Back to Journals » Cancer Management and Research » Volume 12
The Prognostic Value of Combination of Plasma Fibrinogen and CA19-9 in Non-Distant Metastatic Breast Cancer Patients Undergoing Surgery
Authors Hu W , Zheng C, Quan R, Dai X, Zhang X
Received 8 July 2020
Accepted for publication 13 August 2020
Published 23 September 2020 Volume 2020:12 Pages 8875—8886
DOI https://doi.org/10.2147/CMAR.S270385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Wenjing Hu, Chen Zheng, Ruida Quan, Xuanxuan Dai, Xiaohua Zhang
Department of Surgical Oncology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China
Correspondence: Xiaohua Zhang; Xuanxuan Dai
Department of Surgical Oncology, The First Affiliated Hospital of Wenzhou Medical University, 3 Fuxue Road, Lucheng, Wenzhou, Zhejiang 325000, People’s Republic of China
Tel +86 577 5557 9462
Email [email protected]; [email protected]
Purpose: This article aimed to study the prognostic value of preoperative plasma fibrinogen and CA19-9 in non-distant metastatic breast cancer (BC).
Patients and Methods: A total of 343 non-distant metastatic BC patients were included in this study. The optimal cut-off values of plasma fibrinogen and CA19-9 were obtained by receiver operating characteristic (ROC) curve analysis. Univariate and multivariate Cox regression analyses were used to evaluate prognostic factors for overall survival (OS). Survival data were assessed using Kaplan–Meier survival analysis with the Log-rank test. Based on the cut-off values, we classified the fibrinogen-CA19-9 score as follows: 2 (both hyperfibrinogenemia and high CA19-9), 1 (either hyperfibrinogenemia or high CA19-9), and 0 (neither hypefibrinogenemia nor high CA19-9).
Results: Our follow-up time totaled 10 years, the median follow-up time was 77 months (range=2– 119 months), and 82 (23.9%) of 343 patients died during the follow-up period. The optimal cut-off values of plasma fibrinogen and CA19-9 were 2.805 g/L and 11.85 U/mL, respectively. The multivariate Cox analysis results suggested that there was a significant association between worse OS and elevated preoperative plasma fibrinogen and CA19-9 levels (HR=2.016, 95% CI=1.216– 3.342, P=0.007; and HR=2.042, 95% CI=1.282– 3.253, P=0.003). The area under the ROC curve (AUC) increased from 0.589 (for plasma fibrinogen) and 0.594 (for CA19-9) to 0.640 when these two parameters were combined. When we added this combined factor to the multivariate analysis, it was an independent prognostic factor for BC (P< 0.001). According to the above results, we chose four prognostic factors to construct our nomogram. The AUC was 0.724, which indicates that the nomogram performs well.
Conclusion: The combination of plasma fibrinogen and CA19-9 could be used as a valid independent prognostic factor for non-distant metastatic BC compared with either parameter alone and could easily be applied in clinical practice.
Keywords: non-distant metastatic BC, fibrinogen, CA19-9, survival, nomogram
Introduction
Breast cancer (BC) has gradually become the most common malignancy affecting women’s health, accounting for nearly 30% of new cancer cases in women according to a report.1 In China, approximately 270,000 women were diagnosed with BC per year.2 With the development of the medical industry, the prognosis of BC patients has been enhanced by comprehensive treatment based on surgery.3,4 At the same time, a number of prognostic factors of BC and biomarkers have been proven to relate to BC.5 But the high lethality is still worrying, BC has caused 570,000 deaths in 1 year.6 Therefore, our understanding of BC prognosis still needs to be explored further.
Recent studies have shown that the coagulation system plays a key role in cancer progression. Fibrinogen is a 340-kDa glycoprotein in plasma consisting of three pairs of polypeptide chains (Aα, Bβ, and γ), cycles at a rate of 2–3 mg/mL.7 Cancer cells can secrete vascular endothelial growth factor (VEGF), which will result in continuous extravasation of fibrinogen.8 High fibrinogen level was defined correlating with adverse prognostic outcome in various malignancies,9 digestive system cancer cells,10–13 respiratory system cancer cells,14,15 and gynecological cancer cells.16–19 To our knowledge, a previous study showed a fibrinogen-to-albumin ratio (FAR) was significantly linked to prognostic factor in BC.20 CA19-9 is a tumor biomarker, which is used as an auxiliary tool for clinical diagnosis. The study on the prognostic value of CA19-9 in BC is rare. Xu et al21 put forward that the combination of preoperative fibrinogen and CA19-9 has a good ability to predict prognosis in gallbladder cancer patients. Based on the above knowledge, we guessed that the combination of plasma fibrinogen and CA19-9 may also improve our ability to estimate the risk of progression and increase prognostic accuracy in non-distant metastatic BC patients.
