Back to Journals » OncoTargets and Therapy » Volume 13
The Regulatory Mechanism and Biological Significance of Mitochondrial Calcium Uniporter in the Migration, Invasion, Angiogenesis and Growth of Gastric Cancer
Authors Wang X , Song X, Cheng G, Zhang J, Dong L, Bai J, Luo D, Xiong Y, Li S, Liu F, Sun Y, Wang X, Li Y, Huang Y
Received 14 May 2020
Accepted for publication 27 September 2020
Published 17 November 2020 Volume 2020:13 Pages 11781—11794
DOI https://doi.org/10.2147/OTT.S262049
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Xiaofei Wang,1,* Xudong Song,1,* Guang Cheng,2,* Jingwen Zhang,3 Liru Dong,1 Jie Bai,1 Dan Luo,1 Yanjie Xiong,1 Shuang Li,1 Fang Liu,1 Yuanyuan Sun,1 Xin Wang,1 Yuyang Li,1 Yunning Huang4
1Department of Pathology, North China University of Science and Technology Affiliated Hospital, Tangshan 063000, Hebei, People’s Republic of China; 2Central Laboratory of Clinical Medical College, North China University of Science and Technology Affiliated Hospital, Tangshan 063000, Hebei, People’s Republic of China; 3School of Basic Medical Science, NHC Key Laboratory of Metabolic Cardiovascular Diseases Research, NingXia Medical University, Yinchuan 750004, NingXia, People’s Republic of China; 4Department of Gastrointestinal Surgery, People’s Hospital of Ningxia Hui Autonomous Region, Yinchuan 750001, Ningxia, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yunning Huang
Department of Gastrointestinal Surgery, People’s Hospital of Ningxia Hui Autonomous Region, Yinchuan 750001, Ningxia, People’s Republic of China
Email [email protected]
Objective: Increasing evidences suggest that mitochondrial calcium uniporter (MCU), a selective channel responsible for mitochondrial Ca2+ uptake, is involved in the progression of several cancers. In this study, we aimed to observe the clinical implications and biological functions of MCU in gastric cancer.
Methods: The expression of MCU in 90 pairs of gastric cancer tissues and adjacent normal tissues was examined using immunohistochemistry and correlation between MCU expression and clinical features was analyzed. After construction of stable MCU knockdown or overexpression gastric cancer cells, mitochondrial membrane potential (MMP), wound healing and transwell assays were performed to examine MMP levels, migration and invasion. Subcutaneous xenograft tumors induced by gastric cancer cells transfected with MCU siRNAs or controls were constructed. Immunofluorescence was used to detect CD34 expression. Western blot was used to detect the expression of hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF), epithelial-mesenchymal transition (EMT)-related proteins.
Results: MCU had a higher expression in gastric cancer tissues than normal tissues. Compared to gastric cancer tissues, its expression was significantly higher after omental metastasis. MCU expression was significantly correlated with depth of invasion (p=0.048), lymph metastasis (p=0.027), TNM stage (p=0.036) and distant metastasis (p=0.029). Patients with high MCU expression indicated a worse prognosis than those with its low expression (p=0.0098). MCU significantly increased the MMP levels of gastric cancer cells. Wound healing and transwell assay results showed that MCU promoted migration and invasion of gastric cancer cells. In vivo, MCU knockdown significantly inhibited tumor growth and angiogenesis. Both in vitro and in vivo, silencing MCU suppressed the expression of HIF-1α and VEGF as well as activity of EMT processes.
Conclusion: Our findings suggested that highly expressed MCU could promote migration, invasion, angiogenesis and growth of gastric cancer, which could become a potential therapeutic marker for gastric cancer.
Keywords: gastric cancer, mitochondrial calcium uniporter, invasion, angiogenesis, epithelial–mesenchymal transition
Introduction
Gastric cancer is one of the most common malignant tumors and the third leading cause of cancer-related deaths worldwide.1 Despite much progress in surgery, immunotherapy, targeted therapy and adjuvant chemotherapy in the treatment of gastric cancer, the 5-year overall survival rate of gastric cancer is still unsatisfactory.2 More than 80% of patients are diagnosed as advanced and the main cause of deaths of gastric cancer patients is distant metastasis that relies on tumor angiogenesis.2 Dissection of the molecular mechanism regulating gastric cancer metastasis can promote the development of clinical treatment. Epithelial-mesenchymal transition (EMT) is a key cellular process involved in cancer metastasis and progression.3 During EMT, tumor cells lose epithelial (E)-cadherin expression and cell adhesion, and can invade and metastasize.4 Therefore, it is of importance to further understand the molecular mechanism of gastric cancer progression for diagnosis and treatment of gastric cancer.
