Back to Journals » Neuropsychiatric Disease and Treatment » Volume 12
The reduction of CSF tumor necrosis factor alpha levels in schizophrenia: no correlations with psychopathology and coincident metabolic characteristics
Received 25 May 2016
Accepted for publication 18 July 2016
Published 3 November 2016 Volume 2016:12 Pages 2869—2874
DOI https://doi.org/10.2147/NDT.S113549
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Wai Kwong Tang
Haibing Zhu,1 Danfeng Wang,2 Xiuqin Liu3
1Department of Psychiatry, Panyu Central Hospital, 2Department of Psychiatry, Guangzhou Psychiatric Hospital, 3Department of Nuclear Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China
Aim: The aim of this study was to assess whether tumor necrosis factor alpha (TNF-α) levels are correlated with the behavioral syndrome of schizophrenia and/or metabolic abnormalities.
Methods: Sixty patients with first-onset schizophrenia were recruited. The concentrations of TNF-α in the cerebrospinal fluid (CSF) were determined in 22 schizophrenia patients and ten patients with nonsuppurative appendicitis using a radioimmunoassay. Physiological characteristics such as fasting blood glucose, fasting insulin, triglycerides, corrected QT interval, waist circumference, and body mass index were measured prior to CSF collection. Subjects were screened for insulin resistance using the homeostasis model assessment. The extent of positive and negative behavioral symptoms was scored using the Positive and Negative Syndrome Scale.
Results: The CSF TNF-α levels in schizophrenic patients were significantly lower than those in the control group. The age of disease onset was positively correlated with the CSF TNF-α level using Pearson correlation analysis (r=0.37, P<0.05). There were no significant differences in CSF TNF-α levels in terms of age, duration of schizophrenia, or systolic and diastolic blood pressure. Furthermore, the CSF TNF-α levels were not significantly correlated with fasting blood glucose, fasting insulin, insulin resistance index, triglycerides, corrected QT interval, waist circumference, or body mass index. No significant correlation was found between CSF TNF-α levels and the Positive and Negative Syndrome Scale total scores or other factors scores. There were also no significant differences in CSF TNF-α levels between patients with schizophrenia types I and II.
Conclusion: CSF TNF-α levels are decreased in schizophrenia, although this reduction does not correlate with the psychopathology or coincident metabolic characteristics of this disease.
Keywords: schizophrenia, tumor necrosis factor alpha, cerebrospinal fluid, psychopathology, metabolic abnormalities
Introduction
Schizophrenia is a severe mental illness that affects ~781/100,000 persons in the People’s Republic of China.1 This is a complex disorder, and the pathogenesis remains unclear. In recent decades, accumulating neuroimmunological evidence has led to the hypotheses that autoimmunity or central nervous system infection contributes to the development of schizophrenia.2–5 Cytokines, such as tumor necrosis factor alpha (TNF-α), play an important immunomodulatory role in schizophrenia. Indeed, cytokines display immunological, neurochemical, and neuroendocrine activities that impact the brain, with consequent effects on behavior. Signs of inflammation and microglia activation in postmortem brains, cerebrospinal fluid (CSF), dysfunctional blood–brain barrier, the increased retroviral activity, are all strong indicators of the immunological basis of the etiopathology in schizophrenia. In psychiatric disorders, Bechter et al6 found signs of low level central nervous system inflammation in >40% of patients with affective and schizophrenic disorders using modern CSF analysis, and supported the mild encephalitis hypothesis in the pathogenesis of schizophrenia. The immune response of T-helper cells in schizophrenia in the literature is characterized by the imbalance between Th1 and Th2, pro- and anti-inflammatories, respectively, depending on the estimated type of cytokines. Whether the shift is toward Th1 or Th2 side is not confirmed yet. TNF-α is a low-molecular-weight polypeptide with multiple biological activities. Since its discovery in 1975 by Carswell et al,7 many studies on TNF-α have revealed physiological functions in addition to the antineoplastic activity for which it was named. On the one hand, as a proinflammatory cytokine, TNF-α mediates cellular immunity and participates in neuroimmunological regulation and autoimmune processes in the central nervous system, such as regulating growth, differentiation and repair; signaling feedback during early brain development of nerve cells and glial cells; and maintaining normal brain morphology.8,9 As an endogenous pyrogen, it can also influence sleep, feeding, exercise, and behavior.10 On the other hand, TNF-α can enhance the inflammatory reaction and immune response by activating the hypothalamic–pituitary–adrenal axis and by directly targeting the neuroendocrine system.11,12 Moreover, by binding to a brain opioid receptor to exert an endorphin-like effect, TNF-α also possesses the ability to attenuate insulin action and promote melanin generation.13
