Back to Journals » Infection and Drug Resistance » Volume 10
The "black evil" affecting patients with diabetes: a case of rhino orbito cerebral mucormycosis causing Garcin syndrome
Authors Narayanan S , Panarkandy G, Subramaniam G, Radhakrishnan C, Thulaseedharan NK, Manikath N, Ramaswamy S, Radhakrishnan S, Ekkalayil D
Received 22 December 2016
Accepted for publication 27 February 2017
Published 28 March 2017 Volume 2017:10 Pages 103—108
DOI https://doi.org/10.2147/IDR.S130926
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Santhosh Narayanan,1 Geetha Panarkandy,1 Gomathy Subramaniam,2 Chandni Radhakrishnan,1 NK Thulaseedharan,1 Neeraj Manikath,1 Sreejith Ramaswamy,1 Suma Radhakrishnan,3 Danish Ekkalayil1
1Department of General Medicine, 2Department of Radiodiagnosis, 3Department of Otorhinolaryngology, Government Medical College, Kozhikode, Kerala, India
Abstract: Mucormycosis is a life-threatening infection affecting patients with diabetes. It is an angioinvasive disease often resistant to treatment with a debilitating course and high mortality. Here, we report a case of a 45 year old woman with type 2 diabetes mellitus who presented to us with history of right-sided ptosis and facial palsy, and subsequently developed loss of vision and palatal palsy. She was in diabetic ketoacidosis. Nervous system examination revealed involvement of right second, third, fourth, sixth, seventh, ninth, and tenth cranial nerves, suggestive of Garcin syndrome. The hard palate had been eroded with formation of black eschar. Computed tomography of paranasal sinuses revealed right maxillary and ethmoid sinusitis, with spread of inflammation to infratemporal fossa and parapharynygeal neck spaces. Debridement of sinus mucosa was done, and culture of the same yielded growth of rhizopus species. Histopathological examination of the tissue showed angioinvasion and fungal hyphae suggestive of mucormycosis. She was treated with amphotericin B, posaconazole, and periodic nasal sinus debridement, but her general condition worsened after 8 weeks due to secondary sepsis and she succumbed to death.
Keywords: diabetes, rhinoorbitocerebral, mucormycosis, garcin syndrome
Introduction
Rhinocerebral mucormycosis is an acute opportunistic mycosis that predominantly occurs in immunocompromised patients. In its early stage, it may masquerade as a less serious illness and often mimics sinusitis or orbital cellulitis. Imaging methods are of little use during the early stages of rhinocerebral mucormycosis, revealing only inflammatory changes in sinuses. Early diagnosis of rhinocerebral mucormycosis and initiation of prompt and effective treatment are very important for good outcome of the patient. Here, we report the case of a 45 year old lady with diabetes mellitus who presented with multiple unilateral cranial nerve palsies, suggestive of Garcin syndrome, which was eventually proven to be due to mucormycosis. Garcin syndrome is commonly seen with malignancies of head and neck, with mucormycosis being a rare cause.
Case report
Written informed consent was taken from the patient’s next of kin for the publication of this report and accompanying images. The institutional ethics committee of the Government Medical College, Kozhikode, Kerala, has provided permission to publish this report. (Ref no.GMCKKD/RP 2016/IEC/16/08)
A 45 year old woman with history of type 2 diabetes mellitus for 12 years and systemic hypertension for 2 years presented to us with complaints of drooping of right eyelid, drooling of saliva, and facial deviation to left side of 2 days duration (Figure 1).There was no history of headache, fever, altered sensorium, seizures, weakness of limbs, or sensory disturbances. After admission to our hospital, she developed complete loss of vision in the right eye, swelling and redness of right periorbital area, and coughing while having food. She was obese, and her body mass index was 30.3 kg/m2. She had no pallor, icterus, clubbing, lymphadenopathy, or pedal edema. Vital signs were within normal limits. Nervous system examination revealed congested retinal vessels in the right optic fundus, complete ptosis and ophthalmoplegia of right eye, and chemosis and proptosis of right eye, which was suggestive of involvement of second (optic), third (oculomotor), fourth (trochlear), and sixth (abducens) cranial nerves (Figure 2). She had reduced sensations in V1, V2 distribution of right trigeminal nerve. She also had lower motor neuron type of facial palsy on the right side. Movements and sensations were absent on the right side of palate, and the uvula was deviated to the left side. This was indicative of involvement of the ninth (glossopharyngeal) and tenth cranial nerve (vagus). Shrugging of shoulders and lateral neck movement was weak on right side (spinal accessory nerve involvement). She also developed right twelfth nerve (hypoglossal) palsy, with deviation of tongue to the right (Figure 3). Thus, there was involvement of ten cranial nerves on right side, with only the first and eighth cranial nerves being spared. There was a black eschar with erosion of the hard palate on the right side (Figure 4). Cranial nerves on the left side were normal. The sensorimotor system was normal. There were neither cerebellar signs (dysdiadochokinesia, dysmetria, dyssynergia) nor signs of meningeal irritation. Involvement of more than seven cranial nerves on the same side in the absence of raised intracranial tension and sensorimotor signs is called Garcin syndrome, and this is discussed in the following paragraphs. Other systems were normal.
