Back to Journals » Cancer Management and Research » Volume 10
The prognostic value and clinicopathological features of sarcomatoid differentiation in patients with renal cell carcinoma: a systematic review and meta-analysis
Authors Zhang L, Wu B, Zha Z, Zhao H, Feng Y
Received 27 February 2018
Accepted for publication 28 April 2018
Published 22 June 2018 Volume 2018:10 Pages 1687—1703
DOI https://doi.org/10.2147/CMAR.S166710
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Lijin Zhang, Bin Wu, Zhenlei Zha,* Hu Zhao,* Yejun Feng*
Department of Urology, Affiliated Jiang-Yin Hospital of the Southeast University Medical College, Jiang-Yin 214400, China
*These authors contributed equally to this work
Background and purpose: Numerous studies have demonstrated that sarcomatoid differentiation is linked to the risk of renal cell carcinoma (RCC). However, its actual clinicopathological impact remains inconclusive. Therefore, we undertook a meta-analysis to evaluate the pathologic and prognostic impacts of sarcomatoid differentiation in patients with RCC by assessing cancer-specific survival, overall survival, recurrence-free survival, progression-free survival, and cancer-specific mortality.
Materials and methods: In accordance with the preferred reporting items for systematic reviews and meta-analysis statement, relevant studies were collected systematically from PubMed, Embase, and Web of Science to identify relevant studies published prior to January 2018. The pooled effects (hazard ratios, odds ratios, and standard mean differences) and 95% confidence intervals were calculated to investigate the association of sarcomatoid differentiation with cancer prognosis and clinicopathological features.
Results: Thirty-five studies (N=11,261 patients [n=59–1,437 per study]) on RCC were included in this meta-analysis. Overall, the pooled analysis suggested that sarcomatoid differentiation was significantly associated with unfavorable cancer-specific survival (HR=1.46, 95% CI: 1.26–1.70, p<0.001), overall survival (HR=1.59, 95% CI: 1.42–1.78, p<0.001), progression-free survival (HR=1.61, 95% CI: 1.35–1.91, p<0.001), recurrence-free survival (HR=1.60, 95% CI: 1.29–1.99, p<0.001), and cancer-specific mortality (HR=2.36, 95% CI: 1.64–3.41, p<0.001) in patients with RCC. Moreover, sarcomatoid differentiation was closely correlated with TNM stage (III/IV vs I/II: OR=1.84, 95% CI: 1.12–3.03, p=0.017), Fuhrman grade (III/IV vs I/II: OR=8.37, 95% CI: 2.92–24.00, p<0.001), lymph node involvement (N1 vs N0: OR=1.88, 95% CI: 1.08–3.28, p=0.026), and pathological types (clear cell RCC-only vs mixed type: OR=0.48, 95% CI: 0.29–0.80, p=0.005), but was not related to gender (male vs female, OR=0.86, 95% CI: 0.58–1.28, p=0.464) and average age (SMD=−0.02, 95% CI: −0.20–0.17, p=0.868).
Conclusion: This study suggests that sarcomatoid differentiation in histopathology is associated with poor clinical outcome and advanced clinicopathological features in RCC and could serve as a poor prognostic factor for RCC patients.
Keywords: sarcomatoid differentiation, renal cell carcinoma, prognosis, meta-analysis
Introduction
As the 8th most common cancer worldwide, renal cell carcinoma (RCC) accounts for 2–3% of all adult malignancies1 and causes approximately 140,000 deaths per year.2 Although most patients with RCC can be cured by surgical resection, more than 25% of patients still experience local recurrence or distant metastasis.3 Given that clear cell RCC (ccRCC) accounts for approximately 80% of all RCCs,4 it should be noted that a particular histologic subtype is accompanied by different manifestations and pharmacologic consequences.5 Therefore, ideally, the clinical significance of a particular prognostic factor should always be independently validated for each histologic subtype.
RCC with sarcomatoid differentiation is a rare variant of RCC that accounts for 1–8% of all RCC histologic subtypes.6 Histologically, sarcomatoid is a term used to describe morphologic changes within an RCC. Previous research demonstrates that sarcomatoid differentiation is associated with a more aggressive disease and poor outcome after surgical resection or immunotherapy.7,8 The International Society of Urological Pathology recommended that the presence of sarcomatoid differentiation should be classified as Grade 4 regardless of the histological subtype or nuclear grade.9 Given small sample sizes and different conditions, minimal evidence is available on the prognostic role of sarcomatoid differentiation for RCC.