More recently, TNF-α has been implicated in the pathogenesis of schizophrenia.14,15 However, this relationship remains controversial. To date, studies focused on TNF-α have mainly utilized two approaches: TNF-α gene expression analysis (such as the G308A polymorphism in the upstream promoter of the TNF-α gene) and measurement of TNF-α protein levels in body fluids. Nevertheless, associations between polymorphisms in the TNF-α gene and schizophrenia have not been clearly demonstrated.8,16,17 Many studies have measured the serum levels of TNF-α; however, the results from studies taking this approach have also not been consistent.18–20 Studies measuring the level of TNF-α in the CSF are relatively rare, and these levels are commonly below the threshold of detection. The following factors are known to influence patients with schizophrenia: age, gender, body mass index (BMI), smoking, the first episode/relapse, stress, diagnostic criteria, past or current infections, current drug use, and others.19,21,22 Indeed, some studies have taken factors such as the patient’s condition, and the drug dosage and classification into account.23–25 After analyzing a variety of cytokines, Potvin et al26 demonstrated the importance of inflammatory symptoms in patients with schizophrenia.
Moreover, metabolic syndrome is frequently observed in patients with schizophrenia. Experts at the International Diabetes Federation have not only emphasized that central obesity and insulin resistance (IR) are important risk factors but also proposed that inflammatory indicators, such as TNF-α, may be related to metabolic syndrome and may predict the development of cardiovascular disease or diabetes. Indeed, evidence for the relationship between serum TNF-α levels and IR, central obesity, and high BMI has increased.27–31 In a variety of endocrine metabolic diseases, TNF-α can regulate insulin by influencing the insulin signaling pathway, which thus regulates obesity and IR. In a meta-analysis of schizophrenia and cytokine studies, Potvin et al26 also noted that weight gain should be given greater attention in the interpretation of the interaction between schizophrenia and TNF-α. However, no studies have evaluated the relationship between central obesity, IR, and TNF-α in schizophrenia.
In this study, we sought to verify the relationship between schizophrenia and the immune system by measuring the concentrations of TNF-α in the CSF. In particular, we assessed the relationship between changes in TNF-α and the psychopathology of schizophrenia, as well as any coincident metabolic characteristics such as obesity and IR.
Methods
Subjects
The study was conducted at Guangzhou Psychiatric Hospital from January 2011 to December 2012. Sixty patients with schizophrenia who were antipsychotic-naive were recruited, and a total of 50 apparently healthy controls were recruited during regular health screenings. All subjects were of Han Chinese descent, and all the enrolled patients met the International Classification of Diseases 10 (ICD 10) criteria for schizophrenic disorders. Patients and controls were excluded if they had a history of any neurological or immune disease, showed current substance abuse or dependence, or had used medications such as anti-inflammatory or antiviral agents before the study. Everyone involved must be in good physical health based on complete physical, neurological, and psychiatric evaluations, and none had somatic disorders, substance abuse, or dependence except nicotine. The patients were diagnosed and divided into type I or II schizophrenia according to the criteria by Crow.32
The patients gave informed consent to participate in the study. The institutional review board of Guangzhou Psychiatric Hospital approved the study protocol, and this study was performed according to the hospital’s code of conduct.
In accordance with the informed consent form, CSF was collected from 22 patients with schizophrenia. Control CSF was obtained from ten patients, including five males and five females, with nonsuppurative appendicitis who received lumbar spinal anesthesia before surgery.