MCU is a selective channel responsible for mitochondrial Ca2+ uptake.5–7 It has been reported that MCU is involved in the development of several cancers. For example, MCU expression is associated with tumor size and lymph node infiltration of triple negative breast cancer (TNBC).8 In addition, MCU significantly promotes the proliferation and invasiveness of TNBC cells and lung metastasis. MCU silencing reduces the proliferation of glioma cells.9 Abnormally expressed MCU is associated with poor prognosis of colon cancer patients.10 MCU knockdown can promote the migration and metastasis of colon cancer cells and reduce the mitochondrial membrane potential. MCU is dysregulated in hepatocellular carcinoma cells and is closely related to the metastasis and poor prognosis of patients with hepatocellular carcinoma.11 Up-regulation of MCU obviously enhances the invasion and migration ability of hepatocellular carcinoma cells and distant metastasis. MCU has been identified as a prognostic biomarker for pancreatic ductal adenocarcinoma.12 The mechanisms by which MCU plays a role in these tumors have been widely studied. For instance, knockdown of MCU down-regulates the activity of mROS and HIF-1α signaling pathways in TNBC cells.8 Furthermore, MCU suppresses the NAD+/SIRT3/SOD2 pathway, thereby inducing ROS production as well as hepatocellular carcinoma metastases.11 MCU regulated by HINT2 promotes the effect of gemcitabine on apoptosis of pancreatic cancer cells.13 Thus, MCU participates in tumor progression through multiple channels. However, the expression, prognostic value and potential function of MCU in gastric cancer progression remain to be elaborated.
In this study, we identified a novel prognostic biomarker MCU for gastric cancer. MCU was highly expressed in gastric cancer and could promote migration, invasion, angiogenesis and growth of gastric cancer. Our findings suggested that MCU could become a potential therapeutic target for gastric cancer.
Materials and Methods
Human Tissue Specimens
A total of 90 gastric cancer patients were enrolled between 2014 and 2016. All patients did not receive any systemic or local chemotherapy before surgery. During surgery, 90 pairs of gastric cancer tissues and adjacent normal tissues were collected and immediately stored at −80 °C. At least two pathologists independently confirmed the diagnosis of gastric cancer. Our study was in strictly line with the guidelines in the Declaration of Helsinki. All patients provided written informed consent and the study was approved by the Ethics Committee of North China University of Science and Technology Affiliated Hospital (2,014,032).
Immunohistochemistry
Fresh tissue samples were fixed with 4% paraformaldehyde overnight, and then dehydrated with different concentrations of alcohol. The tissue samples were transparent in a 1:1 mixture of 100% alcohol and xylene for 30 min, and xylene for 30 min. The samples were then placed in paraffin wax for embedding and cut to a thickness of 4 μm. The sections were incubated with 3% hydrogen peroxide at room temperature for 20 min to eliminate endogenous peroxidase activity. Then, the sections were blocked with 2% BSA at 37 °C for 2 h. The sections were incubated overnight at 4 °C with primary antibodies (including anti-MCU (1:100; sc-515,930; Santa Cruz, USA), anti-HIF-1α (1:100; 20,960-1-AP; Proteintech, China), anti-VEGF (1:100; ab52917; Abcam, USA), MMP-2 (1:100; 10,373-2-AP; Proteintech, China), anti-Vimentin (1:150; ab92547; Abcam, USA)), followed by secondary antibody for 1 h at 37 °C. The sections were stained with DAB for 5 min and hematoxylin for 2 min. After dehydration and transparency, neutral gum was added dropwise to the tissue sections, gently covered with a coverslip and air-dried. Pathological sections were analyzed with ImageScope software. Immunohistochemical scoring standards were as follows: Firstly, according to the staining intensity, 0 point was negative, 1 point was weak positive, 2 points were medium positive, and 3 points were strong positive. Secondly, according to the percentage of positive staining, 0–10% was 1 point, 11% −50% was 2 points, 51% −80% was 3 points, and >80% was 4 points. Then, immunohistochemical scores were calculated by the staining intensity × the percentage of positive staining.
Immunofluorescence
Paraffin-embedded tissue sections were incubated with primary antibodies labeled with fluorescent substance including anti-MCU (1:100; sc-515,930; Santa Cruz, USA) and anti-CD34 (1:100; ab81289; Abcam, USA) overnight at 4 °C. The sections were then incubated with Alexa Fluor® 488 Conjugate (ZSGB-BIO, #ZF-0512, 1:100, China) and Alexa Fluor® 594 Conjugate (ZSGB-BIO, #ZF-0513, 1:100, China) secondary antibodies at room temperature for 2 h. The results were investigated under an immunofluorescence microscope.
