Back to Journals » Drug Design, Development and Therapy » Volume 14
The Regulating Mechanism of Chrysophanol on Protein Level of CaM-CaMKIV to Protect PC12 Cells Against Aβ25-35-Induced Damage
Authors Ye T, Gao HW, Xuan WT, Ye S, Zhou P, Li XQ, Wang Y , Song H, Liu YY , Cai B
Received 9 January 2020
Accepted for publication 30 June 2020
Published 13 July 2020 Volume 2020:14 Pages 2715—2723
DOI https://doi.org/10.2147/DDDT.S245128
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Ting Ye,1,* Hua-Wu Gao,1,* Wei-Ting Xuan,1 Shu Ye,1– 3 Peng Zhou,1– 3 Xin-Quan Li,1 Yan Wang,1– 3 Hang Song,1,2 Yan-Yan Liu,1,2 Biao Cai1– 3
1School of Integrated Chinese and Western Medicine, Anhui University of Chinese Medicine, Hefei 230012, People’s Republic of China; 2Institute of Integrated Chinese and Western Medicine, Anhui Academy of Chinese Medicine, Hefei 230012, People’s Republic of China; 3Anhui Province Key Laboratory of Chinese Medicinal Formula, Hefei 230012, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan-Yan Liu; Biao Cai Email [email protected]; [email protected]
Objective: To investigate the neuroprotective effect of chrysophanol (CHR) on PC12 treated with Aβ25-35, and the involved mechanism.
Methods: After the establishment of an AD cell model induced by Aβ25-35, the cell survival rate was detected by MTT, cell apoptosis was assayed by Hoechst 33342 staining, mRNA expressions of calmodulin (CaM), calcium/calmodulin-dependent protein kinase kinase (CaMKK), calcium/calmodulin-dependent protein kinase IV (CaMKIV) and tau (MAPT; commonly known as tau) were determined by qRT-PCR, and protein levels of CaM, CaMKK, CaMKIV, phospho-CaMKIV (p-CaMKIV), tau and phospho-tau (p-tau) were detected by Western blot analysis.
Results: When pretreated with CHR before exposure to Aβ25-35, PC12 cells showed that increased cell viability and reduced apoptosis. The qRT-PCR results indicated that the deposition of Aβ25-35 triggers a decrease in levels of CaM, CaMKK, CaMKIV, and tau in PC12 cells. In addition, Western blot results also suggested that Aβ25-35 decreases the protein expression of CaM, CaMKK, CaMKIV, p-CaMKIV, and the ratio of p-tau to tau in PC12 cells. However, the above effects were significantly alleviated after the treatment of CHR.
Conclusion: CHR plays a neuroprotective role in AD though decreasing the protein level of CaM-CaMKK-CaMKIV and the expression of p-tau downstream.
Keywords: chrysophanol, Alzheimer’s disease, CaM-CaMKK-CaMKIV, p-tau
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease and the most common cause of dementia, emerging as a global health issue. Epidemiological studies have revealed that the number of people living with AD would be expected to triple from 50 million to 152 million by 2050.1 AD’s main pathological process is the accumulation of the peptide amyloid-β (Aβ) in the hippocampus,2 which triggers a series of processes called the amyloid cascade leading eventually to neuronal cell death and dementia.3,4
Calmodulin-dependent protein kinase IV (CaMKⅣ) plays a significant role in the pathogenesis of AD.5,6 Deposition of Aβ in neuronal cells causes an increase in Ca2+ concentration, and the combination of overloaded Ca2+ and calmodulin (CaM) activates the activity of calcium calmodulin complex Ca2+/CaM.7 On arrival in the nucleus, Ca2+/CaM activates calcium/calmodulin-dependent protein kinase kinase (CaMKK) and its substrate CaMKIV, the cAMP-response element binding protein (CREB) kinase,8,9 which can phosphorylate tau (MAPT; commonly known as tau) protein.10 Hyperphosphorylated tau protein is the main constituent of abnormal neurofibrillary tangles (NFTs), which is an important factor in the clinical expression of AD.11 Therefore, inhibition of tau hyperphosphorylation via the CaM–CaMKIV signaling pathway is an important method for the prevention and treatment of AD.
