Back to Journals » International Journal of Women's Health » Volume 14
The Quality of Informed Consent in Caesarean Section at a Tertiary Hospital in Addis Ababa, Ethiopia
Authors Ababulgu SN , Ethiopia SS , Bekele D
Received 25 May 2022
Accepted for publication 25 August 2022
Published 19 September 2022 Volume 2022:14 Pages 1361—1369
DOI https://doi.org/10.2147/IJWH.S376037
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Sitra Nuredin Ababulgu,1 Samrawit Solomon Ethiopia,2 Delayehu Bekele3
1St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 2School of Public Health. St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 3Department of Obstetrics and Gynecology, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
Correspondence: Sitra Nuredin Ababulgu, Tel +251920157625, Email [email protected]
Purpose: The absence of high-quality and timely informed consent creates a barrier between the health-care provider and the patient that reinforces a negative view of the healthcare system, deters utilization of health-care services and increases malpractice lawsuits. This research aimed to assess the quality of informed consent in cesarean section (CS) at a large tertiary care center in Ethiopia.
Patients and Methods: An institutional cross-sectional study was conducted on 288 women who underwent planned or emergency CS. A structured questionnaire for respondents with standard indicators was developed as per the recommendations of the Royal College of Surgeons for the evaluation of the completeness of the informed consent document on the medical records.
Results: The median (IQR) age of the participants was 28 (25.0– 32.0) years and 203 (70.5%) has undergone emergency CS. More than half of the respondents 172 (59.7%) were unaware of who would perform the surgery and only 50 (17.4%) of respondents stated they were informed of complications of the CS. A total of 157 (56.3%) of responses fulfilled the criteria for adequate subjective informed consent with an affirmative response while only 109 (37.9%) of responses fulfilled the criteria for adequate objective informed consent. Only educational status of the patient was associated with subjective adequacy of informed consent with those who have some formal education having 2.05 times odds of having adequate subjective consent as compared to those with no formal education.
Conclusion: In this study, we have found that women undergoing CS receive inadequate informed consent. This inadequate informed consent occurs across planned and emergency CS. The results highlight the need for better consent process to increase patient awareness and promote patient-centered-care.
Keywords: quality, informed consent and cesarean section
Introduction
Informed consent is a mechanism by which a health-care provider advises a patient on the risks, benefits, complications and alternatives of a given procedure or intervention enabling a patient to make a voluntary and informed decision as to whether to undergo a procedure or an intervention.1,2 It is an ethical and legal obligation of medical practitioners as treatment cannot be provided without the valid consent of a competent adult.1–3 This is in line with the principle of autonomy in the ethics that govern healthcare.1,4
The decision of performing a cesarean section (CS) must be followed by a legitimate informed consent from the patient or her guardian. However, informed consent documents are frequently generic, containing legal-approved language that complies with laws and hospital policies.4,5 The documents are often used to affirm consent and thereby minimize risks for litigation rather than provide meaningful information that is specific to the procedure or the patient.5,6
The information is often shared minutes before the start of a procedure, when patients are vulnerable and less likely to ask questions, leaving little room for informed decision-making.4,7 Little or no explanation is given regarding the indication for surgery, procedure-related risk, or the postoperative trajectory, leaving patients vulnerable.7
Informed consent is particularly important in obstetrics as the explanation of procedures and seeking consent is associated with an improved rating of the birth experience, while non-consented care is seen as a deterrent to skilled birth care utilization.7,8
Researches on the quality of informed consent concerning obstetric interventions are scanty in developing countries.9–11 This research aims to assess the quality of informed consent in women undergoing CS on both emergency and planned basis and to identify factors associated with quality consent.
Materials and Methods
Study Design and Setting
An institutional-based cross-sectional study was carried out from September to November 2021 at St. Paul’s Hospital Millennium Medical College, a tertiary level hospital in Addis Ababa, Ethiopia.
Population and Sampling Techniques
The study populations were women who underwent emergency or planned CS during the study period. The sample size was determined using a formula for single proportion, N = Zα/22 *p*(1-p)/MOE2, and Zα/2 is the critical value of the normal distribution at α/2, MOE is the margin of error, p is the sample proportion, and N is the population size. A prior study had shown 62.4% of women report having counseling on their condition before giving consent.12 Thus, using a margin of error 0.05, a power of 80% and correcting for a non-response rate of 5% the sample size was determined to be 288. All consecutive patients who underwent CS were included till the target sample size was achieved.