Clinical data collections
General population data were collected, and obesity was determined according to waist circumference and BMI. IR was evaluated using the index of the homeostasis model assessment (HOMA-IR) based on the formula HOMA-IR = (fasting insulin [mU/L] × fasting plasma glucose [FPG mmol/L])/22.5. The IR percentile was obtained by translating this continuous variable into a categorical variable; IR was observed in one-quarter of the subjects regardless of subgroup.33 The Positive and Negative Syndrome Scale (PANSS) was used to assess the psychopathology of patients with schizophrenia at the time of admission. All patients with schizophrenia were divided into type I (n=15 patients) and II (n=7 patients) groups according to their main symptoms.31
Blood draw and TNF-α assays
All venous blood samples were collected using the standard venipuncture technique. Venous blood (5 mL) was drawn between 6.30 and 7.30 am after the subjects had fasted for 12 hours. Then, the blood was centrifuged at 2,600× g for 7 minutes to isolate plasma. The serum was stored at −76°C. The CSF and serum TNF-α were assayed using a radioimmunoassay in accordance with the manufacturer’s instructions (the Department of Nuclear Medicine in the First Affiliated Hospital, Sun Yat-sen University, Guangzhou, People’s Republic of China). All the assays were performed. The intra- and inter-assay coefficients of variation for TNF-α were 5% and 8%, respectively. The lower limit of detection for TNF-α was 0.3 ng/mL, according to the information provided by the supplier. All measurements were performed in duplicate and expressed as ng/mL.
Statistics
Categorical data, such as gender, were analyzed using the chi-squared test and Fisher’s exact test, if necessary. Case–control differences in continuous variables were evaluated using two-tailed independent samples t-tests or one-way analysis of variance, followed by the least significant difference multiple range test for between-group comparison. Data are presented as the mean ± standard deviation (SD). A general linear model was used to consider case–control differences after adjusting for other factors and covariates on transformed data. Correlations were calculated with Spearman’s correlation matrix. P<0.05 was considered statistically significant. For the correlations between CSF TNF-α levels and fasting blood glucose, fasting insulin, and IR parameters, the zero-order partial correlation coefficient test was applied.
Results
Twenty-two patients with schizophrenia (males/females: 14/8, mean age 30.48 [SD 8.91] years) and 50 controls (males/females: 25/25, mean age 30.47 [SD 7.98] years) were included; 22 patients and ten controls (including five males and five females, mean age 29.96 [SD 8.84] years) donated CSF. The mean duration of the illness was 3.4 years (range, 0.25–6 years). The mean age and the proportion of gender were similar between the two groups (P>0.05).
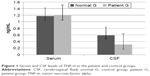
Comparison of serum and CSF levels of TNF-α in the patient and control groups
Figure 1 shows that the CSF TNF-α was lower in the patient group than in the control group (P<0.05). No significant difference in the serum level of TNF-α was observed between the patient group and the control group (P>0.05).
Analysis of CSF TNF-α levels and physiological characteristics within the group of patients with schizophrenia
CSF TNF-α were 0.35±0.18 ng/mL (14 cases) and 0.30±0.25 ng/mL (eight cases), respectively, in the male and female patients. No significant difference was found between the patients and control groups in terms of gender (P>0.05). The onset age was positively correlated with CSF TNF-α levels by Pearson correlation analysis (r=0.37, P<0.05). There was no significant difference on CSF TNF-α levels in terms of age, duration, systolic, and diastolic blood pressure, which was analyzed by Pearson correlation analysis in schizophrenia patients group (P>0.05).
With the Pearson correlation analysis, the results also indicated that the CSF TNF-α levels had no correlations with fasting blood glucose, FINS, IR index, triglycerides, corrected QT interval, waist circumference, and BMI (P>0.05).
Analysis of CSF TNF-α levels and psychopathology in schizophrenia
No significant correlation was found between CSF TNF-α levels and the PANSS total scores, P scores, N scores, or G scores (P>0.05). The schizophrenia type I (n=9) and II (n=13) groups showed PANSS total scores of 77.29±9.90 and 80.32±15.58, respectively, which indicated that the overall levels of disease severity were similar for the subgroups, and the data were comparable. The schizophrenia type I and II groups showed CSF TNF-α levels of 0.32±0.04 ng/mL and 0.30±0.05 ng/mL. There was no significant difference in CSF TNF-α levels between patients with schizophrenia type I and II (P>0.05).