Chrysophanol (CHR) is an anthraquinone found in Rheum palmatum, which was used in the preparation of medicines in ancient China. Recently, CHR has been found to play a central role in improving the abilities of learning and memory and protecting against the injury of hippocampal neuronal cells.12 Furthermore, it has long-term neuroprotective effects against brain injury.13 Previous studies have shown that CHR can resist neurotoxic effects and improve learning and memory impairment in Aβ25-35-induced AD rats, thus indicated that CHR may be a promising therapeutic strategy to protect neuronal cells from further damage and alleviate the progression of those neurological diseases.
Donepezil (Don), a medication approved by the FDA, is most commonly used for the treatment of AD.14 According to previous reports, pretreatment with Don suppressed TNFα-induced intracellular Ca2+ elevation by the phosphatidylinositol 3-kinase (PI3K) pathway in rodent microglial cells, which indicated that Don could reduce calcium concentration.15 Thus, Don was selected as a positive control drug in this study.
Based on preliminary experiments, we speculated that the CaM-CaMKIV would be involved in the protective effects of CHR on Aβ25-35-induced injury in PC12 cells. The purpose of the current work was to explore the mechanism of CaM-CaMKIV in the pathogenesis of AD, which would provide a basis for therapeutic intervention in AD.
Materials and Methods
Materials
The Aβ25-35 was purchased from Sigma-Aldrich (St Louis, MO, USA), and dissolved in deionized water, which was incubated at 37°C for 3 days and used to induce aggregation. The stock solution was diluted to the working concentration immediately, and we added it to the cell culture medium before use. Chrysophanol (Yuanye Bio-Technology Co. Ltd, Shanghai, China) was prepared into the different solutions by adding to the cell culture medium for the further use, and the tablets (Eisai, Jiangsu, China) were dissolved in cell culture medium to a concentration of 10 μM before use.
Cell Culture and Treatment
The PC12 cells were purchased from the Shanghai Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences). After resuscitation, PC12 cells were cultured in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, Inc., Waltham, USA) supplemented with 10% fetal bovine serum (Bio-Rad Laboratories, Inc., Hercules, USA) and 1% penicillin/streptomycin (Gibco Ltd., NY, USA), incubated in a humidified incubator containing 5% CO2 at 37°C and passaged every 3 to 4 days. After 24 h, cells were pretreated with CHR for 12 h and incubated with Aβ25-35 (30 μM) for another 24 h.
Cell Viability Analysis
In vitro, cell viability was assessed by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (Solarbio Technology Company, Beijing, China) reduction assay. The MTT assay was performed to evaluate the biocompatibility and toxicity of CHR on PC12 cells. PC12 cells were adjusted to 2×105 cells/mL and seeded in 96-well plates (100 μL per well). After incubation for 24 h, cells were stimulated with 1‰ (v/v) dimethyl sulfoxide (DMSO) or different doses of CHR (2.5, 5, 10, 25, 50, and 100 μM) for 12 h, respectively. MTT solution (20 μL, 5 mg/mL) was added to 96-well plates and incubated for 4 h at 37°C, then was replaced with DMSO to solubilize the precipitated dye. The absorbance was read at 490 nm in a microplate reader and the above experiments were replicated 3 times. The relative cell viability was expressed as a percentage relative to the untreated control cells.
Morphological Observation Under an Inverted Microscope
PC12 cells were adjusted to a concentration of 2×105 cells/mL and inoculated in 6-well plates (2 mL/well), followed by pretreatment of CHR (10, 25, 50 μM) for 12 h before exposure to 30 mM Aβ25–35 for another 24 h. When appropriate confluency was reached, we observed the morphology and structure of the cells in the clear field of view by light microscope.
Hoechst 33342 Staining
Studies of apoptosis were performed to confirm morphologic changes by the chromatin dye Hoechst 33342 (Solarbio Technology Company, Beijing, China). The morphological changes involved the condensation and margination of nuclear chromatin, and were observed by Hoechst staining and electron microscopy. Cells were fixed in 4% of paraformaldehyde for 10 min at 4°C and washed twice with phosphate buffer saline (PBS), following by incubation with 10 μg/mL Hoechst 33342 for 20–30 min in a humidified incubator in the dark. To adherent cells, a small amount of Hoechst 33342 staining solution was added to cover the sample. To the suspended cells, 3 times the volume of the sample was added to the stain and mixed well, and left at room temperature for 3–5 minutes. Hoechst 33342 staining solution was absorbed and washed with PBS for 2–3 times (each time for 3–5 minutes). Cells were observed and photographed with fluorescence microscopy (Olympus IX71).