Data Collection Tools, Quality Assurance and Pretest
A pre-tested structured questionnaire for the interview of patients and evaluation of patient medical records was used for data collection (Supplementary File). The questionnaire for subjective components of informed consent was prepared based on the recommendations of the Royal College of Surgeons on Consented Care.13 The parameters for the evaluation of patient records were developed from the existing informed consent form used at St. Paul’s Hospital Millennium Medical College. The questionnaire consisted of 3 parts; 1) Basic Demographic Information; 2) Subjective Components of informed consent, and 3) Objective Assessment of informed consent.
The original questionnaire was prepared in English and translated to Amharic, the national language used widely in the area. The Amharic version was then back translated into English to check for consistency. Prior to the data collection, 2 rounds of pretest were done in a comparable setting, and the necessary amendments were made to refine the questionnaire based on the results. Final year medical students collected data after receiving training on the data collection tools. Moreover, 10% random sample was taken weekly by the research team and cross referenced with the respective cards to check for completeness, accuracy and clarity of the collected data. The institutional ethical review board of St. Paul’s Hospital Millennium Medical College gave ethical clearance prior to the commencement of data collection. Written informed consent was obtained from each study participant. The study was conducted in compliance with the Declaration of Helsinki.
Variables and Measurement
The dependent variable was adequacy of informed consent; the independent variables included socio-demographic variables that comprise age, educational status, marital status, place of residence and occupation. Due to the lack of universally set standards for high quality informed consent in cesarean section, for the purpose of this research we have put an operational definition of adequate subjective assessment as an affirmative response in 10 and above out of 20 components and an affirmative response of 7 and above out of 9 components on as adequate objective assessment of informed consent.
Data Analysis
Data was entered using EPi Info version 3.5.3 and imported into, cleaned and analyzed using IBM SPSS Statistics 23 for Microsoft Windows. Mean (±SD) was calculated for continuous variables while percentages were calculated for categorical (nominal) variables. The Student’s t-test was used to assess the mean differences between groups while the chi-square test was used to determine the association between categorical variables. A binary logistic regression model was fitted for the binary outcome (adequacy of informed consent) to control for other confounding variables. First, a univariate analysis was performed. Consequently, those with a p-value ≤0.2 were included in multivariable model. Results were presented in tables and narrative forms. A p-value of <0.05 was used as a threshold to declare statistical significance.
Results
Socio-Demographic Characteristics of Study Participants
A total of 299 participants were eligible for the study during the study period. Of these, 11 women declined to take part in the study, giving a 96.3% response rate. All patient charts were available for data collection. The median age of the participants was 28 with an age range of 18–45. Over fifty percent of them were in the age group 25–30. One-fifth (n=57: 19.8%,) received no formal education and nearly half 124 (43.1%) reported they were housewives. The majority 272 (94.4%) were married and nearly three-quarters (72.6%, n = 209) were from Addis Ababa (Table 1).
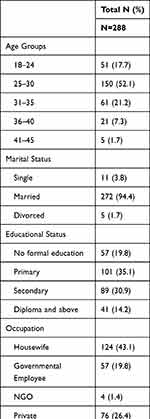 |
Table 1 Characteristics of Study Subjects |
Informed Consent
Subjective Assessment of Informed Consent
Prior to their CS operation, 261 (90.6%) of the 288 respondents understood what the procedure was, and 235 (81.6%) knew about informed consent paperwork. The majority of respondents (96.9%) said they were asked to grant consent prior to procedure, however 173 (60.9%) said they were not given an overview of the surgery. Furthermore, more than half of the respondents 172 (59.7%) were unaware of who would perform the surgery, its estimated duration 199 (69.1%), alternative choices 193 (67%), or the type of anesthesia 173 (60.1%) (Table 2).
 |
Table 2 Participants’ Response to Subjective Assessment of Informed Consent |
Eighty-four percent (243) of respondents were informed why a CS was necessary, and 223 (77.4%) reported they understood why. Concerning the information provided on complications of having a CS, 224 (77.8%) said they had not been informed of any risks. However, of the 50 (17.4%) who said they were informed of complications, the most frequently noted complication was postoperative bleeding and the requirement for transfusion 18 (37.5%), followed by an increased risk of thrombosis 11 (22.92%).
Over half 157 (54.5%) of respondents said they were not given enough time to decide and sign informed consent documents, and 177 (61%) said they were not given an opportunity to ask questions about the CS. Furthermore, 230 (80%) said that the setting was unfavorable to refuse the CS. Overall 162 (56.3%) of the study participants fulfilled the criteria for adequate subjective informed consent.