To further clarify the prognostic and clinicopathological value of sarcomatoid differentiation in RCC, we conducted a systematic review and meta-analysis to evaluate whether the presence of sarcomatoid differentiation has a prognostic impact on cancer-specific survival (CSS), overall survival (OS), recurrence-free survival (RFS), progression-free survival (PFS), and cancer-specific mortality (CSM).
Materials and methods
Literature search
In accordance with the preferred reporting items for systematic reviews and meta-analysis guideline,10 we systematically searched for relevant studies in PubMed, Embase, and Web of Science until January 2018. The following terms were included in the search strategy: “sarcomatoid differentiation,” “renal cell cancer OR renal cell carcinoma”, and “prognostic factor OR oncologic outcome.” These terminologies were used in all possible combinations, and the language of publications was restricted to English. Moreover, the reference lists of the included articles were scanned manually for additional potentially relevant studies.
Inclusion and exclusion criteria
Studies eligible for inclusion in our meta-analysis had to meet the following criteria: 1) studies that included RCC and where the expression of sarcomatoid differentiation was pathologically confirmed; 2) studies in which the association between sarcomatoid differentiation and the prognosis of RCC (CSS, OS, RFS, PFS, and CSM) were reported; and 3) studies wherein HRs and their 95% CIs for survival analysis were reported or could be computed from given data. The exclusion criteria were as follows: 1) reviews, case reports, conference records, and comments and non-original articles; 2) studies that did not analyze the sarcomatoid differentiation, clinicopathological features, and survival outcome; 3) studies with insufficient data to estimate the HRs and 95% CIs; and 4) studies that were not published in English. In addition, when multiple reports describing the same population were published, the most recent or most complete report was used.
Data extraction and quality assessments
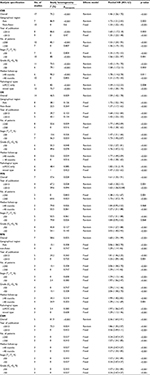
According to the inclusion and exclusion criteria, 2 investigators independently extracted the following data from eligible studies: first author’s name, year of publication, country, period of recruitment, study design, age of patients, gender ratio, number of patients, follow-up time, histology, nuclear grade, pathology tumor (pT) stage, and survival end point. If multivariate and univariate analyses were both conducted in the same study, only the results of multivariate analysis were extracted because this information is more accurate as it accounts for confounding factors. When disagreement occurred, the issue was resolved through discussion among the authors. The quality in prognosis studies11 tool was used to assess the methodological quality of each included study. Each study can be assessed by 6 important bias domains: study participation, study attrition, prognostic factor measurement, study confounding, outcome measurement, and statistical analysis and reporting. Studies from the analysis that are at high risk for any important bias were defined as low quality.
Statistical analysis
The statistical processes in this meta-analysis were undertaken using Stata 12.0 (StataCorp, College Station, TX, USA). Dichotomous variables were calculated by HRs, and pooled HRs with 95% CIs were used to evaluate the association of sarcomatoid differentiation with RCC prognosis (CSS, OS, RFS, PFS, and CSM). Furthermore, we studied the associations between sarcomatoid differentiation and clinical parameters of RCC. Data about Fuhrman grade (III/IV vs I/II), pT stage (pT3–4 vs pT1–2), lymph node involvement (N1 vs N0), pathological types (ccRCC-only vs mixed type), and gender (male vs female) were continuous variables whereas average age was a dichotomous variable. Comparisons of continuous and dichotomous variables were pooled as standard mean differences (SMDs) and ORs.
Statistical heterogeneity among studies was assessed using Cochran’s Q test and Higgins I2 statistic. When I2 <50% or pheterogeneity >0.1, which indicates that no obvious heterogeneity existed among studies, the fixed effects (FE) model was applied; otherwise, the random-effects (RE) model was applied. To obtain a more precise evaluation of heterogeneity, subgroup analyses were conducted for CSS, OS, RFS, PFS, and CSM by geographical region, year of publication, pathological types, pT stage, Fuhrman grade, number of patients, and median follow-up. Publication bias was assessed using funnel plots and Egger’s linear regression test. In addition, sensitivity analyses were used to estimate the robustness of the results by sequential omission of individual studies. A 2-tailed p-value <0.05 was considered statistically significant.
