Back to Journals » Journal of Inflammation Research » Volume 13
The Prognostic Significance of 5-Fluorouracil Induced Inflammation and Immuno-Modulation in Colorectal Cancer Patients
Authors Abdellateif MS , Salem SE , Badr DM, Shaarawy S, Hussein MM, Zekri AN, Fouad MA
Received 21 September 2020
Accepted for publication 18 November 2020
Published 31 December 2020 Volume 2020:13 Pages 1245—1259
DOI https://doi.org/10.2147/JIR.S283069
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Mona S Abdellateif,1 Salem E Salem,2 Doaa M Badr,3 Sabry Shaarawy,1 Marwa M Hussein,2 Abdel-Rahman N Zekri,4 Mariam A Fouad3
1Medical Biochemistry and Molecular Biology, Cancer Biology Department, National Cancer Institute, Cairo University, Cairo, Egypt; 2Medical Oncology Department, National Cancer Institute, Cairo, Egypt; 3Pharmacology and Experimental Oncology Unit, Cancer Biology Department, National Cancer Institute, Cairo University, Cairo, Egypt; 4Virology and Immunology Unit, Cancer Biology Department, National Cancer Institute, Cairo, Egypt
Correspondence: Mariam A Fouad
Pharmacology and Experimental Oncology Unit, Cancer Biology Department, National Cairo Institute, Cairo University, 1Kasr AlAini Street, Fom Elkalig, Cairo 11796, Egypt
Email [email protected]
Aim: The change in the levels of peripheral inflammatory markers together with EGFR in relation to 5- fluorouracil (5-FU) therapy was evaluated for their prognostic significance in colorectal cancer (CRC) patients.
Patients and Methods: Expression levels of COX2, IL6, IL1β, EGFR, IL10, and TNFα were determined with quantitative real-time PCR (qPCR) in the peripheral blood of 90 CRC patients. The inflammatory response was correlated with patients’ clinical features, disease-free survival (DFS), and overall survival (OS).
Results: After 6 months of 5-FU therapy, increased inflammatory response was found to be associated with smoking, T3 or T4 tumors, performance status (PS) III, positive lymph nodes, distant metastasis, and gastrointestinal (GIT) toxicity. The combination of COX2 with interleukins in a predictive equation for DFS was significant in patients with over-expression of EGFR. DFS and OS rates were reduced in patients with increased COX2, IL6, IL10, and TNFα expression with 5-FU therapy. Significant hazard of disease progression was associated with smoking (HR=1.27, P=0.004), 5-FU induction of COX2, and IL6 expression (HR=1.35, P=0.001 and HR=1.27, P=0.004, respectively). Moreover, smoking, 5-FU induction of IL6, TNFα, and IL10 expression are found to be independent prognostic factors for OS (P=0.003, 0.003, 0.002, and 0.002, respectively).
Conclusion: The peripheral effects of 5-FU therapy have shown a significant impact on the treatment outcome of CRC patients.