Experimental Grouping
Based on the viability of analysis, the morphology, and the apoptosis assays, the subsequent experiments were divided into four groups, which were the control group (Control), AD model group (Model, Aβ25-35 30 μM), chrysophanol group (CHR, 50 μM), and donepezil group (Don, 10 μM).
Quantitative RT-PCR
The mRNA expression level of CaM, CaMKK, CaMKIV, and tau were tested by quantitative RT-PCR. All treated cells were collected and washed twice with PBS solution. Then, 1 mL TRIZOL and 200 μL chloroform were added and the mixture was vortexed for 15 s. After leaving at room temperature for 10 minutes, phases were separated by centrifugation (12000 g for 15 minutes at 4°C). The upper layer was transferred to a new tube and was added with an equal volume of isopropanol with a vigorous shake, then followed by incubating at room temp for 10 minutes. The RNA pellets were collected, followed by centrifugation (12000 g for 10 minutes at 4°C) and resolved in 20 μL DEPC H2O. The reverse transcription was conducted by a reverse transcription kit following the manufacture’s protocol. Quantitative RT-PCR was performed on the 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) by SYBR green kit (TOYOBO, Japan) with β-actin as control. A mixed cDNA sample control was incorporated into each PCR cycle and repeated three holes. All target gene primers were designed by the comparative Ct (2−ΔΔCt) (cycle threshold) method. The specific primers used for the current study are shown in Table 1.
 |
Table 1 Primer Sequences in Quantitative RT-PCR |
Western Blotting
In this study, the expression levels of CaM, CaMKK, CaMKIV, p-CaMKIV, tau, and p-tau protein were detected by Western blotting. According to the manufacturer’s instructions, all protein samples were extracted by the extraction kit and quantified by bicinchoninic acid kit (Best Bio, Shanghai, China). Samples were detected by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 10% gel), and then transferred onto nitrocellulose membranes (Pall Corporation, Pensacola, USA). The membranes were blocked in TBS tween 20 (TBS-T, 0.1% tween 20) contained 5% non-fat dry milk for 2 h at room temperature with rotation. After blocking, the membranes were incubated with the rabbit anti-rat monoclonal primary antibody CaM, CaMKK (1:5000, Cambridge, UK), CaMKIV, p-CaMKIV, tau, p-tau (1:1000, Cell Signaling Technology, MA, USA) at 4°C overnight. After incubation, the membranes were washed by TBST 3 times (each time for 10 min). Then, the goat anti-rabbit IgG (1:20,000; ZSGB-BIO, Beijing, China) was added to combine with the primary antibody for 2 h at room temperature with rotation and washed for 10 minutes (three times with TBST). The antigen-antibody bands were detected by ECL chemiluminescence and automatic exposure using the gel imaging device (ProteinSimple, CA, USA). The immunoblots of proteins were corrected to the bands of β-actin. All the experiments were repeated 3 times.
Statistical Analysis
All data were expressed as the mean ± standard deviation (x±s), and analyzed statistically using SPSS 23.0 software (SPSS, Chicago, IL, USA). Multi-group means were compared by one-way analysis of variance which followed the least significant difference test, and the value of P<0.01 was statistical significance.
Results
The Effect of CHR on Survival Rate of Aβ25-35-Induced Injury in Cells
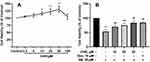
The cytotoxic and neuroprotective effects of CHR on PC12 cells were assessed by MTT assay (Figure 1). Compared to the control group, cell survival rates were significantly increased by treatment with a concentration of CHR at 10, 25, and 50 μM (P<0.01). However, inconsistent with the above findings, treating cells with a higher concentration of CHR (100 μM) resulted in a decrease in survival rates. So the concentrations of CHR (10, 25, and 50 μM) were selected for subsequent experiments (Figure 1A). To circumvent the neuroprotective effect of CHR on Aβ25-35-induced PC12 cell injury, PC12 cells were pre-incubated with different concentrations of CHR (10, 25, 50 μM) for 12 h, followed by incubating with Aβ25–35 for 24 hours. Although these cells’ exposure to the Aβ25–35 markedly decreased cell viability, negative effects induced by Aβ25–35 were diminished in the presence of CHR (P<0.01) (Figure 1B). Altogether the above results suggested CHR is beneficial to Aβ25-35-induced PC12 cell injury, especially in the high-dose group (50 μM).