Results
Literature search
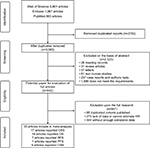
The flowchart depicting the study selection procedure in this meta-analysis is shown in Figure 1. After the initial search of relevant databases, 5,848 potentially relevant citations were retrieved. In total, 4,906 studies were excluded by reviewing the title and abstract, including 2,783 duplicate reports, 1,770 irrelevant studies, and 353 non-research articles (non-human studies, letters, case reports, meeting records, and reviews). The full-texts of the 942 remaining articles were assessed, and 907 papers were excluded due to insufficient survival information or duplicated cohorts. Finally, in accordance with the inclusion criteria, 35 articles published from 2004 to 2017 about the association of sarcomatoid differentiation and RCC survival were eligible for the meta-analysis.
Study characteristics
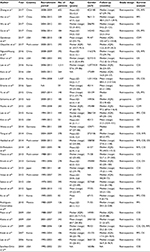
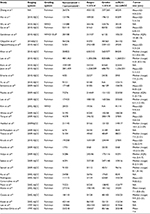
The characteristics of the 35 eligible studies12–46 are presented in Table 1. These studies enrolled 11,261 patients (59–1,437 per study), with a median follow-up ranging from 12.6 to 102 months. Most of the included studies had a retrospective design. Among the included studies, 10 were conducted in America, 7 in China, 6 in Korea, 5 in Europe, 4 at multiple centers, 1 in Mexico, 1 in Egypt, and 1 in Japan. CSS was evaluated in 17 studies, and OS was reported in 14 studies. Both PFS and RFS were reported in 7 studies, and CSM was reported in 5 studies. The characteristics, including tumor features and pathologic outcomes, are summarized in Table 2. Sarcomatoid differentiation was detected in (792/11,261) 7.03% of pathological specimens of the included patients. Ten of the included studies were limited to ccRCC, whereas 25 studies involved various tumor types, including ccRCC, papillary RCC, chromophobe RCC, and unclassified variants. The quality in prognosis studies tool was applied to assess the methodological quality of the included studies, demonstrating that all studies were of high quality (Table S1).
Meta-analysis results
Our meta-analysis demonstrated that sarcomatoid differentiation expression in RCC was associated with poor CSS (RE model, HR=1.46, 95% CI: 1.26–1.70; p<0.001; I2=75.2%; Figure 2A), OS (RE model, HR=1.59, 95% CI: 1.42–1.78, p<0.001; I2=46.5%; Figure 2B), PFS (RE model, HR=1.61, 95% CI: 1.35–1.91; p<0.001; I2=57.6%; Figure 2C), RFS (RE model, HR=1.60, 95% CI: 1.29–1.99, p<0.001; I2= 58.6%; Figure 2D), and CSM (RE model, HR=2.36, 95% CI: 1.64–3.41; p<0.001; I2=81.9%; Figure 2E). To explore the heterogeneity between these studies, the significance of sarcomatoid differentiation was evaluated further via subgroup analysis based on the main features, including geographical region, year of publication, pathological types, pT stage, Fuhrman grade, number of patients, and median follow-up (Table 3). The results of subgroup analysis suggested sarcomatoid differentiation as a prognostic factor despite heterogeneity among some groups. Of note, heterogeneity decreased significantly in some models, such as geographical region in non-Asian (CSS, OS, and RFS), year of publication before 2013 (CSS, OS, RFS, and CSM), number of patients <250 (CSS, OS, RFS, and CSM), (pT3–4) % ≥50 (CSS, OS, PFS, and CSM), median follow-up <40 months (CSS, OS, RFS, and CSM), and mixed type pathology (OS and RFS).
To explore the significance of sarcomatoid differentiation in pathologic diagnosis, we evaluated the relationship between the expression of sarcomatoid differentiation and clinicopathological features. As shown in Table 4, sarcomatoid differentiation was significantly related to TNM stage (III/IV vs I/II: OR=1.84, 95% CI: 1.12–3.03, p=0.017, Figure S1A), Fuhrman grade (III/IV vs I/II: OR=8.37, 95% CI: 2.92–24.00, p<0.001, Figure S1B), lymph node involvement (N1 vs N0: OR=1.88, 95% CI: 1.08–3.28, p=0.026, Figure S1C), and pathological type (ccRCC-only vs mixed type: OR=0.48, 95% CI: 0.29–0.80, p=0.005, Figure S1D), However, no significant correlations were observed with regard to gender (male vs female, OR=0.86, 95% CI: 0.58–1.28, p=0.464, Figure S1E) and average age (SMD=−0.02, 95% CI: −0.20–0.17, p=0.868, Figure S1F). No significant heterogeneity was observed in those groups.