All in all, this article aimed to evaluate the roles of the combination plasma fibrinogen and CA19-9 in predicting prognostic in non-distant metastatic BC. In the meantime, we expected that the combination of these two common parameters can provide a basis for individualized treatment plans for postoperative BC treatment.
Materials and Methods
Patients Selection
This study was a regression cohort study. We retrospectively analyzed the medical records of female BC patients who underwent radical mastectomy in the First Affiliated Hospital of Wenzhou Medical University, from January 2010 to December 2014. The enrollment criteria were: 1) complete baseline data; 2) without any preoperative treatment; 3) no clinical evidence of distant metastasis; 4) no history of other cancers; and 5) non-ductal carcinoma in situ. The clinic ethics committee approval was supported by the ethics committees of The First Affiliated Hospital of Wenzhou Medical University. This study was conducted in light of the Declaration of Helsinki.
Data Collection
The information of enrollment patients were obtained from hospital databases, and their outpatient records were extracted for data abstraction. Baseline characteristics including age, pathological diagnosis, TNM stage, hormone receptor, and human epidermal growth factor receptor-2 (HER2) status, adjuvant therapy, preoperative plasma fibrinogen, CA19-9, CEA, CA125, and CA15-3 level, and date of last follow-up or death were collected.
The hematological parameters were measured within 3 days before surgery. Fasting blood (2 mL) was collected from the cubital vein in the morning and serum was collected by centrifugation. CA19–9, CEA, CA125, and CA15-3 were detected using an electrochemiluminescence immunoassay system (Elecsys, Roche, Basel Switzerland) according to the manufacturer’s instructions. The fibrinogen concentration was measured based on the Clauss22 method. The reference range was 2–4 g/L for plasma fibrinogen, 0–35 U/mL for CA19–9, 0–9 ng/mL for CEA, 0–35 U/mL for CA125, and 0–31.3 U/mL for CA15-3. The pathological grading was assessed according to the TNM staging system of the AJCC 7th edition based on imaging results and postoperative pathological examination. The status of Estrogen receptor (ER), progesterone receptor (PR), and HER2 were defined referencing to the results of immunohistochemistry (IHC). Patients with 2+IHC staining for HER2 need further use fluorescence in situ hybridization (FISH) to determine whether they were positive or negative. Survival duration was counted from the date of surgery, and overall survival was defined as the period until the day of death or the last follow-up date. The survival information was obtained by soliciting patients on the telephone or inquiring medical records. The last follow-up was in December 2019.
Statistical Analysis
Overall survival (OS) refers to the time from surgery to death because of any cause or the last follow-up. The patients’ characteristics were analyzed by descriptive statistics and crosstab. Chi-square analysis was also performed to count differences in categorical variables. By calculating the Youden index using the receiver operating characteristic (ROC) curve to define the cut-off threshold for plasma fibrinogen and CA19-9, the area under the curve (AUC) was used to calculate the ability to predict. Meanwhile, Cox proportional hazards regression methodology was used to conduct the univariate and multivariate analyses, whose consequence can reveal the effect of potential confounders on OS. Survival data was obtained using the Kaplan–Meier survival analysis with Log-rank test. The nomogram was conducted using the statistical package R (the R foundation; http://www.r-project.org;version3.4.3). P<0.05 was deemed statistically significant. Data analysis was performed using GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA) and SPSS version 26.0 (SPSS, Inc, Chicago, IL, USA) in this study.