Overall, a total of 157 (56.3%) of responses fulfilled the criteria for adequate subjective informed consent with an affirmative response in 10 and above out of 20 components. On univariable logistic regression age, marital status, education, occupation, place of residence and type of CS was evaluated. Educational status, occupation, place of residence and type of CS were found to be associated with subjective informed consent at a P-value of 0.2. Multivariable logistic regression was employed on these variables and only education was found to be associated with receiving quality informed consent. Those with some formal education has a 2.05 times odds of having an adequate subjective consent compared to those with no formal education (Table 3).
 |
Table 3 Multivariable Association of Baseline Variables with Patient-Reported Adequacy of Informed Consent |
Objective Assessment of Informed Consent
Regarding the objective assessment of informed consent, the full name and age of the patient was recorded in 270 (93.8%) and 256 (88.9%) of informed consent documents, respectively. In addition, three quarters of the documents stated the name of the surgery as well as the reason for the surgery and 260 (90.3%) of the documents contain the name and signature of the surgeon. Similarly, the patient’s signature was found on 279 (96.1%) of the informed consent forms. In the objective assessment of informed consent 109 (37.8%) of documents fulfilled the criteria for adequacy with an affirmative response of 7 and above out of 9 components (Table 4).
 |
Table 4 Objective Assessment of Informed Consent |
A total of 109 (37.9%) of responses fulfilled the criteria for adequate objective informed consent. On univariable logistic regression marital status, education, occupation, place of residence and type of CS was evaluated age and occupation were associated with objective informed consent at a p-value of 0.2 but on multivariable logistic regression no factors were found to be associated (Table 5).
 |
Table 5 Multivariable Association of Baseline Variables with Objective Adequacy of Informed Consent |
Consent in Emergency versus Planned CS
In terms of planned versus emergency cesarean section, our findings show that 107 (52.7%) of the 203 women who underwent emergency CS met the criteria for adequate subjective informed consent, while 55 (64.7%) of women who underwent planned CS also met the criteria for adequate subjective informed consent. However, in terms of objective informed consent only 77 (37.9%) of emergency CS respondents and 32 (37.6%) of planned respondents fulfilled the criteria. On univariable logistic regression type of surgery (planned versus emergency) was associated with subjective adequacy of informed consent. However, on multivariable logistic regression no factors were found to be associated.
Discussion
Informed consent is an ethical and legal obligation of medical practitioners to obtain valid consent of a competent and informed adult in order to proceed with a given treatment and/or intervention.1,4,6,14,15 Obstetric patients pose multiple challenges to the provision of adequate informed consent; however, explanation of procedures and seeking consent are associated with improved rating of birth experience, while non-consented care is seen as a deterrent to skilled birth care utilization.7,8
The results of our study showed that a majority of the respondents (90.6%) knew what a CS was and 84% stated that they were informed the indication for the cesarean section. The results are similar to a study done in Nigeria which found that 93% knew what a CS was and 87% were informed of the indication.14 A study in India also yielded similar response with 97.4% and 91.3% response in each question, respectively.15 This shows that communication of the reason for the cesarean section was carried out prior to the CS. Regarding the communication of the risks and complications of the caesarean section, in this study only 17.4% of respondents stated that they were informed of complications related to the CS. This is much lower when compared to a study done in Malawi and India where 31.3% and 29% of respondents, respectively, received information on the risks of CS.7,15 This indicates that there are important gaps in communication during the informed consent process. This can have significant legal implications if the patient has any intraoperative or postoperative complications.6 One possible explanation that can be given is the urgency of care provision that is required in managing most obstetric patients. Another explanation is the informed consent document itself, which is not specific to any procedure and seems merely to avoid legal liability than to aid in patient decision-making. Preparing a checklist or guide for counseling that contains information on information that should be provided such as type of anesthesia, estimated time, and common complications can be one possible solution.16
In relation to the planned versus emergency cesarean section and the provision of informed consent, our study found that there was no statistically significant relationship between patients who had planned caesarian section versus emergency. This is similar to a study in Hawassa on obstetric and gynecologic surgery patients that showed that the schedule of surgery had no statistically significant association with the number of components received.12 This suggests that the emergent nature that differentiates planned and emergency CS did not have an impact on provision of informed consent. This indicates gaps in how health professionals provide informed consent, suggesting a basic disregard for the importance of the informed consent process.
Fifty four percent of respondents that took part in this study stated that they were not given adequate time to decide whether to undergo the surgery and 81% stated that the environment was unfavorable to refuse the surgery. This is similar to another study done in Hawassa University Hospital, where 69.1% stated that they were not given adequate time and 93.4% stating that the environment was unfavorable to decline the surgery.12,17 In comparison, a study was done in the Zambia where 50% were informed of their right to decline the procedure.18 The reasons for this difference could be time constraints, need for immediate decision in certain cases with regard to individual clinical conditions and a stressful environment for both the patient and health-care professional that would have made provision and comprehension of informed consent challenging.