The Effect of CHR on Cell Morphology of Aβ25-35-Induced Cells
The observations of cells’ morphology could be executed by the light microscope (Figure 2). In control groups, cells intertwined into a network which had a good refractive index. In model groups, some cells were detached and exhibited morphological change. In morphological results, shorter protrusions were detected in cells, and the number of cells decreased significantly. However, CHR pre-treatment reversed the morphological alterations induced by Aβ25–35, including significantly increased protrusions, regular morphology, and increased refractive index. Consistent with the above findings, CHR pre-treatment at a concentration of 50 μM has shown the best effect. The above results indicated that the CHR can ameliorate Aβ25-35-induced cell damage, while the high concentration of Aβ25-35 can inhibit the proliferation of PC12 cells.
Effect of CHR on Apoptosis of Aβ25-35-Induced Cells
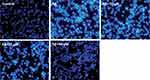
Immunofluorescence analysis of PC12 cells was conducted by Hoechst 33342 (fluorescent DNA dye). The result of Figure 3 suggested that the model group exhibits typical characteristics of apoptosis, which included chromatin condensation, nuclear shrinkage, and nuclear fragmentation. Obviously, the 10, 25, and 50 μM CHR efficiently prevented the condensation of chromatin, and the CHR (50 μM) dose group result was even more striking. As a result, these findings indicated that 10, 25, and 50 μM CHR can protect the Aβ25-35-induced PC12 cells against apoptosis.
The Results of mRNA Expression in Aβ25-35-Induced Cells
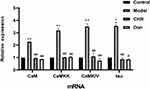
Quantitative RT-PCR was conducted to detect the effects of different dosages of CHR. The results are shown in Figure 4. The mRNA expression of CaM, CaMKK, CaMKIV, and tau was significantly increased in the model group compared to the control group (P<0.01). However, the expression of CaM, CaMKK, CaMKIV, and tau were significantly decreased in the CHR and Don groups (P<0.01). These findings have important implications for mRNA expression of CaM, CaMKK, CaMKIV, and downstream tau, which were significantly decreased after CHR pretreatment in Aβ25-35-induced cells.
The Effect of CHR on p-tau Protein Expression in Aβ25-35-Induced Cells
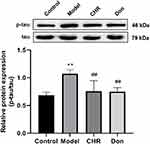
The expression of p-tau and tau protein was assessed by Western blotting. As shown in Figure 5, the ratio of p-tau to tau was significantly increased in the model group compared to the control group (P<0.01). Meanwhile, the ratio of p-tau to tau was significantly decreased in the CHR and Don groups (P<0.01). Therefore, our results indicated that CHR could significantly reduce the ratio of p-tau to tau in Aβ25-35-induced cells. Here, CHR showed inhibitory effects on p-tau.
The Effect of CHR on CaM, CaMKK, CaMKIV and p-CaMKIV Protein Expression in Aβ25-35-Induced Cells
We identified the protein expression of CaM, CaMKK, CaMKIV, and p-CaMKIV by Western blotting. As shown in Figure 6, the protein expression levels of CaM, CaMKK, CaMKIV, and p-CaMKIV were significantly increased in the model group compared to the control group (P<0.01). Meanwhile, the expression levels of CaM, CaMKK, CaMKIV, and p-CaMKIV were significantly decreased in the CHR and Don groups (P<0.01, P<0.05). Therefore, our results indicated that CHR could significantly reduce the levels of CaM, CaMKK, CaMKIV, and p-CaMKIV in Aβ25-35-induced cells. Thus, CHR showed inhibitory effects on CaM, CaMKK, CaMKIV, and p-CaMKIV.
Discussion
Alzheimer’s disease, the commonest cause of dementia, is a growing global health concern with huge implications for individuals and society and is characterized by synapse loss, memory impairment, and other cognitive problems.16,17 These characteristic pathological changes were cerebral cortex atrophy, Aβ deposition, neurofibrillary tangles (NFTs), senile plaque (SP), and reduced numbers of memory neurons. Aβ is a fragment of amyloid precursor protein (APP) which accumulated in AD. Aβ accumulation is believed to contribute strongly to the pathogenesis of AD, which leads to cognitive dysfunction and memory loss, and synaptic impairments.18–20 As a result, Aβ25-35 was added to establish the AD cell model in our study.