Results
Patient Clinic Characteristics
In the light of the inclusion criteria, this study included 343 consecutive patients histopathological diagnosed with BC between 2010 and 2014 in The First Affiliated Hospital of Wenzhou Medical University. Detailed baseline characteristics of enrolled patients are shown in Table 1. The median follow-up time was 77 months (range=2–119 months), and death occurred 82 (23.9%) of the 343 BC patients. The median age was 45, 18.9% patients’ OS <60 (64 in 343). Approximately 91.8% of patients had invasive ductal carcinoma (315, 91.8%). In total, 175 cases of T1 (51%), 156 cases of T2 (45.5%), six cases of T3 (1.7%), and six cases of T4 (1.7%) stage were determined by postoperative pathology. Meanwhile, pathological N stage was 178 (51.9%), 125 (36.4%), 16 (4.7%), and 24 (7%) patients for N0, N1, N2, and N3 stage, respectively. Among the 343 patients, 110 (32.1%), 187 (54.5%), and 46 (13.4%) were classified as TNM stages I, II, and III, respectively. ER status was available for 95% of cases, with 204 ER-positive (59.5%), 122 ER-negative (35.6%), and 5% remained unknown. PR status varied from positive (169, 49.3%) to negative (158, 46.1%) to unknown (16, 4.7%). About 85 (24.8%) of all subjects are positive for HER2 status.
 |
Table 1 Baseline Characteristics of 343 Non-Metastatic Breast Cancer Patients, n (%) |
Correlations Between Plasma Fibrinogen and Clinicopathological Characteristics
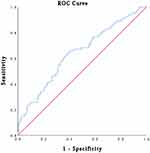
The optimal cut-off value for plasma fibrinogen calculating by the ROC curve was 2.805 g/L (sensitivity of 0.659 and specificity of 0.51) (Figure 1A); the AUC was 0.589 (95% CI=0.516–0.661). Patients were classified into two groups according to the cut-off: group A, hyperfibrinogenemia ≥2.805 g/L, included 182 patients (53.1%); group B, hypofibrinogenemia <2.805 g/L, included 161 patients (46.9%) (Table 1). As shown in Table 2, an elevated plasma fibrinogen level was significantly correlated with age (P<0.001), HER2 status (P=0.029), CEA level (P=0.038), and ki67 (P=0.003).
 |
Table 2 Association Between Fibrinogen Concentration Level and Clinicopathological Characteristics in Breast Cancer Patients |
Associations Between Preoperative CA19-9 and Clinicopathological Characteristics
The optimal cut-off value for CA19-9 intercepted from the ROC curve was 11.85 U/mL (sensitivity of 0.439 and specificity of 0.747) (Figure 1B); the AUC was 0.594 (95% CI= 0.521–0.667). Patients were classified into two groups according to the cut-off: group A, high CA19-9 level ≥11.85 U/mL, included 102 patients (29.7%); group B, low CA19-9 level <11.85 U/mL, included 241 patients (70.3%) (Table 1). Table 3 shows that the level of CA19-9 was significantly correlated with CEA level (P=0.042).
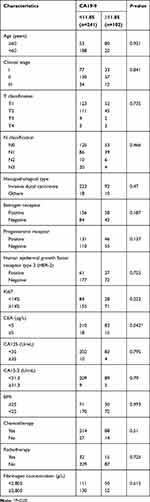 |
Table 3 Association Between CA19-9 and Clinicopathological Characteristics in Breast Cancer Patients |
Analysis of Prognostic Factor
The consequence of univariate COX analysis of OS suggested that Histopathological type (HR=0.453, 95% CI=0.246–0.837, P=0.011), CEA level (HR=1.389, 95% CI=1.065–1.812, P=0.015), CA19-9 level (HR=2.609, 95% CI=1.338–3.201, P=0.001), and plasma fibrinogen level (HR= 2.007, 95% CI: 1.27–3.174, P = 0.003) were associated with worse OS significantly (Table 4).The OS curve displayed by plasma fibrinogen level showed that non-distant metastatic breast cancer patients with lower level of plasma fibrinogen (< 2.805 g/L) had more favorable outcome than those with hyperfibrinogenemia (≥ 2.805 g/L) (Figure 2A). Furthermore, the OS curve displayed by CA19-9 showed that low CA19-9 level patients (< 11.85 U/mL) had longer survival time than those with high CA19-9 level (≥ 11.85 U/mL) (Figure 2B). Meanwhile, multivariate Cox regression analysis also showed that CA19-9 level (HR = 1.827, 95% CI: 1.135–2.942, P = 0.001) and plasma fibrinogen level (HR = 2.067, 95% CI: 1.332–3.207, P = 0.001) were both independent prognostic factors in non-distant metastatic breast cancer patients. (Table 5).