Sensitivity analysis
In sensitivity analysis by sequential omission of individual studies, the pooled HR for CSS ranged from 1.37 (95% CI: 1.22–1.54) to 1.49 (95% CI: 1.28–1.74) (Figure S2A). Similarly, the pooled HR for OS ranged from 1.54 (95% CI: 1.37–1.72) to 1.62 (95% CI: 1.46–1.80) (Figure S2B), for PFS from 1.53 (95% CI:1.31–1.79) to 1.68 (95% CI: 1.41–2.00) (Figure S2C), for RFS from 1.47 (95% CI: 1.23–1.75) to 1.73 (95% CI: 1.39–2.16) (Figure S2D), and for CSM from 2.06 (95% CI: 1.48–2.87) to 2.72 (95% CI: 1.83–4.04) (Figure S2E). These results indicated that the findings were reliable and robust.
Publication bias
Egger’s tests and funnel plots were conducted to estimate publication bias in the present meta-analysis. As shown in Figure 3, the funnel plots indicated that the included studies (CSS, OS, RFS, and PFS) had no evident asymmetry. The p-values of the Egger’s tests were all greater than 0.05 in CSS (p-Egger=0.723, Figure 3A), OS (p-Egger=0.925, Figure 3B), PFS (p-Egger=0.443, Figure 3C), and RFS (p-Egger=0.108, Figure 3D). However, a statistically significant publication bias was founded in CSM (p-Egger=0.003, Figure 3E).
Discussion
The rate of incidence of RCC has rapidly increased by approximately 2% worldwide during the last decade.47 Although significant advancements have been made in managing renal masses, long-term survival remains unsatisfactory, and the vast majority of patients with RCC still die of their disease. Therefore, RCC patients should be closely followed up, and reliable prognostic biomarkers that evaluate postoperative risks and allow individualized treatment for RCC patients are necessary. In recent years, numerous studies have investigated a wide variety of prognostic factors, such as TNM stage,13 Fuhrman’s grade, and tumor size.48 However, these prognostic variables cannot always make accurate predictions due to the limitation of significant tumor heterogeneity in RCC patients.14 Therefore, novel biomarkers that can distinguish high-risk RCC patients and improve clinical outcomes are desperately needed.
An RCC with sarcomatoid differentiation is a distinct subtype that is defined by the presence of atypical spindle cells and is similar to all forms of sarcoma.49 The reported incidence of sarcomatoid differentiation is between 0.7% and 13.2% of all RCCs,50 which is consistent with our result of 7.03% (792/11,261). Clinically, sarcomatoid differentiation in RCC is associated with more aggressive tumor biology, increased rates of recurrence, and poor survival.28 Furthermore, RCC with sarcomatoid differentiation demonstrates unfavorable responses to targeted therapy.8 According to the 2016 World Health Organization Classification, RCC with sarcomatoid differentiation should not recognized as a separate and distinct entity, indicating that the sarcomatoid component could occur in all types of RCC.51
To date, several studies have examined the prognostic value of sarcomatoid differentiation for RCC patients. However, these results were not consistent. Gu et al15 demonstrated that the presence of sarcomatoid differentiation was significantly associated with poor oncologic outcomes (OS and PFS) for surgically treated RCC patients. Furthermore, Keegan et al5 confirmed that the sarcomatoid component was associated with poor survival even when encountered in low-stage disease. However, a study by Tosco et al33 found that sarcomatoid differentiation failed to independently predict CSM in surgically treated RCC patients. Similarly, Chen et al48 found that the sarcomatoid feature is not a prognostic factor of pT3 RCC for PFS and CSS. Zhang et al49 demonstrate that the presence of rhabdoid differentiation does not confer an increased risk of death from the largest study, to date, of patients with Grade 4 RCC. Although sarcomatoid differentiation is commonly recognized by clinicians as being associated with poor outcomes, no commonly accepted prognostic system for sarcomatoid RCC is currently available due to the low morbidity and lack of study data. With this objective in mind, we first sought to confirm that sarcomatoid differentiation is an independent prognostic feature for RCC patients.