In our experiment, cell viability analysis, light microscope observation, and Hoechst 33342 staining were conducted after adding Aβ25-35 (30 μM, 24 h). We found that there was significant cell damage in PC12 cells, while the cell damage was significantly reduced with different concentrations of CHR (10, 25, and 50 μM) in advance. These results indicated that different dosages of CHR (10, 25, and 50 μM) can eliminate Aβ deposition, and had a good neuroprotective effect on Aβ25-35-induced PC12 cells. At the same time, we found that the effect of the 50 μM concentration of CHR was better than the other concentrations of CHR when CHR was applied in Aβ25-35-induced PC12 cells.
The CaM-CaMKIV protein family is mainly composed of CaM, CaMKK, and CaMKIV signal molecules. One function of CaM is activating the members of a family of S/T protein kinases, called Ca2+/CaM-dependent protein kinases or CaM kinases. This family includes several kinases, such as phosphorylase kinase, myosin–light chain kinase (MLCK), CaM kinases I, II, III, and IV.21,22 CaMKK is a downstream substrate of Ca2+/CaM and is activated when CaM is phosphorylated by Ca2+; then, CaMKK can phosphorylate and activate CaMKIV. In addition, previous studies showed that AD is associated with the accumulation of calcium ions (Ca2+), which was responsible for the phosphorylation of tau.23 The intracellular accumulation of phosphorylated tau was detected in the brains of AD patients, which can trigger nuclear CaMKIV. In turn, CaMKIV can also aggravate hyperphosphorylation of intracellular tau. The increased Ca2+ concentration may induce a self-perpetuating harmful loop to promote neurodegeneration.24
According to previous reports, tau is well established as a microtubule-associated protein, which can receive various post-translational modifications. Also, changes in its phosphorylation pattern were reported in the pathogenesis of AD.25 When the tau protein is phosphorylated, the excessive phosphate binding inhibited tau protein and microtubules to a large extent, and caused the formation of NFT, finally leading to neuronal degeneration and AD. Therefore, the inhibition of tau hyperphosphorylation can meaningfully improve neuronal damage and cognitive function.
Chrysophanol (CHR) is a member of the anthraquinone family, which was originally extracted from Rheum palmatum and showed a variety of pharmacological effects, such as anti-inflammatory, antioxidant, antiapoptotic, anti-aging, and neuroprotective effect.26–30 In the prevention and treatment of AD, previous studies demonstrated that CHR could attenuate hippocampal neuronal cytoplasmic edema and increase memory and learning ability in mice.12 In addition, CHR could suppress hippocampal neuronal cell death via inhibition of Drp1-dependent mitochondrial fission.31 Moreover, CHR could improve the memory ability of the AD model rat by inhibiting tau hyperphosphorylation and has a neuroprotective effect.32 But the underlying mechanism of CHR functioning as a treatment for AD has not been elucidated.
In this study, the mRNA levels of CaM, CaMKK, CaMKIV, and tau in Aβ25-35-induced PC12 cells were significantly increased. CHR was added into Aβ25-35-induced PC12 cells and could significantly decrease the mRNA expression of CaM, CaMKK, CaMKIV, and tau. Similarly, CHR could also decrease the protein expression of CaM, CaMKK, CaMKIV, p-CaMKIV, and p-tau in Aβ25-35-induced PC12 cells. These results suggested that the neuroprotective effect of CHR is closely related to the down-regulation of CaM-CaMKIV and the expression of p-tau downstream (shown in Figures 4–6).
The above results of the analysis indicated that CHR reduces phosphorylation of tau in Aβ25-35-induced PC12 cells by inhibiting the CaM-CaMKIV protein family which can alleviate the damage of Aβ25-35-induced AD and has a good protection in neuronal cells. Thus, our findings suggested the potential use of CHR for the treatment of neuronal cell damage by mediating neurodegenerative diseases.