Western Blot
Tissues or cells were lysed using 100 μL RIPA lysate supplemented with protease inhibitor cocktail on the ice for 30 min, which were then centrifuged at 12,000 g for 15 min at 4 °C. The supernatant was harvested and stored at −80 °C. BCA method was used to measure the concentration of total protein. Protein samples were transferred onto the PVDF membrane (Millipore, USA) and blocked by 5% skimmed milk powder for 2 h. The membranes were incubated with primary antibodies including anti-E-cadherin (1:1000; ab76055; Abcam, USA), anti-Vimentin (1:2000; ab92547; Abcam, USA), anti-N-cadherin (1:1000; ab18203; Abcam, USA), anti-HIF-1α (1:1000; 20,960-1-AP; Proteintech, China), anti-MCU (1:400; sc-515,930; Santa Cruz, USA), anti-VEGF (1:1000; ab52917; Abcam, USA), anti-TGF-β (1:1000; ab92486; Abcam, USA), anti-ITGB1 (1:1000; 12,594-1-AP; Proteintech, China), anti-MMP-2 (1:100; 10,373-2-AP; Proteintech, China) and anti-β-actin (1:1000; 20,536-1-AP; Proteintech, China) for 4 °C overnight. After that, the membranes were incubated with horseradish peroxidase IgG antibodies for 30 min at room temperature. Protein blots were visualized using Chemiluminescence kit (ECL).
Cell Culture
Two gastric cancer cell lines including HGC-27 and SNU-1 that were purchased from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (Shanghai, China) were cultured in Gibco DMEM medium (SH30084.03, Hyclone, USA) plus 10% fetal bovine serum (11,885,084, Gibco, USA). All cells were grown at a humidified incubator with 5% CO2 at 37°C.
Transfection
According to manufacturer’s instructions, MCU-siRNA (sense: 5ʹ-CAUAAAGGAGCCAAAAAGUCA-3ʹ, antisense: 5ʹ-ACUUUUUGGCUCCUUUAUGGA-3ʹ) and its control lentiviral particles were transfected into HGC-27 and SNU-1 cells. HGC-27 and SNU-1 cells were treated with different concentrations of MCU agonist Spermine (0, 12.5μM, 25μM, 50μM, 100μM, 200μM, 500μM); (18,041, Cayman, USA). Green fluorescent protein (GFP)-PURO-MCU and its control were detected using Western blot and immunofluorescence.
Cell Counting Kit-8 (CCK-8)
HGC-27 and SNU-1 cells were seeded into 96-well plates and treated with different concentrations of Spermine (0μM; 12.5μM; 25μM; 50μM; 100μM; 200μM; 500μM) for 48h. The cell viability was detected with CCK-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). The 450 nm of absorbance was tested using a multiscan spectrum.
Flow Cytometry Assay
HGC-27 and SNU-1 cells (5×105 cells/well) were seeded into a 6-well plate and incubated with different concentrations of Spermine (0μM; 25μM; 50μM; 100μM; 200μM; 500μM) for 48 h. 400μL Annexin V-fluorescein isothiocyanate (FITC) binding buffer (BestBio, Shanghai, China) was used to mark the re-suspended cells, followed by digestion with 0.25% trypsin. After centrifugation at 1000 × g at room temperature for 5 min, the cells were incubated with 5 μL Annexin V-FITC for 20 min and 10 μL propidium iodide for 5 min at 4°C. Finally, the stained cells were analyzed using flow cytometry (BD Biosciences, San Jose, CA, USA).
Mitochondrial Membrane Potential (MMP)
Transfected HGC-27 and SNU-1 cells were seeded into 6-well plates supplemented with serum and phenol red, followed by treatment with test compounds for 24h. Then, the cells were stained with JC-1 dye (M8650, Solarbio, China). Under a fluorescence microscope (IX71; OLYMPUS, Japan), the results were observed and quantified using Image-Pro Plus software (IPP 6.0, USA). The mitochondrial uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP) was used as a positive control.
Transwell Assay
Transwell chambers (3412; Corning, USA) were placed in a 24-well plate. Matrigel and serum-free medium were mixed well on ice at a ratio of 1:20. Then, the mixture was added vertically to the cells in a 37 °C incubator for 30 min. The cells were seeded into the upper chamber. Six hundred microliter 1640 medium plus 10% fetal bovine serum were added to the lower chamber. After 48 h, the transwell chambers were harvested, fixed with methanol for 40 min and stained using 0.5% crystal violet for 20 min. The results were observed under an optical microscope (IX71; OLYMPUS, Japan), and 5 fields were randomly photographed and counted (400 ×).