Moreover, this study’s inconsistent association with subjective and objective adequacy. This is a possible indicator of how health-care provider-related factors play a significant role in the observed inadequacy of informed consent suggesting the possible global nature of the problem. Further research is needed to investigate this point.
Conclusion
In conclusion, our findings indicate that women who undergo cesarean section receive inadequate informed consent with inconsistent associations. It can be concluded that efforts need to be made to improve the consent process for CS in order to increase patient awareness and promote patient-centered-care.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Glennon K, Tower C, Gillham J, Myers J. An audit of informed consent for cesarean section and instrumental delivery in a tertiary referral center in the United Kingdom. Clin Audit. 2011;3:1–5. doi:10.2147/ca.s13413
2. Ochieng J, Ibingira C, Buwembo W, et al. Informed consent practices for surgical care at university teaching hospitals: a case in a low resource setting. BMC Med Ethics. 2014;15(1). doi:10.1186/1472-6939-15-40
3. Paterick TJ, Carson GV, Allen MC, Paterick TE. Medical informed consent: general considerations for physicians. Mayo Clin Proc. 2008;83(3):313–319. doi:10.4065/83.3.313
4. Drolet B, Brower J. I. Consent and the reasonable-patient standard. JAMA. 2016;316(9):993. doi:10.1001/jama.2016.10558
5. Spatz E, Suter L, George E, et al. An instrument for assessing the quality of informed consent documents for planned procedures: development and testing. BMJ Open. 2020;10(5):e033297. doi:10.1136/bmjopen-2019-033297
6. Watkins R. Understanding quality improvement is more important now than ever before. N C Med J. 2014;75(3):220–223. doi:10.18043/ncm.75.3.220
7. Zethof S, Bakker W, Nansongole F, Kilowe K, van Roosmalen J, van den Akker T. Pre-post implementation survey of a multicomponent intervention to improve informed consent for caesarean section in Southern Malawi. BMJ Open. 2020;10(1):e030665. doi:10.1136/bmjopen-2019-030665
8. Spatz ES, Bao H, Herrin J. Quality of informed consent documents among us. Hospitals: a cross-sectional study. BMJ Open. 2020;10(5):e033299. doi:10.1136/bmjopen-2019-033299
9. Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF. Ethiopia Mini Demographic and Health Survey 2019: Key Indicators. Rockville: EPHI and ICF; 2019.
10. Abubeker F, Gashawbeza B, Gebre T, et al. Analysis of cesarean section rates using Robson ten group classification system in a tertiary teaching hospital, Addis Ababa, Ethiopia: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1). doi:10.1186/s12884-020-03474-x
11. Tripathy S, Shubhashree T, Sajeetha Kumari R, Mohapatra S. Informed consent process before caesarean section: a study of patient’s perspective regarding adequacy of consent process. Indian J Obstet Gynecol Res. 2020;7(2):239–242. doi:10.18231/j.ijogr.2020.049
12. Teshome M, Wolde Z, Gedefaw A, Tariku M, Asefa A. Surgical informed consent in obstetric and gynecologic surgeries: experience from a comprehensive teaching hospital in Southern Ethiopia. BMC Med Ethics. 2018;19(1). doi:10.1186/s12910-018-0293-2
13. Royal College of Surgeons of England. Good Surgical Practice domain 3: communication, partnership and teamwork; 2014. Available from: https://www.rcseng.ac.uk/standards-and-research/gsp/domain-3/.
14. Coulter A, Entwistle V, Gilbert D. Sharing decisions with patients: is the information good enough? BMJ. 1999;318(7179):318–322. doi:10.1136/bmj.318.7179.318
15. Nanda S, Duhan N, Malik R. Study of adequacy of informed consent in caesarean section in a tertiary care, teaching and research institute of Northern India. Int J Reprod Contracept Obste. 2015;4(3):780–785. doi:10.18203/2320-1770.ijrcog20150091
16. Swende TZ. Emergency caesarean section in a Nigerian tertiary health centre. Niger J Med. 2008;17(4). doi:10.4314/njm.v17i4.37419
17. Chane W, Birhanu B, Suga Y. quality of informed consent among patients who underwent major surgical procedure in a tertiary care hospital, Addis Ababa, Ethiopia. Open Access Surg. 2020;13:27–33. doi:10.2147/oas.s250532
18. Lubansa DC. A study of adequacy of informed consent for caesarean section at the university teaching Hospital [Dissertation]. Lusaka: University Teaching Hospital; 2012.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