Conclusion
With pretreatment with CHR before exposure to Aβ25-35, PC12 cells showed increased cell viability and reduced apoptosis. mRNA levels of CaM, CaMKK, CaMKIV, and tau were tested by qRT-PCR, and protein expression of CaM, CaMKK, CaMKIV, p-CaMKIV, tau, and p-tau were tested by Western blotting. As a whole, our study highlights the point that CHR can effectively reduce the mRNA expression of CaM, CaMKK, CaMKIV, and tau in Aβ25-35-induced PC12 cells. Similarly, CHR can also reduce the protein expression of CaMKK, CaMKIV, p-CaMKIV, and p-tau. In conclusion, CHR had a nerve protective effect by regulating the expression of the p-tau protein through regulating CaMKIV in Aβ25-35-induced PC12 cells. This may be one of the protective mechanisms of CHR, which regulates Aβ25-35-induced neurotoxicity by regulating tau hyperphosphorylation and the CaM–CaMKIV signal pathway, thus providing a new mechanism for the prevention of AD.
Acknowledgments
We sincerely thank all the staff who participated in this study. This work was supported by the National Natural Science Foundation of China (Nos. 81574040 and 81873351), the Key Project Foundation of Support Program for the Excellent Young Faculties in Universities of Anhui Province in China (Nos. gxyq ZD2018053 and gxyg2019033), and the Key Natural Research Projects of Anhui University of Traditional Chinese Medicine (No. 2019zrzd01).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Patterson C. World Alzheimer Report 2018. The State of the Art of Dementia Research. London: New frontiers; 2018:32–36.
2. Hung AS, Liang Y, Chow TC, et al. Mutated tau, amyloid and neuroinflammation in Alzheimer disease-a brief review. Prog Histochem Cytochem. 2016;51:1–8. doi:10.1016/j.proghi.2016.01.001
3. Götz J, Schild A, Hoerndli F, et al. Amyloid-induced neurofibrillary tangle formation in Alzheimer’s disease: insight from transgenic mouse and tissue-culture models. Int J Dev Neurosci. 2004;22:453–465. doi:10.1016/j.ijdevneu.2004.07.013
4. Kirkitadze MD, Kowalska A. Molecular mechanisms initiating amyloid beta-fibril formation in Alzheimer’s disease. Acta Biochim Pol. 2005;52:417–423. doi:10.18388/abp.2005_3454
5. Fujisawa H. Regulation of the activities of multifunctional Ca2+/calmodulin-dependent protein kinases. J Biochem. 2001;129:193–199. doi:10.1093/oxfordjournals.jbchem.a002843
6. Müller M, Cárdenas C, Mei LJ, et al. Constitutive cAMP response element binding protein (CREB) activation by Alzheimer’s disease presenilin-driven inositol trisphosphate receptor (InsP3R) Ca2+ signaling. Proc Natl Acad Sci USA. 2011;108:13293–13298. doi:10.1073/pnas.1109297108
7. Wayman GA, Tokumitsu H, Davare MA, et al. Analysis of CaM-kinase signaling in cells. Cell Calcium. 2011;50:1–8. doi:10.1016/j.ceca.2011.02.007
8. McCullough LD, Tarabishy S, Liu L, et al. Inhibition of calcium/calmodulin-dependent protein kinase kinase β and calcium/calmodulin-dependent protein kinase IV is detrimental in cerebral ischemia. Stroke. 2013;44:2559–2566. doi:10.1161/STROKEAHA.113.001030
9. Kang H, Sun LD, Atkins CM, et al. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–783. doi:10.1016/S0092-8674(01)00497-4
10. Yin YL, Gao D, Wang YL, et al. Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc Natl Acad Sci USA. 2016;113:E3773–81. doi:10.1073/pnas.1604519113
11. Abd Al EN, Wesam M. The role of MAPK signaling pathway in selenium amelioration of high fat/high cholesterol diet-induced tauopathy in rats. Chem Biol Interact. 2019;302:108–116. doi:10.1016/j.cbi.2019.01.022
12. Zhang J, Yan C, Wang S, et al. Chrysophanol attenuates lead exposure-induced injury to hippocampal neurons in neonatal mice. Neural Regen Res. 2014;9:924–930. doi:10.4103/1673-5374.133141
13. Zhao YM, Fang YL, Li JC, et al. Neuroprotective effects of Chrysophanol against inflammation in middle cerebral artery occlusion mice. Neurosci Lett. 2016;630:16–22. doi:10.1016/j.neulet.2016.07.036
14. Li HC, Luo KX, Wang JS. Extrapyramidal side effect of donepezil hydrochloride in an elderly patient: a case report. Medicine. 2020;99(11):e19443. doi:10.1097/MD.0000000000019443
15. Haraguchi Y, Mizoguchi Y, Ohgidani M, et al. Monji A donepezil suppresses intracellular Ca2+ mobilization through the PI3K pathway in rodent microglia. J Neuroinflammation. 2017;214(1):258. doi:10.1186/s12974-017-1033-0
16. Weiner Michael W, Veitch Dallas P, Aisen Paul S, et al. 2014 update of the Alzheimer’s disease neuroimaging initiative: a review of papers published since its inception. Alzheimers Dement. 2015;11:e1–120.