Using the largest sample size to date, this meta-analysis is the most comprehensive study to systematically analyze the prognostic power of sarcomatoid differentiation in patients with RCC. We found that sarcomatoid differentiation was significantly associated with CSS (HR=1.46, p<0.001), OS (HR=1.59, p<0.001), PFS (HR=1.61, p<0.001), RFS (HR=1.60, p<0.001), and CSM (HR=2.36, p<0.001) in RCC patients. In addition, subgroup analyses demonstrated that sarcomatoid differentiation remained a good biomarker regardless of the background of ethnic background, pT stage, nuclear grade, and tumor type. Given the lower sample size of the subgroup (PFS and median follow-up ≥40 months) with a different result (2 studies involving 1,720 patients), we can ignore the inconsistent result to some extent.
Our findings, furthermore, demonstrated that RCC cases exhibiting sarcomatoid differentiation are prone to experiencing a higher nuclear grade (OR=8.37, p<0.001), increased pathological T stage (OR=1.84, p=0.017), lymph node involvement (OR=1.88, p=0.026), and mixed histologic types (OR=0.48, p=0.005). However, sarcomatoid differentiation is not associated with gender (OR=0.86, p=0.464) and average age (SMD= −0.02, p=0.868). Interestingly, although RCCs differ among histological subtypes, we observed no differences on comparing the positive expression of sarcomatoid differentiation between ccRCC and mixed type (CSS, OS, and RFS). In other words, sarcomatoid differentiation may be independently validated as a prognostic factor for each histologic subtype, and this information reflects the risk stratification in the clinical treatment of RCC.
However, several limitations of this study need to be acknowledged. First, significant heterogeneity was detected for several parameters. Although we selected random-effect or fixed-effect models based on heterogeneity, it still existed due to the differences in the included studies. Second, although a comprehensive search strategy was applied to determine eligible studies, it is possible that some eligible studies were not included, which may cause selection bias. Third, the criteria for the presence of sarcomatoid differentiation in pathologic specimens were inconsistent, which may potentially contribute to potential bias. Thus, rigorous morphological criteria should be conducted to standardize the diagnosis of sarcomatoid differentiation. Additionally, a publication bias existed in CSM, thus inflating the estimate for the association of sarcomatoid differentiation with CSM risk.
Conclusion
Our results demonstrated that sarcomatoid differentiation expression was associated with poor pathological features and prognosis. These findings indicate that sarcomatoid differentiation is a potential adverse prognostic marker that could be utilized to divide risk stratification and formulate individualized treatments for patients with RCC. Considering the limitations of the present analysis, larger studies using standardized methods and criteria are required to verify the prognostic roles of sarcomatoid differentiation expression in RCC.
Author contributions
LJZ and BW designed the research. ZLZ and HZ undertook the literature search. HZ and YJF analyzed the data and interpreted the results. LJZ wrote the paper. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Calvo E, Schmidinger M, Heng DY, Grünwald V, Escudier B. Improvement in survival end points of patients with metastatic renal cell carcinoma through sequential targeted therapy. Cancer Treat Rev. 2016;50:109–117. | ||
Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387(10021):894–906. | ||
MacLennan S, Imamura M, Lapitan MC, et al. Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol. 2012;61(5):972–993. | ||
Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924. | ||
Keegan KA, Schupp CW, Chamie K, Hellenthal NJ, Evans CP, Koppie TM. Histopathology of surgically treated renal cell carcinoma: survival differences by subtype and stage. J Urol. 2012;188(2):391–397. | ||
Thomas AZ, Adibi M, Slack RS, et al. The role of metastasectomy in patients with renal cell carcinoma with sarcomatoid dedifferentiation: a matched controlled analysis. J Urol. 2016;196(3):678–684. | ||