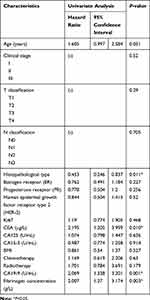 |
Table 4 Univariate Analysis of Overall Survival in Breast Cancer Patients |
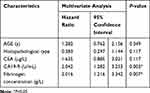 |
Table 5 Multivariate Analysis of Overall Survival in Breast Cancer Patients |
The Prognostic Ability of Combination of Plasma Fibrinogen and CA19-9
According to the above results, the level of plasma fibrinogen and CA19-9 could be independent prognostic factors in non-distant metastatic BC patients, but the problem we pointed out that the prognostic ability of the combination of fibrinogen and CA19-9 was not solved. The patients who own both hyperfibrinogenemia (≥ 2.805) and high CA19-9 (≥ 11.85) were allocated a score of 2, those with either hyperfibrinogenemia (≥ 2.805) or high CA19-9 (≥ 11.85) were allocated a score of 1, those with neither hyperfibrinogenemia (≥ 2.805) nor high CA19-9 (≥ 11.85) allocated a score of 0. The AUC of combination of fibrinogen and CA19-9 (AUC=0.640; 95% CI=0.571–0.71) (Figure 3) was higher than that of plasma fibrinogen (AUC=0.589; 95% CI=0.516–0.661) (Figure 1A), and that of CA19-9 (AUC=0.594; 95% CI=0.521–0.667) (Figure 1B). When the combination of plasma fibrinogen and CA19-9 added into Cox regression analysis, both univariate and multivariate Cox regression analyses of survival suggested that the combination of plasma fibrinogen and CA19-9 was valuable for predicting prognosis in non-distant metastatic BC patients after surgery (Table 6). Figure 4 shows the results of the OS curve. The above results show that the combination of plasma fibrinogen and CA19-9 could be an independent prognostic factor exceeding either plasma fibrinogen or CA19-9 alone.
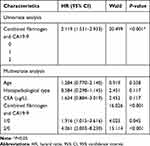 |
Table 6 Univariate and Multivariate Analysis of Overall Survival According to the Combination of Fibrinogen and CA19-9 |
Development and Validation of a Nomogram
Prognostic factors which were significantly associated with overall survival were used to conduct a nomogram to predict the 5-year OS in patients with non-distant metastatic BC, as shown in Figure 5. The AUC values were 0.724 (95% CI=0.648–0.769) for 5-year OS indicating the good predict ability of the nomogram, as shown in Figure 6.
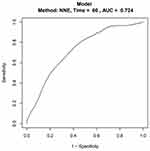 |
Figure 6 Receiver operating characteristic curve analysis based on the Nomogram. The area under the curve (AUC) was 0.724 (95% CI=0.648–0.769) indicating the ability of the nomogram. |
Discussion
Increasing incidence of BC brings about a heavy public health and economic burden. Currently, there are a number of articles studying the independent factors related to the prognosis of breast cancer. For example, the albumin-to-alkaline phosphatase ratio (AAPR) was proposed to be an independent prognostic factor, the preoperative AAPR value associates with the OS in non-distant metastatic breast cancer patients.23 Another norm, systemic inflammation response index (SIRI), was considered to a reliable predictor of OS to provide personalized prognostication for breast cancer patients.24 But there is still a lot of room for us to find indicators that are simpler and easier to practice clinically.
In this article, the survival duration of 343 non-distant metastatic BC patients undergoing radical surgery was retrospectively analyzed, and the associations between preoperative plasma fibrinogen, CA19-9, and survival was investigated. According to our results, plasma fibrinogen and CA19-9 level were related to the survival of BC patients, when they were combined, the AUC was greater than either parameter alone. The above findings showed that the combination of fibrinogen and CA19-9 could be an independent predictor for OS in non-distant metastatic BC patients. Patients with hyperfibrinogenemia and high level of CA19-9 would have unfavorable overall survival. To the best of our knowledge, it was the first time to combine the plasma fibrinogen and CA19-9 to study their correlation with breast cancer prognosis. Exhilaratingly, we found that, when the plasma fibrinogen and CA19-9 were combined, the efficiency was better than those of a single index.