Discussion
In neurological diseases, the cross talk of immune activation in peripheral blood versus CSF is of special interest as results may explain the involvement of the peripheral immune system to induce or control neuroinflammation. Even biomarker pattern analysis has led to a breakthrough in disease diagnosis and treatment.34 As reported earlier, we did not observe any difference in the serum levels of TNF-α between schizophrenic patients and healthy controls, but TNF-α levels were significantly lower in the CSF of patients with schizophrenia compared to healthy individuals. This finding in the serum is consistent with several earlier reports.4,21,23,24,26 It is not consistent with the other studies, such as Erbağci et al,22 Theodoropoulou et al,25 Upthegrove et al,35 Haack et al,36 and Naudin et al.37 They have found increased TNF-α in the serum of schizophrenic patients compared to normal control subjects. In the CSF, Paterson et al20 found that CSF TNF-α levels were higher in patients with schizophrenia compared to healthy controls. Even Maxeiner et al34 pointed out that affective and schizophrenic disorders clearly present with an inflammatory phenotype in the CSF and also in serum, the cytokines determined were, in general, higher in schizophrenia. Thus, our study suggests a reduction in the TNF-α level in the central nervous system of patients with schizophrenia. The concentrations of TNF-α indicating monocyte inflammation differed from the other findings. At this point, several other investigations should be considered. Sharief and Hentges38 report that TNF-α is important to the disease process in schizophrenia. In particular, these authors showed that TNF-α helps to restore the blood–brain barrier. Low levels of CSF TNF-α result from the inability of the cytokine to enter the central nervous system from the circulation, leaving only protein that is generated intrathecally. In general, the level of TNF-α is proportional to the abundance of functional CD4+ T-helper 1 (Th1) cells, and studies have shown a depletion of Th1 cells in schizophrenia with coincident enrichment in Th2 cells.39,40 Similar to Th1 cells, Th2 cells can also secrete TNF-α.41,42 Zhang et al43 suggested dysregulation in the cytokine system with schizophrenia and found that positive symptoms of schizophrenia may be linked to abnormally high levels of free radicals perhaps generated from dopamine (DA) metabolism and excessive DA release through an immune disturbance in cytokines, it remains unknown whether cytokines other than TNF-α also promote the pathological symptoms characteristic of schizophrenia and related psychiatric disorders. Moreover, it is not clear how unchanged TNF-α levels in the CSF is contributing to the schizophrenic phenotype. Müller et al,8 Potvin et al,26 and Kim et al44 mentioned that an imbalance of Th1 and Th2 cells was a critical factor related to the onset of the disease. Indeed, we infer that immune activation appears to be more restricted to CSF; insufficient TNF-α activity may explain the relatively weak cell-mediated immune response observed during the first episode and untreated schizophrenia, and support the hypothesis that mild inflammation is a unique characteristic of a subgroup of psychiatric diseases.
In addition to the age of disease onset, we did not observe correlations between the CSF TNF-α concentration and variables, such as gender, duration of schizophrenia, corrected QT interval, or PANSS score. Moreover, we did not observe a correlation between TNF-α and various metabolic parameters in patients with schizophrenia. It seemed to indicate a change of CSF TNF-α in the early stage of disease occurrence. But the age range of both the patients and the control group should be widened, also the number of participants should be increased dramatically to obtain a full picture of the ratios involved and produce clearer results and indicators. We must caution, however, that TNF-α may be important to neural and immune processes even if it is not related to the severity of psychosis and is not a good indicator of disease severity. While TNF-α may contribute to the early stages of brain degeneration or to the onset of abnormal neuroimmunologic reactivity, its relative abundance may not be directly related to the outward manifestations of schizophrenia.24,26,40 Some studies have proposed that TNF-α might participate in the pathophysiological processes of both central obesity and the occurrence of IR, which are closely related to metabolic diseases such as diabetes and cardiovascular disease. Grigsby and Dobrowsky45 proposed that TNF-α might interfere with insulin information transfer upstream of the insulin receptor, at the receptor, or downstream of the insulin receptor. In addition, Wärnberg et al28 suggested that obesity is related to TNF-α and other inflammatory factors in healthy Japanese adolescents. Park et al29 and Moon et al30 showed that the serum TNF-α level was significantly related to weight, waistline circumference, and BMI; therefore, interventions to reduce obesity may prevent increased levels of this cytokine. In 2008, Bo et al31 proposed that visfatin may increase the waist circumference and that TNF-α and inflammatory cytokines are involved in this process. However, our data showed no significant and direct correlations between CSF TNF-α and various metabolic abnormalities in patients with schizophrenia.
Current study showed that CSF TNF-α levels are decreased in schizophrenia, although this reduction does not correlate with the psychopathology or coincident metabolic characteristics of this disease. It would be useful to examine the postmortem brain literature for this protein/gene. There have been several known weaknesses in this study, due to the participation of only 22 enrolled schizophrenic samples and particularly small number of CSF subjects (n=10) in the control group. Another primary factor was cultural and laboratory resources that were unavailable to us during this study. Moreover, the levels of other proinflammatory cytokines such as interleukin 1 (IL 1) and interleukin 6 (IL 6) were not determined, and the association between serum TNF-α levels and physiological characteristics should also be examined.