Wound Healing Assay
After 48 h treatment with MCU siRNA, Spermine or corresponding controls, HGC-27 and SNU-1 cells were seeded in 6-well plates. A 200 μL sterile plastic pipette tip was used to lightly line. After rinsing the cells with PBS for 3 times, 2 mL of medium containing 10% FBS was added to each well. After 72 h, the results were observed and taken photos.
Xenograft Model
Balb/c female nude mice (5 weeks old, weight 16–20g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China, https://www.vitalriver.com/). All nude mice were randomly divided into two groups including control group and MCU siRNA group (4/each group). HGC-27 or SNU-1 cells (2×106 cells) stably transfected with MCU siRNA or control were subcutaneously injected into the left flank of the mice. The tumor volumes and survival time of each mouse were recorded. All animal experiments were performed in line with NIH Guidelines for the Care and Use of Laboratory Animals. Our study was approved by the Ethics Committee of North China University of Science and Technology Affiliated Hospital (2,014,032).
Statistical Analyses
All statistical analyses were carried out using GraphPad Prism 8.0 software (GraphPad Inc., La Jolla, CA, USA) and SPSS. The experiments were repeated at least three times. All data were expressed as the mean ± standard deviation (SD). Comparisons between two groups were performed using student’ t-test. Multiple comparisons were performed using ANOVA followed by Tukey’s test. Correlation between MCU expression and gastric cancer clinical features was analyzed using Chi-Square test. Pearson correlation between MCU expression and other proteins (HIF-1α, VEGF and E-cadherin) was analyzed in gastric cancer. P<0.05 was considered significant.
Results
Correlation Between MCU Expression and Clinical Characteristics and Prognosis of Gastric Cancer
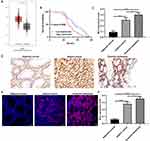
Using the GEPIA database (http://gepia2.cancer-pku.cn/), 408 gastric cancer tissues and 211 normal tissues were retrieved in this study. As shown in Figure 1A, MCU expression was significantly higher in gastric cancer tissues than normal tissues. In our cohort of 90 gastric cancer patients, they were divided into high- and low-expression groups according to the median value of MCU. We found that patients with high MCU expression indicated a poorer prognosis than those with low MCU expression (p=0.0098; Figure 1B). We collected 90 gastric cancer tissue sections and MCU expression was detected using immunohistochemistry. Compared to adjacent normal tissues, MCU was significantly highly expressed in gastric cancer tissues (Figure 1C and D). Intriguingly, the expression of MCU was significantly higher in omental metastasis tissues than gastric cancer tissues (Figure 1C and D). Immunofluorescence results showed that red fluorescent marked MCU was mainly expressed in cell membrane and cytoplasm (Figure 1E). Consistent with the immunohistochemistry results, high MCU expression was found in gastric cancer tissues (Figure 1F). As shown in Table 1, 64 of 90 gastric cancer tissue sections had high MCU expression. We also analyzed the correlation between MCU expression and clinical features. MCU expression was significantly correlated with depth of invasion (p=0.013), lymph metastasis (p=0.002), TNM stage (p=0.001) and distant metastasis (p=0.002). However, there was no correlation between MCU expression and gender (p=0.835) and age (p=0.905).
 |
Table 1 Correlation Between MCU Expression and Clinical Characteristics of Patients with Gastric Cancer |
Abnormal Expression of MCU, HIF-1α, VEGF and E-Cadherin Proteins in Gastric Cancer
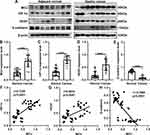
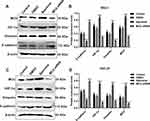
As previous studies, MCU expression is associated with HIF-1α signaling pathway in TNBC8 and colon cancer.10 Thus, in this study, we detected the expression of MCU, HIF-1α, VEGF and E-cadherin in gastric cancer. Twenty-four pairs of gastric cancer tissues and corresponding adjacent normal tissues were randomly selected for Western blot. Consistent with our immunohistochemistry and immunofluorescence results, MCU expression was significantly higher in gastric cancer tissues than adjacent normal tissues (Figure 2A and B). As expected, the expression levels of HIF-1α and VEGF proteins were both significantly higher in gastric cancer tissues than adjacent normal tissues (Figure 2C and D). Moreover, lower E-cadherin expression was detected in gastric cancer tissues compared to adjacent normal tissues (Figure 2E). Our correlation analysis results showed that MCU was positively correlated to HIF-1α (Figure 2F) and VEGF (Figure 2G), and negatively correlated to E-cadherin (Figure 2H) in gastric cancer.