The following biological mechanisms may explain the reason for the prognostic significance of plasma fibrinogen levels in BC patients. First, a previous study suggested that fibrinogen not only participates in wound healing, but also plays a role in the formation of tumor stroma under pathological conditions. The generation of tumor stroma both start with spillover of plasma fibrinogen, fibronectin, and plasminogen.25 Then fibrinogen converted to fibrin providing an initial matrix for the formation of the tumor stroma.26 Generated stroma furnishes connective tissue and new blood vessels which assist malignant tumor to absorb nutrients, grow, and spread.27 Second, fibrinogen can bind to aIIbβ3 integrin to mediate the aggregation of tumor cells and platelets to form microthrombi. The microthrombi can serve as a barrier to protect tumor cells from natural killer cell elimination.28 Third, the endothelial barrier permeability can be induced by VE-cadherin binding domain of fibrinogen, meanwhile, the transendothelial migration of malignant breast epithelial cells also increased.29
Apart from the above mechanism research, many scholars have also done reports on fibrinogen and solid cancer prognosis, such as colorectal, lung cancer.30,31 As far as we know, there are several studies to explore the prognostic significance of plasma fibrinogen in BC patients. Mei et al19 posed elevated level of preoperative plasma fibrinogen was associated with poor prognosis in operable BC patients (HR=1.475, 95% CI=1.177–1.848, P=0.001). The cut-off value of fibrinogen they found was 2.83, while the AUC value was 0.595 (95% CI=0.560–0.630). In line with our findings, the optimal cut-off value of fibrinogen was 2.805, the AUC value was 0.589 (95% CI=0.516–0.661). The result we obtained was similar to their findings. Another paper found that the risk of death in BC patients with elevated plasma fibrinogen (>2.83 g/L) levels was 1.475 times those with low fibrinogen levels.32 There was also a study suggesting that preoperative plasma fibrinogen level associates with disease specific survival in breast cancer patients (HR=1.71; 95% CI= 1.02–2.85; P=0.042).33 Based on the above mechanism and case studies, we have reason to believe that fibrinogen can indeed indicate the prognosis of BC.
As for CA19-9, it was first reported by Koprowski et al34 that CA19-9 was a carbohydrate tumor-associated antigen which could isolate from a human colorectal cancer cell line. Since 1979, CA19-9 has become an important tumor marker in various cancers, especially in pancreatic cancer.35 Nowadays, CA19-9 has also been used in early breast cancer diagnosis.36 It is worth noting that an increase of CA19-9 could be regarded as a sign of Ileocecal valve metastasis from BC.37 In this study, our survival analysis of CA19-9 showed that the patients with low CA19-9 level (<11.85 U/mL) had longer survival time. Perhaps the increase of CA19-9 after surgery may indicate distant metastasis of breast cancer in the future, especially related to digestive tract metastasis, which needs more research to verify.
All in all, fibrinogen and CA19-9 are convenient to get via routine preoperative laboratory tests, and could easily be applied in clinical practice to predict the prognosis of BC patients. The nomogram we performed may easily be applied in clinical practice to predict the overall survival in non-distant metastatic BC patients. However, more related studies are warranted to validate these conclusions.
This study is the first to analyze the prognostic value of the combination of plasma fibrinogen and CA19-9. Inevitably, there were many limitations existing: 1) Our study was a single center study, meanwhile our sample size was small, the conclusions may be biased. 2) The combination of plasma and CA19-9 has been shown to be an independent prognostic factor of non-distant metastatic BC; however, the outcome of sensitivity and specificity are not good, and further studies are needed to measure the correct cut-off value. 3) This is a retrospective study and we did not explore the molecular mechanisms.