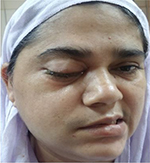  | Figure 1 Drooping of right eyelid, drooling of saliva, and facial deviation to left side. |
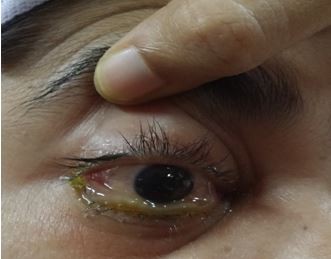
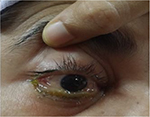  | Figure 2 Chemosis, proptosis and ophthalmoplegia of right eye. |
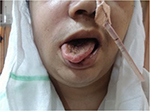  | Figure 3 Deviation of tongue to the right (right hypoglossal nerve palsy). |
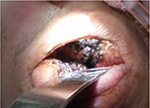  | Figure 4 Black eschar with erosion of the hard palate on the right side. |
Hematological and biochemical investigations revealed anemia, leucocytosis, and high blood sugar with ketoacidosis (Table 1).
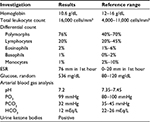  | Table 1 Hematological and biochemical investigations Abbreviation: ESR, erythrocyte sedimentation rate. |
Her electrocardiogram did not reveal any abnormalities. Chest X-ray was within normal limits.
Contrast-enhanced computed tomography of paranasal sinuses revealed right maxillary and right ethmoid sinusitis, with inflammation extending to zygomatic, pterygoid spaces, and infratemporal fossa and cavernous sinus (Figure 5). Culture of the debrided tissue from sinus mucosa yielded rhizopus growth in Sabouraud’s dextrose agar (Figure 6), and microscopy showed filamentous branching aseptate hyphae with sporangia (Figure 7). Histopathological examination confirmed the presence of angioinvasion by fungal hyphae (Figure 8).
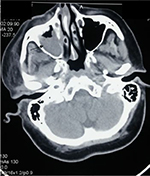  | Figure 5 Computed tomography of paranasal sinuses showing right maxillary sinusitis with spread of inflammation to infratemporal fossa. |
  | Figure 6 Rhizopus growth in Sabouraud’s dextrose agar. |
  | Figure 7 Filamentous branching aseptate hyphae with sporangia. Note: Magnification: 40×. |
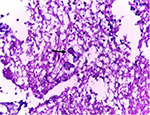  | Figure 8 Angioinvasion by fungal hyphae (arrow). |
She was treated with intravenous liposomal amphotericin B, posaconazole, and timely endoscopic nasal debridements. Blood sugar was controlled with insulin. She improved symptomatically after 6 weeks of treatment, as evidenced clinically by resolution of periorbital edema and chemosis. Unfortunately she developed fever, headache, and altered sensorium following the intracranial extension of the disease. Her hospital course was complicated by secondary bacterial sepsis, and she succumbed to her illness.