Acknowledgments
This work was supported by Grant 2006-YB-114 and 2007A23 from the Medical and Health Science and Technologies in Guangzhou Fund Project, and by Grant 2007213 from the Guangdong Province Traditional Chinese Medicines Agencies Building Strong Scientific Research Projects. We thank Miss Xiao Lin, who kindly assisted with the preparation and proofreading of the manuscript.
Disclosure
No conflict of interest exists in the submission of this manuscript. The author does not have shares in any pharmaceutical company, nor do family members. The author does not accept any personal retainer from any pharmaceutical company.
References
Cai S, Lu H, Bai Z, Wu R, Zhao J. Paliperidone extended-release tablets in Chinese patients with schizophrenia: meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat. 2015;11:1817–1834. | ||
Tanaka S, Matsunaga H, Kimura M. Autoantibodies against four kinds of neurotransmitter receptors in psychiatric disorders. J Neuroimmunol. 2003;141(1–2):155–164. | ||
Eaton WW, Byrne M, Ewald H. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163(3):521–528. | ||
Brown AS. The risk for schizophrenia from childhood and adult infections. Am J Psychiatry. 2008;165(1):7–10. | ||
Koponen H, Rantakallio P, Veijola J, Jones P, Jokelainen J, Isohanni M. Childhood central nervous system infections and risk for schizophrenia. Clin Neurosci. 2004;254(1):9–13. | ||
Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321–330. | ||
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72(9):3666–3670. | ||
Müller N, Riedel M, Ackenheil M, Schwarz MJ. The role of immune function in schizophrenia: an overview. Eur Arch Psychiatry Clin Neurosci. 1999;249:462–468. | ||
Boin F, Zanardini R, Pioli R, Altamura CA, Maes M, Gennarelli M. Association between -G308A tumor necrosis factor alpha gene polymorphism and schizophrenia. Mol Psychiatry. 2001;6(1):79–82. | ||
Trapali M, Liapi C, Perelas, et al. Affect of isocaloric diets and sibutramine on food intake, body mass variation and serum TNF-alpha levels in rats. Pharmacology. 2008;82(1):15–21. | ||
Richards LJ, Chover, Gonzalez A, Harbuz MS, Jessop DS. Protective effects of endotoxin in a rat model of chronic inflammation are accompanied by suppressed secretion of pro-inflammatory cytokines and biphasic alteration in hypothalamo- pituitary-adrenal axis activity. J Neuroendocrinol. 2006;18(11):875–882. | ||
Kariagina A, Romanenko D, Ren SG, Chesnokova V. Hypothalamic-pituitary cytokine network. Endocrinology. 2004;145(1):104–112. | ||
Ko WC, Liu TP, Cheng JT, Tzeng TF, Liu IM. Effect of opioid mu-receptors activation on insulin signals damaged by tumor necrosis factor alpha in myoblast C2C12 cells. Neurosci Lett. 2006;397(3):274–278. | ||
Figiel I. Pro-inflammatory cytokine TNF-alpha as a neuroprotective agent in the brain. Acta Neurobiol Exp (Wars). 2008;68(4):526–534. | ||
Watanabe Y, Muratake T, Kaneko N, Fukui N, Nara Y, Someya T. No association between the tumor necrosis factor-alpha gene promoter polymorphisms and schizophrenia in a Japanese population. Psychiatry Res. 2007;153(1):1–6. | ||
Czerski PM, Rybakowski F, Kapelski P, et al. Association of tumor necrosis factor -308G/A promoter polymorphism with schizophrenia and bipolar affective disorder in a Polish population. Neuropsychobiology. 2008;57(1–2):88–94. | ||
Pae CU. Potential role of lymphotoxin-alpha (tumor necrosis factor-beta) in the development of schizophrenia. Med Hypotheses. 2007;68(6):1359–1362. | ||
Liu L, Jia FJ, Li HF. The mRNA expression levels of IL-1beta, TNF-alpha and tyrosine hydroxylase in peripheral blood of paranoid schizophrenic patients. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2007;23(11):1043–1045. | ||
Mittleman BB, Castellanos FX, Jacobsen LK, Rapoport JL, Swedo SE, Shearer GM. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. J Immunol. 1997;159(6):2994–2999. | ||
Paterson GJ, Ohashi Y, Reynolds GP, Pratt JA, Morris BJ. Selective increases in the cytokine, TNFalpha, in the prefrontal cortex of PCP-treated rats and human schizophrenic subjects: influence of antipsychotic drugs. J Psychopharmacol. 2006;20(5):636–642. | ||