Conclusion
In conclusion, the present study showed that BC patients who had both hyperfibrinogenemia and high CA19-9 had short OS. Therefore, the combination of plasma fibrinogen and CA19-9 could be used as a valid independent prognostic factor for non-distant metastatic BC compared with either parameter alone and could easily be applied in clinical practice.
Ethics Approval and Informed Consent
The clinic ethics committee approval was supported by the ethics committees of The First Affiliated Hospital of Wenzhou Medical University. This study was conducted in the light of the Declaration of Helsinki. All individual participants included in the study signed informed consent.
Acknowledgments
This study was funded by National Natural Science Foundation of China (81902692) and Zhejiang Medical and Health Science and Technology Project (2019RC204).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Raghunath A, Desai K, Ahluwalia MS. Current treatment options for breast cancer brain metastases. Curr Treat Options Oncol. 2019;20(3):19.
4. Fish ML, Grover R, Schwarz GS. Quality-of-life outcomes in surgical vs nonsurgical treatment of breast cancer-related lymphedema: a systematic review. JAMA Surg. 2020;155(6):513. doi:10.1001/jamasurg.2020.0230
5. Zhu QL, Xu WH, Tao MH. Biomarkers of the metabolic syndrome and breast cancer prognosis. Cancers (Basel). 2010;2(2):721–739. doi:10.3390/cancers2020721
6. Shoombuatong W, Schaduangrat N, Nantasenamat C. Towards understanding aromatase inhibitory activity via QSAR modeling. EXCLI J. 2018;17:688–708. doi:10.17179/excli2018-1417
7. Byrnes JR, Duval C, Wang YM, et al. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin alpha-chain crosslinking. Blood. 2015;126(16):1940–1948. doi:10.1182/blood-2015-06-652263
8. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
9. Tianxing G, Xiaojie P, Lihuan Z, Yangyun H. Combination of preoperative fibrinogen and neutrophil to lymphocyte ratio is a predictive prognostic factor in ESCC and AEG systematic review. Biosci Rep. 2019;39(10). doi:10.1042/BSR20190480
10. Zhang SS, Lei YY, Cai XL, et al. Preoperative serum fibrinogen is an independent prognostic factor in operable esophageal cancer. Oncotarget. 2016;7(18):25461–25469. doi:10.18632/oncotarget.8171
11. Wang GY, Jiang N, Yi HM, et al. Pretransplant elevated plasma fibrinogen level is a novel prognostic predictor for hepatocellular carcinoma recurrence and patient survival following liver transplantation. Ann Transplant. 2016;21:125–130. doi:10.12659/AOT.895416
12. Yamamoto M, Kurokawa Y, Miyazaki Y, et al. Usefulness of preoperative plasma fibrinogen versus other prognostic markers for predicting gastric cancer recurrence. World J Surg. 2016;40(8):1904–1909. doi:10.1007/s00268-016-3474-5
13. Hong T, Shen D, Chen X, Wu X, Hua D. Preoperative plasma fibrinogen, but not D-dimer might represent a prognostic factor in non-metastatic colorectal cancer: a prospective cohort study. Cancer Biomark. 2017;19(1):103–111. doi:10.3233/CBM-160510
14. Kim KH, Park TY, Lee JY, et al. Prognostic significance of initial platelet counts and fibrinogen level in advanced non-small cell lung cancer. J Korean Med Sci. 2014;29(4):507–511. doi:10.3346/jkms.2014.29.4.507
15. Ghanim B, Hoda MA, Klikovits T, et al. Circulating fibrinogen is a prognostic and predictive biomarker in malignant pleural mesothelioma. Br J Cancer. 2014;110(4):984–990. doi:10.1038/bjc.2013.815
16. Zhao K, Deng H, Qin Y, Liao W, Liang W. Prognostic significance of pretreatment plasma fibrinogen and platelet levels in patients with early-stage cervical cancer. Gynecol Obstet Invest. 2015;79(1):25–33. doi:10.1159/000365477
17. Seebacher V, Polterauer S, Grimm C, et al. The prognostic value of plasma fibrinogen levels in patients with endometrial cancer: a multi-centre trial. Br J Cancer. 2010;102(6):952–956. doi:10.1038/sj.bjc.6605547