Discussion
Mucormycosis, also termed zygomycosis, is a fungal infection caused by organisms of the order mucorales, the genera of which consist mainly of rhizopus, mucor, and rhizomucor fungi.1 Patients affected with this infection always have some predisposing factor, with diabetes mellitus being the commonest.2 Other factors include hematological malignancies, glucocorticoid treatment, iron overload, and deferoxamine treatment. These microorganisms have the enzyme ketone reductase, which helps them grow in a medium with high glucose and acidity; hence, the special predilection toward individuals with diabetic ketoacidosis. Increased concentrations of free iron also supports the growth of these microorganisms in an acidic medium.3,4 Increased use of voriconazole as a prophylactic measure for aspergillosis has resulted in emergence of mucormycosis as an important infection in stem cell transplant recipients.5 Pathologically, mucormycosis is characterized by infarction and necrosis due to angioinvasion by hyphae.6
Guillain-Alajouanine-Garcin syndrome, or Garcin syndrome, was first described in 1926. It is defined by multiple ipsilateral cranial nerve palsies (at least 7) in the absence of long tract signs or raised intracranial pressure. Mutsukura et al7 have reported a case of Garcin syndrome in mucormycosis, in which the patient developed long tract signs due to internal carotid artery occlusion. This condition is very rare and is usually due to malignancies affecting the skull base and also due to primary nasopharyngeal carcinoma. Nonmalignant etiologies include pachymeningitis secondary to otitis media, rhinocerebral mucormycosis, leptomeningeal carcinomatosis, and aneurysm of the internal carotid artery.8 Tuberculous meningitis is a close mimicker of rhinocerebral mucormycosis as the former often presents as multiple cranial palsies as illustrated in a case reported by Yang et al.9
Rhinoorbitocerebral infection is the most common clinical presentation. Infection occurs through inhalation of fungal spores. Symptoms include acute sinusitis with fever, nasal discharge, headache. Spread to the surrounding structures – orbit, palate, infratemporal region, parapharyngeal region – occurs very rapidly. A blackish eschar occurs in the palate and nasal mucosa due to tissue necrosis. Downward spread of infection to the infratemporal fossa and parapharyngeal neck spaces results in ninth, tenth, eleventh, and twelfth cranial nerve palsies. Orbital involvement results in periorbital edema, chemosis, and orbital apex syndrome with the involvement of the third, fourth, and sixth cranial nerves. Blindness occurs when optic canal is invaded. Involvement of the trigeminal nerve and facial nerve occurs by direct perineural spread as well as due to infarction of the blood vessels supplying the nerve. Spread to cavernous sinuses and internal carotid artery occurs through sphenoid sinus.10 Intracranial extension occurs through ethmoid sinus and results in altered sensorium. Pulmonary, gastrointestinal, and cutaneous involvement are other presentations of mucormycosis. The pulmonary form presents with massive hemoptysis. Abdominal pain and hemetemesis are the features of the gastrointestinal type. Cutaneous infection is often associated with trauma and wounds in the immunocompromised, with cellulitis and tissue necrosis being the manifestations. These forms occur less commonly in diabetics and more commonly in patients with other risk factors.11,12
Mucormycosis is diagnosed by identification of organisms in culture and histopathological examination. Polymerase chain reaction based-techniques on specimens are under trial.13 Nasal endoscopy will provide an appropriate specimen for rhinocerebral infection. Pulmonary mucormycosis can mimic pneumonia. Bronchoalveolar lavage fluid culture often gives a better yield than sputum.14 The gastrointestinal form is diagnosed by endoscopic biopsy. Imaging modalities show inflammatory process in sinus and neck spaces. In MRI, this is evident as isointense lesions in T1-weighted images with absence of contrast enhancement. Nonenhancement of turbinate, also known as “black turbinate sign,” is a predictor of impending cavernous sinus thrombophlebitis.15 Treatment of rhinocerebral mucormycosis involves a combination of antifungal therapy and timely surgical debridement. Surgical debridement to remove all necrotic tissue should be done as soon as the disease is suspected, and it can become quite disfiguring if the disease has extensively spread. Antifungal therapy involves amphotericin B, preferably in liposomal formulation as it is less toxic to the kidneys. Duration of therapy has not been determined. As a general principle, antifungal therapy is continued until resolution of lesions or stabilization of lesions. Antifungal therapy is also continued during the course of neutropenia and throughout the immunosuppressive courses of corticosteroids. Treatment is guided by clinical response and can sometimes take many months. Oral posaconazole can be used as a substitute to amphotericin if the patient shows response to the latter, but it is not recommended as a first-line agent.16,17 Hyperbaric oxygen therapy has been reported to be promising in management of mucormycosis. It improves oxidative metabolism of phagocytic cells and increases oxygen tensions in ischemic tissue.18 Mucormycosis carries a high mortality rate. A few recent series19 have described a mortality of approximately 40% in diabetics with rhinocerebral mucormycosis. Prognosis is better if the disease has not penetrated beyond the sinus. Mortality rates are similar in patients with hematological malignancies and transplant recipients.20 Pulmonary and gastrointestinal forms carry an even higher mortality rate, as they occur mostly in neutropenic patients. The cutaneous forms have better prognosis. Disseminated mucormycosis has the worst prognosis as surgical debridement of infected tissues is often not feasible.