MCU Promotes Mitochondrial Membrane Potential, Migration and Invasion of Gastric Cancer Cells
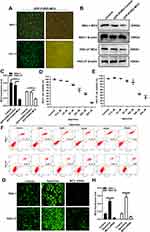
To observe the role of MCU in gastric cancer, we firstly constructed stably gastric cells silencing or overexpressing MCU. As shown in Figure 3A, GFP-PURO-MCU was remarkedly inhibited after HGC-27 and SNU-1 cells transfected with MCU siRNA. Western blot analysis was then performed. As expected, both in HGC-27 and SNU-1 cells, MCU expression was significantly silenced after transfection of MCU siRNA (Figure 3B and C). Different concentrations of MCU agonist Spermine (0, 12.5μM, 25μM, 50μM, 100μM, 200μM, 500μM) were used to activate MCU expression in two gastric cancer cells. The CCK-8 assay results showed that Spermine significantly inhibited the cell viability of SNU-1 (Figure 3D) and HGC-27 cells (Figure 3E), with a concentration manner. However, there was no significant effect of Spermine on gastric cancer cell apoptosis, as shown in flow cytometry (Figure 3F). According to the CCK-8 assay results, 50μM MCU agonist Spermine was used for MCU overexpression in this study. Our immunofluorescence assay results confirmed that the expression of MCU was significantly activated by Spermine and was distinctly silenced by MCU siRNAs both in HGC-27 and SNU-1 cells (Figure 3G and H).
The MMP assay results showed that MCU overexpression induced by Spermine significantly elevated the MMP levels in HGC-27 and SNU-1 cells (Figure 4A and B). Conversely, MCU knockdown remarkedly suppressed the MMP levels in gastric cancer cells (Figure 4A and B). As shown in the transwell results, the invasion ability of gastric cancer cells was significantly promoted after treatment with Spermine, however, it was significantly inhibited after transfection with MCU siRNA (Figure 4C and D). Furthermore, wound healing assays were also carried out. We found that the wound distance was remarkedly decreased after 72 h both in HGC-27 and SNU-1 cells treated with Spermine (Figure 4E and F). In converse, silencing MCU significantly increased the wound distance in gastric cancer cells transfected with MCU siRNA (Figure 4E and F).
MCU Regulates HIF-1α, E-Cadherin and Vimentin in Gastric Cancer Cells
The Western blot results showed that MCU overexpression significantly promoted HIF-1α expression, however, its knockdown significantly inhibited HIF-1α expression both in SNU-1 and HGC-27 cells (Figure 5A–D). Furthermore, we found that Vimentin expression was remarkedly elevated after MCU overexpression, which was remarkedly decreased after MCU knockdown in SNU-1 and HGC-27 cells (Figure 5A–D). Also, the results showed that the expression of E-cadherin had a decreased level in gastric cancer cells treated with MCU overexpression, and had an increased level in gastric cancer cells transfected with MCU siRNA (Figure 5A–D).
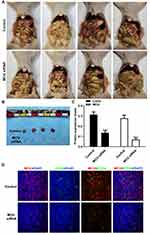
Silencing MCU Inhibits Gastric Cancer Progression and Microvessel Density in vivo
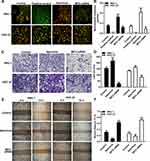
To further confirm the in vitro findings, we investigated the biological functions of MCU in vivo. Similarly, stable transfection of MCU caused a distinct decrease in the growth of subcutaneous xenograft tumors induced by gastric cancer cells in nude mice (Figure 6A and B). The immunofluorescence assay results showed that the expression levels of MCU and CD34 were both significantly decreased in subcutaneous xenograft tumors induced by gastric cancer cells transfected with MCU siRNAs (Figure 6C and D). CD34 has been considered as a marker of microvessel density in the tumor stroma. Thus, silencing MCU could inhibit the formation of microvessel density in gastric cancer.
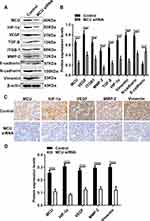
Silencing MCU Suppresses HIF-1α, VEGF and EMT Pathways in vivo
Our Western blot results showed that the expression levels of MCU, HIF-1α, VEGF, TGF-β, ITGB-1, MMP-2, N-cadherin and Vimentin were all significantly decreased in subcutaneous xenograft tumors induced by gastric cancer cells transfected with MCU siRNA (Figure 7A and B). Conversely, the expression of E-cadherin had a distinct increase in subcutaneous xenograft tumors induced by gastric cancer cells transfected with MCU siRNA (Figure 7A and B). Consistent with the Western blot results, our immunohistochemistry results confirmed that the expression levels of MCU, HIF-1α, VEGF, MMP-2 and Vimentin proteins had a significant decrease within subcutaneous xenograft tumors induced by gastric cancer cells transfected with MCU siRNA (Figure 7C and D).