Barak V, Barak Y, Levine J, Nisman B, Roisman I. Changes in interleukin-1 beta and soluble interleukin-2 receptor levels in CSF and serum of schizophrenic patients. J Basic Clin Physiol Pharmacol. 1995;6(1):61–69. | ||
Erbağci AB, Herken H, Köylüoglu O, Yilmaz N, Tarakçioglu M. Serum IL-1beta, sIL-2R, IL-6, IL-8 and TNF-alpha in schizophrenic patients, relation with symptomatology and responsiveness to risperidone treatment. Mediators Inflamm. 2001;10(3):109–115. | ||
Coelho FM, Reis HJ, Nicolato R, et al. Increased serum levels of inflammatory markers in chronic institutionalized patients with schizophrenia. Neuroimmunomodulation. 2008;15(2):140–144. | ||
Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. | ||
Theodoropoulou S, Spanakos G, Baxevanis CN, et al. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr Res. 2001;47(1):13–25. | ||
Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801–808. | ||
Zinman B, Hanley AJ, Harris SB, Kwan J, Fantus IG. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84(1):72–78. | ||
Wärnberg J, Nova E, Moreno LA, et al. AVENA Study Group. Inflammatory proteins are related to total and abdominal adiposity in a healthy adolescent population: the AVENA Study. Am J Clin Nutr. 2006;84(3):505–512. | ||
Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69(1):29–35. | ||
Moon YS, Kim DH, Song DK. Serum tumor necrosis factor-alpha levels and components of the metabolic syndrome in obese adolescents. Metabolism. 2004;53(7):863–867. | ||
Bo S, Ciccone G, Baldi I, et al. Plasma visfatin concentrations after a lifestyle intervention were directly associated with inflammatory markers. Nutr Metab Cardiovasc Dis. 2009;19(6):423–430. | ||
Crow TJ. Molecular pathology of schizophrenia: more than one disease process? Br Med J. 1980;280(6207):66–68. | ||
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. | ||
Maxeiner HG, Marion Schneider E, Kurfiss ST, Brettschneider J, Tumani H, Bechter K. Cerebrospinal fluid and serum cytokine profiling to detect immune control of infectious and inflammatory neurological and psychiatric diseases. Cytokine. 2014;69(1):62–67. | ||
Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155(1–3):101–108. | ||
Haack M, Hinze-Selch D, Fenzel T, et al. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res. 1999;33(5):407–418. | ||
Naudin J, Capo C, Giusano B, Mège JL, Azorin JM. A differential role for interleukin-6 and tumor necrosis factor-alpha in schizophrenia? Schizophr Res. 1997;29(2–3):227–233. | ||
Sharief MK, Hentges ER. Association between tumor necrosis factor-a and disease progression in patients with multiple sclerosis. N Engl J Med. 1991;325(7):467–472. | ||
O’Brien SM, Scully P, Dinan TG. Increased tumor necrosis factor-alpha concentrations with interleukin-4 concentrations in exacerbations of schizophrenia. Psychiatry Res. 2008;160(3):256–262. | ||
Yu B, Becnel J, Zerfaoui M, Rohatgi R. Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. J Pharmacol Exp Ther. 2008;327(2):16–23. | ||
Katsikis PD, Cohen SB, Londei M, Feldmann M. Are CD4+ Th1 cells pro-inflammatory or anti-inflammatory? The ratio of IL-10 to IFN-gamma or IL-2 determines their function. Int Immunol. 1995;7(8): 1287–1294. | ||
Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999;5(4):285–294. | ||
Zhang XY, Zhou DF, Qi LY, et al. Superoxide dismutase and cytokines in chronic patients with schizophrenia: association with psychopathology and response to antipsychotics. Psychopharmacology (Berl). 2009;204(1):177–184. | ||
Kim YK, Myint AM, Lee BH, et al. Th1, Th2 and Th3 cytokine alteration in schizophrenia. See comment in PubMed Commons below. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(7):1129–1134. | ||
Grigsby RJ, Dobrowsky RT. Inhibition of ceramide production reverses TNF-induced insulin resistance. Biochem Biophys Res Commun. 2001;287(5):1121–1124. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.