/WNR.0000000000001385
36. Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Brain imaging reveals that engagement of descending inhibitory pain pathways in healthy women in a low endogenous estradiol state varies with testosterone. Pain. 2013;154(4):515–524. doi:10.1016/j.pain.2012.11.016
37. Lindh I, Ellström AA, Milsom I. The effect of combined oral contraceptives and age on dysmenorrhoea: an epidemiological study. Hum Reprod. 2012;27(3):676–682. doi:10.1093/humrep/der417
38. Larsson G, Milsom L, Lindstedt G, Rybo G. The influence of a low-dose combined oral contraceptive on menstrual blood loss and iron status. Contraception. 1992;46(4):327–334. doi:10.1016/0010-7824(92)90095-B
39. Hauksson A, Ekström P, Juchnicka E, Laudański T, Åkerlund M, Mats Åkerlund D. The influence of a combined oral contraceptive on uterine activity and reactivity to agonists in primary dysmenorrhea. Acta Obstet Gynecol Scand. 1989;68(1):31–34. doi:10.3109/00016348909087685
40. Chan WY, Yusoff Dawood M, Fuchs F. Prostaglandins in primary dysmenorrhea. Am J Med. 1981;70(3):535–541. doi:10.1016/0002-9343(81)90576-3
41. Hutchinson MR, Shavit Y, Grace PM. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63(3):772–810. doi:10.1124/pr.110.004135
42. Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–231. doi:10.1038/nri3621
43. Bouman A, Schipper M, Heineman MJ, Faas M. 17β-estradiol and progesterone do not influence the production of cytokines from lipopolysaccharide-stimulated monocytes in humans. Fertil Steril. 2004;82:1212–1219. doi:10.1016/j.fertnstert.2004.05.072
44. Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain. 2011;152(9):1966–1975. doi:10.1016/j.pain.2011.03.029
45. Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154–162. doi:10.1097/00006842-199903000-00006
46. Herrera AY, Faude S, Nielsen SE, Locke M, Mather M. Effects of hormonal contraceptive phase and progestin generation on stress-induced cortisol and progesterone release. Neurobiol Stress. 2019;10:100151. doi:10.1016/j.ynstr.2019.100151
47. Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biol Psychol. 2013;92(2):257–266. doi:10.1016/j.biopsycho.2012.10.007
48. Keevil BG, Adaway J, Fiers T, Moghetti P, Kaufman J-M. The free androgen index is inaccurate in women when the SHBG concentration is low. Clin Endocrinol (Oxf). 2018;88(5):706–710. doi:10.1111/cen.13561
49. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi:10.1210/jcem.84.10.6079
50. Máximo MM, Silva PS, Vieira CS, et al. Low-dose progestin-releasing contraceptives are associated with a higher pain threshold in healthy women. Fertil Steril. 2015;104(5):1182–1189. doi:10.1016/j.fertnstert.2015.07.1165
51. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy versus ovarian conservation in the nurses. Health Study Obstet Gynecol. 2013;121(4):709–716. doi:10.1097/AOG.0b013e3182864350
52. Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD). Pain Physician. 2012;15(3 Suppl):ES145–ES156.
53. Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with and without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause NYN. 2014;21(6):612–623. doi:10.1097/GME.0000000000000093
54. Bulun SE, Zeitoun KM, Takayama K, Simpson E, Sasano H. Aromatase as a therapeutic target in endometriosis. Trends Endocrinol Metab. 2000;11(1):22–27. doi:10.1016/S1043-2760(99)00216-7
55. Castagnetta LA, Carruba G, Granata OM, et al. Increased estrogen formation and estrogen to androgen ratio in the synovial fluid of patients with rheumatoid arthritis. J Rheumatol. 2020;10.
56. Huhtinen K, Desai R, Ståhle M, et al. Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J Clin Endocrinol Metab. 2012;97(11):4228–4235. doi:10.1210/jc.2012-1154
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.