Discussion
In this study, we found that MCU expression was increased in gastric cancer. Silencing MCU inhibited the migration, invasion, angiogenesis and growth of gastric cancer. Mechanistically, MCU could activate HIF-1α, VEGF and EMT pathways. Our findings highlighted the carcinogenicity of MCU in gastric cancer, which can regulate tumor growth and invasiveness, and could become a promising therapeutic target for gastric cancer.
Previous studies have found that MCU is highly expressed in TNBC,8 colon cancer,10 glioma,9 hepatocellular carcinoma11 and pancreatic ductal adenocarcinoma.12 In this study, high MCU expression was found in gastric cancer tissues. Furthermore, its low expression indicated a poor prognosis for patients with gastric cancer. Local infiltration is the most important process before gastric cancer metastasis. Furthermore, lymph node metastasis also plays a key role in gastric cancer metastasis. Unfortunately, in the early stage of gastric cancer, the rate of lymph node metastasis may exceed 20%.14 We found that MCU expression was significantly correlated with depth of invasion, lymph metastasis, TNM stage and distant metastasis, indicating that MCU expression could be associated with prognosis of gastric cancer. Intriguingly, our results indicated that MCU expression was significantly higher in omental metastasis than gastric cancer tissues, indicating that MCU expression might contribute to gastric cancer metastasis. Combining previous studies, MCU has been confirmed to promote several cancer metastasis, such as colon cancer10 and hepatocellular carcinoma.11 We found that MCU knockdown significantly induced the loss of MMP, and its overexpression remarkedly enhanced the levels of MMP in SNU-1 and HGC-27 gastric cancer cells, indicating that MCU may promote the proliferation of gastric cancer cells by enhancing MMP levels. Also, our findings revealed that MCU significantly promoted the migration and invasion of gastric cancer cells.
In gastric cancer tissues, there was an increase in expression of HIF-1α and Vimentin and loss of E-cadherin. MCU overexpression significantly promoted the expression of HIF-1α and Vimentin, and its knockdown significantly suppressed the expression of E-cadherin in two gastric cancer cells. Hypoxia is a characteristic of tumor microenvironments.15–17 In response to hypoxia, HIF-1α is stabilized. MCU silencing can down-regulate the expression of HIF-1α, thereby attenuating the transcription of HIF-1α target genes related to the progression of TNBC.8 HIF-1α can induce the proliferation, migration and invasion of gastric cancer cells by promoting the expression of VEGF.18 Our results showed that silencing MCU could inhibit the expression of VEGF in subcutaneous xenograft tumors induced by gastric cancer cells in nude mice, indicating that MCU might regulate the expression of VEGF via HIF-1α.
EMT is a process by which cells change from a proliferative epithelial phenotype to a migratory and invasive mesenchymal phenotype.19 During the progress of EMT, gastric cancer cells lost their polarity and adhesion ability, thereby weakening the connection between cells.20–22 Vimentin has been shown to play an important role in the invasion and metastasis of gastric cancer.23 Vimentin promotes gastric cancer progression by inhibiting E-cadherin. Our results indicated that MCU could promote migration and invasion of gastric cancer cells by regulating EMT process in vitro and in vivo. Increasing evidences confirm that TGF-β is a key growth factor driving EMT in various tumors, including gastric cancer.2 Inhibition of TGF-β-induced gastric cancer cells is a promising strategy for treating gastric cancer.24–26 Our findings suggested that MCU could inhibit the expression of TGF-β, thereby relieving the EMT process of gastric cancer.
We further observed the biological functions of MCU in vivo. As expected, silencing MCU distinctly suppressed the growth of subcutaneous xenograft tumors induced by gastric cancer cells in nude mice. Angiogenesis is the process of new capillary formation, which plays a central role in tumor growth and metastasis of gastric cancer.27,28 We found that CD34 was significantly decreased in xenograft tumors induced by gastric cancer cells transfected with MCU siRNA according to our immunohistochemistry, suggesting that MCU knockdown could suppress angiogenesis, thereby blockage of gastric cancer invasion and metastasis.