17. Currais A, Prior M, Dargusch R, et al. Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in Alzheimer’s disease transgenic mice. Aging Cell. 2014;13:379–390. doi:10.1111/acel.12185
18. Ahmad A, Ali T, Park HY, et al. Neuroprotective effect of fisetin against Amyloid-beta-induced cognitive/synaptic dysfunction, neuroinflammation, and neurodegeneration in adult mice. Mol Neurobiol. 2017;54:2269–2285. doi:10.1007/s12035-016-9795-4
19. Zhang LJ, Tu RQ, Wang YW, et al. Early-life exposure to lead induces cognitive impairment in elder mice targeting SIRT1 phosphorylation and oxidative alterations. Front Physiol. 2017;8:446. doi:10.3389/fphys.2017.00446
20. Liu CM, Yang W, Ma JQ, et al. Dihydromyricetin inhibits lead-induced cognitive impairments and inflammation by the adenosine 5ʹ-monophosphate-activated protein kinase pathway in mice. J Agric Food Chem. 2018;66:7975–7982. doi:10.1021/acs.jafc.8b02433
21. Hudmon A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi:10.1146/annurev.biochem.71.110601.135410
22. Means AR. Regulatory cascades involving calmodulin-dependent protein kinases. Mol Endocrinol. 2000;14:4–13. doi:10.1210/mend.14.1.0414
23. Cao LL, Guan PP, Liang YY, et al. Cyclooxygenase-2 is essential for mediating the effects of calcium ions on stimulating phosphorylation of tau at the sites of Ser 396 and Ser 404. J Alzheimers Dis. 2019;68:1095–1111. doi:10.3233/JAD-181066
24. Wei YP, Ye JW, Wang X, et al. Tau-induced ca/calmodulin-dependent protein kinase-IV activation aggravates nuclear tau hyperphosphorylation. Neurosci Bull. 2018;34:261–269. doi:10.1007/s12264-017-0148-8
25. Šimić G, Babić Leko M, Wray S, et al. Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules. 2016;6:6. doi:10.3390/biom6010006
26. Song GH, Zhang Y, Yu SP, et al. Chrysophanol attenuates airway inflammation and remodeling through nuclear factor-kappa B signaling pathway in asthma. Phytother Res. 2019;33:2702–2713. doi:10.1002/ptr.6444
27. Xie L, Tang HL, Song JW, et al. Chrysophanol: a review of its pharmacology, toxicity and pharmacokinetics. J Pharm Pharmacol. 2019;71:1475–1487. doi:10.1111/jphp.13143
28. Park S, Lim W, Song G. Chrysophanol selectively represses breast cancer cell growth by inducing reactive oxygen species production and endoplasmic reticulum stress via AKT and mitogen-activated protein kinase signal pathways. Toxicol Appl Pharmacol. 2018;360:201–211. doi:10.1016/j.taap.2018.10.010
29. Shen CY, Jiang JG, Yang L, et al. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: pharmacological mechanisms and implications for drug discovery. Br J Pharmacol. 2017;174:1395–1425. doi:10.1111/bph.13631
30. Lin F, Zhang C, Chen XZ, et al. Chrysophanol affords neuroprotection against microglial activation and free radical-mediated oxidative damage in BV2 murine microglia. Int J Clin Exp Med. 2015;8:3447–3455.
31. Chae U, Min J-S, Leem HH, et al. Chrysophanol suppressed glutamate-induced hippocampal neuronal cell death via regulation of dynamin-related protein 1-dependent mitochondrial fission. Pharmacology. 2017;100(3–4):153–160. doi:10.1159/000477814
32. Ye T, Li X, Zhou P, et al. Chrysophanol improves memory ability of d-galactose and Aβ25-35 treated rat correlating with inhibiting tau hyperphosphorylation and the CaM-CaMKIV signal pathway in hippocampus. 3 Biotech. 2020;10(3):111. doi:10.1007/s13205-020-2103-z
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.