Joseph RW, Millis SZ, Carballido EM, et al. PD-1 and PD-L1 expression in renal cell carcinoma with sarcomatoid differentiation. Cancer Immunol Res. 2015;3(12):1303–1307. | ||
Golshayan AR, George S, Heng DY, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol. 2009;27(2):235–241. | ||
Delahunt B, Cheville JC, Martignoni G, et al; Members of the ISUP Renal Tumor Panel. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37(10):1490–1504. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. | ||
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. | ||
Zhang H, Liu Y, Xie H, et al. High mucin 5AC expression predicts adverse postoperative recurrence and survival of patients with clear-cell renal cell carcinoma. Oncotarget. 2017;8(35):59777–59790. | ||
Xie Y, Ma X, Li H, et al. Prognostic value of clinical and pathological features in Chinese patients with chromophobe renal cell carcinoma: a 10-year single-center study. J Cancer. 2017;8(17):3474–3479. | ||
Wu CY, Huo JP, Zhang XK, et al. Loss of CD15 expression in clear cell renal cell carcinoma is correlated with worse prognosis in Chinese patients. Jpn J Clin Oncol. 2017;47(12):1182–1188. | ||
Gu L, Li H, Wang H, et al. Presence of sarcomatoid differentiation as a prognostic indicator for survival in surgically treated metastatic renal cell carcinoma. J Cancer Res Clin Oncol. 2017;143(3):499–508. | ||
Gershman B, Moreira DM, Thompson RH, et al. Renal cell carcinoma with isolated lymph node involvement: long-term natural history and predictors of oncologic outcomes following surgical resection. Eur Urol. 2017;72(2):300–306. | ||
Chipollini J, Abel EJ, Peyton CC, et al. Pathologic predictors of survival during lymph node dissection for metastatic renal-cell carcinoma: results from a multicenter collaboration. Clin Genitourin Cancer. 2018;16(2):e443–e450. | ||
NguyenHoang S, Liu Y, Xu L, et al. High mucin-7 expression is an independent predictor of adverse clinical outcomes in patients with clear-cell renal cell carcinoma. Tumour Biol. 2016;37(11):15193–15201. | ||
Khor LY, Dhakal HP, Jia X, et al. Tumor necrosis adds prognostically significant information to grade in clear cell renal cell carcinoma: a study of 842 consecutive cases from a single institution. Am J Surg Pathol. 2016;40(9):1224–1231. | ||
Lee H, Lee SE, Byun SS, Kim HH, Kwak C, Hong SK. Preoperative plasma fibrinogen level as a significant prognostic factor in patients with localized renal cell carcinoma after surgical treatment. Medicine. 2016;95(4):e2626. | ||
Kara O, Maurice MJ, Zargar H, et al. Prognostic implications of sarcomatoid and rhabdoid differentiation in patients with grade 4 renal cell carcinoma. Int Urol Nephrol. 2016;48(8):1253–1260. | ||
Jeon HG, Choi DK, Sung HH, et al. Preoperative prognostic nutritional index is a significant predictor of survival in renal cell carcinoma patients undergoing nephrectomy. Ann Surg Oncol. 2016;23(1):321–327. | ||
Errarte P, Guarch R, Pulido R, et al. The expression of fibroblast activation protein in clear cell renal cell carcinomas is associated with synchronous lymph node metastases. PLoS One. 2016;11(12):e0169105. | ||
Yu X, Guo G, Li X, et al. Retrospective analysis of the efficacy and safety of sorafenib in Chinese patients with metastatic renal cell carcinoma and prognostic factors related to overall survival. Medicine. 2015;94(34):e1361. | ||
Schiavina R, Borghesi M, Chessa F, et al. The prognostic impact of tumor size on cancer-specific and overall survival among patients with pathologic T3a renal cell carcinoma. Clin Genitourin Cancer. 2015;13(4):e235–e241. | ||
Psutka SP, Boorjian SA, Lohse CM, et al. The association between metformin use and oncologic outcomes among surgically treated diabetic patients with localized renal cell carcinoma. Urol Oncol. 2015;33(2):67.e15–e23. | ||
Lee HW, Jeon HG, Jeong BC, et al. Diagnostic and prognostic significance of radiologic node-positive renal cell carcinoma in the absence of distant metastases: a retrospective analysis of patients undergoing nephrectomy and lymph node dissection. J Korean Med Sci. 2015;30(9):1321–1327. | ||
Kim T, Zargar-Shoshtari K, Dhillon J, et al. Using percentage of sarcomatoid differentiation as a prognostic factor in renal cell carcinoma. Clin Genitourin Cancer. 2015;13(3):225–230. | ||