Extracellular matrix (ECM) plays an important role in the process of invasion and metastasis of gastric cancer.29,30 Therefore, the disruption about the dynamic balance of ECM may be closely related to tumor invasion and metastasis. Matrix metalloproteinases (MMPs) are zinc and calcium dependent proteases, which may degrade ECM components, promote angiogenesis and regulate cell adhesion.31 Our Western blot and immunohistochemistry results showed that MCU knockdown significantly decreased the expression of MMP-2 protein in subcutaneous xenograft tumors induced by gastric cancer cells, confirming that MCU could be involved in the development of gastric cancer.
In this study, we analyzed the abnormal expression and biological functions of MCU in gastric cancer. Our results suggested that MCU could promote migration, invasion, angiogenesis and growth of gastric cancer cells in vitro and in vivo. Thus, targeting MCU could be a promising treatment strategy for gastric cancer. In-depth molecular mechanisms of MCU in gastric cancer progression require to be clarified in further studies.
Conclusion
Our research showed the carcinogenic effects of MCU on gastric cancer and silencing MCU can inhibit the migration, invasion, angiogenesis and growth of gastric cancer, which provided a new target for the treatment of gastric cancer.
Abbreviations
MCU, mitochondrial calcium uniporter; TNBC, triple negative breast cancer; MMP, mitochondrial membrane potential; HIF-1α, hypoxia-inducible factor-1α; E-cadherin, epithelial-cadherin; CCK-8, Cell counting kit-8; MMP, mitochondrial membrane potential; ECM, extracellular matrix; MMPs, metalloproteinases.
Data Sharing Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of North China University of Science and Technology Affiliated Hospital (2,014,032).
Consent for Publication
All participants provided written informed consents.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by Project of Hebei Medical Science Research Project (20191127); Project of training excellent clinical medical talents and basic research project funded by the government (361036); Key R & D plan of the autonomous region in 2019 (2019BEG03007).
Disclosure
The authors declare no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. He Z, Dong W, Li Q, Qin C, Li Y. Sauchinone prevents TGF-beta-induced EMT and metastasis in gastric cancer cells. Biomed Pharmacother. 2018;101:355–361. doi:10.1016/j.biopha.2018.02.121
3. Zhang D, Zhou S, Liu B. Identification and validation of an individualized EMT-related prognostic risk score formula in gastric adenocarcinoma patients. Biomed Res Int. 2020;2020:7082408.
4. Song Y, Ye M, Zhou J, Wang Z, Zhu X. Targeting E-cadherin expression with small molecules for digestive cancer treatment. Am J Transl Res. 2019;11(7):3932–3944.
5. Baradaran R, Wang C, Siliciano AF, Long SB. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature. 2018;559(7715):580–584. doi:10.1038/s41586-018-0331-8
6. Fan C, Fan M, Orlando BJ, et al. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature. 2018;559(7715):575–579. doi:10.1038/s41586-018-0330-9
7. Oxenoid K, Dong Y, Cao C, et al. Architecture of the mitochondrial calcium uniporter. Nature. 2016;533(7602):269–273.
8. Tosatto A, Sommaggio R, Kummerow C, et al. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1alpha. EMBO Mol Med. 2016;8(5):569–585. doi:10.15252/emmm.201606255
9. Li X, Spelat R, Bartolini A, et al. Mechanisms of malignancy in glioblastoma cells are linked to mitochondrial Ca2+uniporter upregulation and higher intracellular Ca2+levels. J Cell Sci. 2020;133(6):jcs237503. doi:10.1242/jcs.237503
10. Sun Y, Li M, Liu G, et al. The function of Piezo1 in colon cancer metastasis and its potential regulatory mechanism. J Cancer Res Clin Oncol. 2020;146(5):1139–1152. doi:10.1007/s00432-020-03179-w
11. Ren T, Zhang H, Wang J, et al. MCU-dependent mitochondrial Ca2+ inhibits NAD+/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene. 2017;36(42):5897–5909. doi:10.1038/onc.2017.167
12. Giulietti M, Occhipinti G, Principato G, Piva F. Weighted gene co-expression network analysis reveals key genes involved in pancreatic ductal adenocarcinoma development. Cell Oncol. 2016;39(4):379–388. doi:10.1007/s13402-016-0283-7