18. Bekos C, Grimm C, Brodowicz T, et al. Prognostic role of plasma fibrinogen in patients with uterine leiomyosarcoma - a multicenter study. Sci Rep. 2017;7(1):14474. doi:10.1038/s41598-017-13934-8
19. Mei Y, Zhao S, Lu X, Liu H, Li X, Ma R. Clinical and prognostic significance of preoperative plasma fibrinogen levels in patients with operable breast cancer. PLoS One. 2016;11(1):e0146233. doi:10.1371/journal.pone.0146233
20. Hwang KT, Chung JK, Roh EY, et al. Prognostic influence of preoperative fibrinogen to albumin ratio for breast cancer. J Breast Cancer. 2017;20(3):254–263. doi:10.4048/jbc.2017.20.3.254
21. Xu WY, Zhang HH, Yang XB, et al. Prognostic significance of combined preoperative fibrinogen and CA199 in gallbladder cancer patients. World J Gastroenterol. 2018;24(13):1451–1463. doi:10.3748/wjg.v24.i13.1451
22. Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 1957;17(4):237–246. doi:10.1159/000205234
23. Long ZQ, Hua X, Zhang WW, et al. Prognostic impact of the pretreatment albumin to alkaline phosphatase ratio for nonmetastatic breast cancer patients. Cancer Manag Res. 2019;11:4809–4814. doi:10.2147/CMAR.S200759
24. Hua X, Long ZQ, Huang X, et al. The preoperative systemic inflammation response index (SIRI) independently predicts survival in postmenopausal women with breast cancer. Curr Probl Cancer. 2020;44(4):100560. doi:10.1016/j.currproblcancer.2020.100560
25. Dvorak HF, Underhill LH, Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. doi:10.1056/NEJM198612253152606
26. Staton CA, Brown NJ, Lewis CE. The role of fibrinogen and related fragments in tumour angiogenesis and metastasis. Expert Opin Biol Ther. 2003;3(7):1105–1120. doi:10.1517/14712598.3.7.1105
27. Sudhoff T, Schneider W. Fibrinolytic mechanisms in tumor growth and spreading. Clin Investig. 1992;70(8):631–636. doi:10.1007/BF00180278
28. Zheng S, Shen J, Jiao Y, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100(5):859–865. doi:10.1111/j.1349-7006.2009.01115.x
29. Sahni A, Arevalo MT, Sahni SK, Simpson-Haidaris PJ, The V. E-cadherin binding domain of fibrinogen induces endothelial barrier permeability and enhances transendothelial migration of malignant breast epithelial cells. Int J Cancer. 2009;125(3):577–584. doi:10.1002/ijc.24340
30. Li M, Wu Y, Zhang J, Huang L, Wu X, Yuan Y. Prognostic value of pretreatment plasma fibrinogen in patients with colorectal cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2019;98(37):e16974. doi:10.1097/MD.0000000000016974
31. Zhong H, Qian Y, Fang S, Wang Y, Tang Y, Gu W. Prognostic value of plasma fibrinogen in lung cancer patients: a meta-analysis. J Cancer. 2018;9(21):3904–3911. doi:10.7150/jca.26360
32. Wen J, Yang Y, Ye F, et al. The preoperative plasma fibrinogen level is an independent prognostic factor for overall survival of breast cancer patients who underwent surgical treatment. Breast. 2015;24(6):745–750. doi:10.1016/j.breast.2015.09.007
33. Krenn-Pilko S, Langsenlehner U, Stojakovic T, et al. An elevated preoperative plasma fibrinogen level is associated with poor disease-specific and overall survival in breast cancer patients. Breast. 2015;24(5):667–672. doi:10.1016/j.breast.2015.08.003
34. Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5(6):957–972. doi:10.1007/BF01542654
35. Berger AC, Garcia M, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26(36):5918–5922. doi:10.1200/JCO.2008.18.6288
36. Bayo J, Castano MA, Rivera F, Navarro F. Analysis of blood markers for early breast cancer diagnosis. Clin Transl Oncol. 2018;20(4):467–475. doi:10.1007/s12094-017-1731-1
37. Santini D, Altomare A, Vincenzi B, et al. An increase of CA 19.9 as the first clinical sign of ileocecal valve metastasis from breast cancer. In Vivo (Athens, Greece). 2006;20(1):165–168.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.