Weiss VL, Braun M, Perner S, et al. Prognostic significance of venous tumour thrombus consistency in patients with renal cell carcinoma (RCC). BJU Int. 2014;113(2):209–217. | ||
Teng J, Gao Y, Chen M, et al. Prognostic value of clinical and pathological factors for surgically treated localized clear cell renal cell carcinoma. Chinese Med J. 2014;127(9):1640–1644. | ||
Haddad AQ, Wood CG, Abel EJ, et al. Oncologic outcomes following surgical resection of renal cell carcinoma with inferior vena caval thrombus extending above the hepatic veins: a contemporary multicenter cohort. J Urol. 2014;192(4):1050–1056. | ||
El-Mokadem I, Fitzpatrick J, Bondad J, et al. Chromosome 9p deletion in clear cell renal cell carcinoma predicts recurrence and survival following surgery. Br J Cancer. 2014;111(7):1381–1390. | ||
Tosco L, Van Poppel H, Frea B, Gregoraci G, Joniau S. Survival and impact of clinical prognostic factors in surgically treated metastatic renal cell carcinoma. Eur Urol. 2013;63(4):646–652. | ||
Kruck S, Eyrich C, Scharpf M, et al. Impact of an altered Wnt1/β-catenin expression on clinicopathology and prognosis in clear cell renal cell carcinoma. Int J Mol Sci. 2013;14(6):10944–10957. | ||
Kondo T, Ikezawa E, Takagi T, et al. Negative impact of papillary histological subtype in patients with renal cell carcinoma extending into the inferior vena cava: single-center experience. Int J Urol. 2013;20(11):1072–1077. | ||
Volpe A, Novara G, Antonelli A, et al. Chromophobe renal cell carcinoma (RCC): oncological outcomes and prognostic factors in a large multicentre series. BJU Int. 2012;110(1):76–83. | ||
Sukov WR, Lohse CM, Leibovich BC, Thompson RH, Cheville JC. Clinical and pathological features associated with prognosis in patients with papillary renal cell carcinoma. J Urol. 2012;187(1):54–59. | ||
Sameh WM, Hashad MM, Eid AA, Abou Yousif TA, Atta MA. Recurrence pattern in patients with locally advanced renal cell carcinoma: the implications of clinicopathological variables. Arab J Urol. 2012;10(2):131–137. | ||
Ku JH, Park YH, Myung JK, Moon KC, Kwak C, Kim HH. Expression of hypoxia inducible factor-1α and 2α in conventional renal cell carcinoma with or without sarcomatoid differentiation. Urol Oncol. 2011;29(6):731–737. | ||
Rodríguez-Covarrubias F, Castillejos-Molina R, Sotomayor M, et al. Impact of lymph node invasion and sarcomatoid differentiation on the survival of patients with locally advanced renal cell carcinoma. Urol Int. 2010;85(1):23–29. | ||
Poon SA, Gonzalez JR, Benson MC, McKiernan JM. Invasion of renal sinus fat is not an independent predictor of survival in pT3a renal cell carcinoma. BJU Int. 2009;103(12):1622–1625. | ||
Klatte T, Said JW, de Martino M, et al. Presence of tumor necrosis is not a significant predictor of survival in clear cell renal cell carcinoma: higher prognostic accuracy of extent based rather than presence/absence classification. J Urol. 2009;181(4):1558–1564; discussion 1563–1554. | ||
Coons BJ, Stec AA, Stratton KL, et al. Prognostic factors in T3b renal cell carcinoma. World J Urol. 2009;27(1):75–79. | ||
Kwak C, Park YH, Jeong CW, et al. Sarcomatoid differentiation as a prognostic factor for immunotherapy in metastatic renal cell carcinoma. J Surg Oncol. 2007;95(4):317–323. | ||
Lee SE, Byun SS, Oh JK, et al. Significance of macroscopic tumor necrosis as a prognostic indicator for renal cell carcinoma. J Urol. 2006;176(4 Pt 1):1332–1337; discussion 1337–1338. | ||
Sanchez-Ortiz RF, Rosser CJ, Madsen LT, Swanson DA, Wood CG. Young age is an independent prognostic factor for survival of sporadic renal cell carcinoma. J Urol. 2004;171(6 Pt 1):2160–2165. | ||
Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193–205. | ||
Chen K, Lee BL, Huang HH, et al. Tumor size and Fuhrman grade further enhance the prognostic impact of perinephric fat invasion and renal vein extension in T3a staging of renal cell carcinoma. Int J Urol. 2017;24(1):51–58. | ||
Zhang BY, Thompson RH, Lohse CM, et al. A novel prognostic model for patients with sarcomatoid renal cell carcinoma. BJU Int. 2015;115(3):405–411. | ||
Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategies. Oncologist. 2012;17(1):46–54. | ||
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs–part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.