13. Chen L, Sun Q, Zhou D, et al. HINT2 triggers mitochondrial Ca2+ influx by regulating the mitochondrial Ca2+ uniporter (MCU) complex and enhances gemcitabine apoptotic effect in pancreatic cancer. Cancer Lett. 2017;411:106–116. doi:10.1016/j.canlet.2017.09.020
14. Shi Y, Sun H. Down-regulation of lncRNA LINC00152 suppresses gastric cancer cell migration and invasion through inhibition of the ERK/MAPK signaling pathway. Onco Targets Ther. 2020;13:2115–2124. doi:10.2147/OTT.S217452
15. He C, Wang L, Zhang J, Xu H. Hypoxia-inducible microRNA-224 promotes the cell growth, migration and invasion by directly targeting RASSF8 in gastric cancer. Mol Cancer. 2017;16(1):35. doi:10.1186/s12943-017-0603-1
16. Li Q, Tang H, Hu F, Qin C. Knockdown of A-kinase anchor protein 4 inhibits hypoxia-induced epithelial-to-mesenchymal transition via suppression of the Wnt/beta-catenin pathway in human gastric cancer cells. J Cell Biochem. 2018;119(12):10013–10020. doi:10.1002/jcb.27331
17. Zhou D, Huang L, Zhou Y, Wei T, Yang L, Li C. RON and RONDelta160 promote gastric cancer cell proliferation, migration, and adaption to hypoxia via interaction with beta-catenin. Aging (Albany NY). 2019;11(9):2735–2748. doi:10.18632/aging.101945
18. Zhang J, Xu J, Dong Y, Huang B. Down-regulation of HIF-1α inhibits the proliferation, migration, and invasion of gastric cancer by inhibiting PI3K/AKT pathway and VEGF expression. Biosci Rep. 2018;38(6). doi:10.1042/BSR20180741
19. Zhu X, Chen L, Liu L, Niu X. EMT-mediated acquired EGFR-TKI resistance in NSCLC: mechanisms and strategies. Front Oncol. 2019;9:1044. doi:10.3389/fonc.2019.01044
20. Ge S, Xia X, Ding C, et al. A proteomic landscape of diffuse-type gastric cancer. Nat Commun. 2018;9(1):1012. doi:10.1038/s41467-018-03121-2
21. Xu J, Liu D, Niu H, et al. Resveratrol reverses doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin Cancer Res. 2017;36(1):19. doi:10.1186/s13046-016-0487-8
22. Zou S, Ma C, Yang F, Xu X, Jia J, Liu Z. FBXO31 suppresses gastric cancer EMT by targeting snail1 for proteasomal degradation. Mol Cancer Res. 2018;16(2):286–295. doi:10.1158/1541-7786.MCR-17-0432
23. Mei JW, Yang ZY, Xiang HG, et al. MicroRNA-1275 inhibits cell migration and invasion in gastric cancer by regulating vimentin and E-cadherin via JAZF1. BMC Cancer. 2019;19(1):740. doi:10.1186/s12885-019-5929-1
24. Luo Y, Wu J, Wu Q, et al. miR-577 regulates TGF-β induced cancer progression through a SDPR-modulated positive-feedback loop with ERK-NF-κB in gastric cancer. Mol Ther. 2019;27(6):1166–1182. doi:10.1016/j.ymthe.2019.02.002
25. Zhou X, Men X, Zhao R, et al. miR-200c inhibits TGF-beta-induced-EMT to restore trastuzumab sensitivity by targeting ZEB1 and ZEB2 in gastric cancer. Cancer Gene Ther. 2018;25(3–4):68–76. doi:10.1038/s41417-017-0005-y
26. Zong W, Yu C, Wang P, Dong L. Overexpression of SASH1 inhibits TGF-beta1-induced EMT in gastric cancer cells. Oncol Res. 2016;24(1):17–23. doi:10.3727/096504016X14570992647203
27. Li TJ, Jiang YM, Hu YF, et al. Interleukin-17-producing neutrophils link inflammatory stimuli to disease progression by promoting angiogenesis in gastric cancer. Clin Cancer Res. 2017;23(6):1575–1585. doi:10.1158/1078-0432.CCR-16-0617
28. Tenderenda M, Rutkowski P, Jesionek-Kupnicka D, Kubiak R. Expression of CD34 in gastric cancer and its correlation with histology, stage, proliferation activity, p53 expression and apoptotic index. Pathol Oncol Res. 2001;7(2):129–134. doi:10.1007/BF03032579
29. Gan L, Meng J, Xu M, et al. Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin beta4/FAK/SOX2/HIF-1alpha signaling pathway in gastric cancer. Oncogene. 2018;37(6):744–755. doi:10.1038/onc.2017.363
30. Jang M, Koh I, Lee JE, Lim JY, Cheong JH, Kim P. Increased extracellular matrix density disrupts E-cadherin/beta-catenin complex in gastric cancer cells. Biomater Sci. 2018;6(10):2704–2713. doi:10.1039/C8BM00843D
31. Zhao L, Niu H, Liu Y, et al. LOX inhibition downregulates MMP-2 and MMP-9 in gastric cancer tissues and cells. J Cancer. 2019;10(26):6481–6490. doi:10.7150/jca.33223
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.