Conclusion
Mucormycosis is an aggressive and devastating fungal infection mainly affecting the immunocompromised patients. Garcin syndrome is a rare presentation of the rhinocerebral form, which affects multiple cranial nerves and has high morbidity. A high index of clinical suspicion is required for prompt diagnosis, as radiological investigations may not reveal any abnormality in the initial stages. Treatment involves an integrated approach with antifungal drugs, reversal of predisposing risk factors, and timely surgical debridement.
Disclosure
The authors report no conflicts of interest in this work.
References
Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. | ||
Lanternier F, Dannaoui E, Morizot G, et al. A global analysis of mucormycosis in France: the RetroZygo Study (2005–2007). Clin Infect Dis. 2012;54(Suppl 1):S35–S43. | ||
Gale GR, Welch AM. Studies of opportunistic fungi, Inhibition of Rhizopus oryzae by human serum. Am J Med Sci. 1961;241:604–612. | ||
de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994;47:1843–1850. | ||
Park BJ, Pappas PG, Wannemuehler KA, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg Infect Dis. 2011;17:1855–1864. | ||
Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S23–S34. | ||
Mutsukura K, Tsuboi Y, Imamura A, Fujiki F, Yamada T. [Garcin syndrome in a patient with rhinocerebral mucormycosis]. No To Shinkei. 2004;56:231–235. Japanese. | ||
Letournel F, Lejeune P, Josselin N, Barthelaix A, Dubas F. Malignant non-Hodgkin Lymphoma presenting with Garcin’s syndrome. Rev Neurol (Paris). 2004;160(10):952–955. | ||
Yang H, Wang C. Looks like tuberculous meningitis, but not: a case of rhinocerebral mucormycosis with garcin syndrome. Front Neurol. 2016;7:181. | ||
Del Valle Zapico A, Rubio Suárez A, Mellado Encinas P, Morales Angulo C, Cabrera Pozuelo E. Mucormycosis of the sphenoid sinus in an otherwise healthy patient. Case report and literature review. J Laryngol Otol. 1996;110:471–473. | ||
Warkentien T, Rodriguez C, Lloyd B, et al. Invasive mold infections following combat-related injuries. Clin Infect Dis. 2012;55:1441–1449. | ||
Xia ZK, Wang WL, Yang RY. Slowly progressive cutaneous, rhinofacial, and pulmonary mucormycosis caused by mucor irregularis in an immunocompetent woman. Clin Infect Dis. 2013;56:993–995. | ||
Hammond SP, Bialek R, Milner DA, Petschnigg EM, Baden LR, Marty FM. Molecular methods to improve diagnosis and identification of mucormycosis. J Clin Microbiol. 2011;49:2151–2153. | ||
Al-Abbadi MA, Russo K, Wilkinson EJ. Pulmonary mucormycosis diagnosed by bronchoalveolar lavage: a case report and review of the literature. Pediatr Pulmonol. 1997;23:222–225. | ||
Safder S, Carpenter JS, Roberts TD, Bailey N. The “black turbinate” sign: an early MR imaging finding of nasal mucormycosis. AJNR Am J Neuroradiol. 2010;31:771–774. | ||
van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42:e61–e65. | ||
Spellberg B, Walsh TJ, Kontoyiannis DP, John Edwards Jr, Ibrahim AS. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009;48:1743–1751. | ||
John BV, Chamilos G, Kontoyiannis DP. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect. 2005;11:515–517. | ||
Hong H-L, Lee Y-M, Kim T, et al. Risk Factors for Mortality in Patients with Invasive Mucormycosis. Infection & Chemotherapy. 2013;45(3):292–298. | ||
Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. Outcome of zygomycosis in patients with hematological diseases? Leukemia Lymphoma. 2004;45:1351–1360. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