Keywords: colorectal cancer, 5-fluorouracil, COX2, EGFR, interleukins, survival, TNFα
Introduction
Worldwide, colorectal cancer (CRC) is the second most common malignancy in women and the third in men. Many genetic, epigenetic and long- standing inflammatory conditions have contributed in the development of CRC.1 Treatment with antimetabolite (5-Fluorouracil: 5-FU) is the standard first-line therapy for CRC patients. It can be used either single or combined with oxaliplatin according to the stage of the CRC disease.2 5-FU is a pyrimidine analog, interacts with the nucleic acid sequence, and interferes with the RNA and DNA synthesis in both normal and tumor cells.3
Gastrointestinal (GIT) toxicity is one of the most commonly encountered side-effects experienced during systemic therapy for CRC, and it is one of the major causes of dose limiting serious toxicity in chemotherapy regimens containing 5-FU.4 50% of CRC patients receiving 5-FU as a single agent and up to 40% of patients receiving combination 5-FU therapy can develop severe diarrhea.5 Other common non-GIT toxicity criteria recorded, including anemia, peripheral neuropathy, and hand-foot syndrome; were found to be modulated with some pro-inflammatory and anti-inflammatory cytokines, and have an impact in the survival of CRC patients.6–9
Recent studies demonstrated the stage-dependent alterations in the inflammatory cytokines’ levels in tumor tissues, tumor microenvironment, and/or the peripheral blood of CRC patients.10–12 Interleukins 6 (IL6), IL1β, IL10, cyclooxygenase2 (COX2), and tumor necrosis factor (TNFα) are the most known inflammatory biomarkers involved in the adenoma–carcinoma progression of CRC.13 Peripheral blood serves as an easy, rapid, and reliable method for investigating patients with CRC. In addition, it is considered the richest vehicle with cytokines, either detected in serum or plasma.14,15 These inflammatory cytokines proved to show significant diagnostic,16 prognostic,17 and predictive18 values for CRC patients. It has been reported that the baseline level of cytokines could determine the response of CRC patients to treatment as well as their survival outcomes,19 however their fluctuated serum levels during the treatment regimen also have a contribution in the tolerance (efficacy and toxicity) of patients to treatment and overall outcome.20 Accordingly, this study aimed at investigating the changes in the expression levels of some inflammatory markers (COX2, IL6, IL1β, IL10, and TNFα) together with EGFR in the peripheral blood of CRC patients who were treated with 5-FU based therapy, trying to find a prominent inflammatory response associated with patients` outcomes including response to treatment and survival rates.
Patients and Methods
This prospective cohort study included 90 patients with histo-pathologically confirmed CRC and 30 age- and sex-matched healthy normal controls. Patients were presented at the Egyptian National Cancer Institute (NCI) during the period between November 2013 to October 2014.
All patients received 5-FU based therapy in doses and duration described in the protocols of CRC treatment followed by the Medical Oncologists in NCI, Cairo University. In patients with favorable prognosis, adjuvant capecitabine was administered orally as a single agent in a 1,250 mg/m2 dose twice/day for 2 weeks, followed by a 1-week rest period, given as 3-week cycles for a total of eight cycles. While metastatic patients had received XELOX in 3-week treatment cycles in which intravenous oxaliplatin 130 mg/m2 was given in day 1 followed by oral capecitabine 1,000 mg/m2 twice daily from day 1 evening till day 15 morning. Peripheral blood samples were collected from all patients and control groups to assess the expression levels of COX2, IL6, IL1β, IL10, TNFα, and EGFR using quantitative real time-polymerase chain reaction (qRT-PCR). The expression of the markers was assessed in the peripheral blood of the same patients at baseline (before treatment) and at the end of the 5-FU treatment regimen (after treatment). The expression at baseline was first compared with the healthy control expression level. Then, the change in the expression, either by decrease or increase, was evaluated for their clinicopathological and survival correlations.
RNA Extraction and cDNA Synthesis
Total RNA was extracted from the lymphocytic cell pellet with total RNA purification kit (Direct-Zol RNA Kit, Zymo Research, Germany). cDNA synthesis was performed according to the manufacturer’s instructions using Revert Aid First Strand cDNA synthesis kit (ThermoFisher, UK).
qPCR for Detection of the Expression of Assessed Markers
qPCR was conducted according to manufacturer’s instructions by Applied Biosystems Syber Green PCR Master Mix (USA). Reverse and forward sequences of primers genes encoding for mRNA transcript of COX2, IL6, IL1β, IL10, TNFα, and EGFR genes were designed by NCBI-NIH tool, and the sequences were summarized as follows: COX2 (Sequence ID: NM_000963.4, Region: 698–717) forward primer: CAGCACTTCACGCATCAGTT, reverse primer: TCTGGTCAATGGAAGCCTGT, IL6 (Sequence ID: NM_000600.5, Region: 546–565) forward primer: GAGACTTGCCTGGTGAAAAT, reverse primer: CAGGGGTGGTTATTGCATCT, IL1β (Sequence ID: XM_017003988.2, Region: 467–486) forward primer: GGACAAGCTGAGGAAGATGGC, reverse primer: TTTTTTGCTGTGAGTCCCGG, IL10 (Sequence ID: NM_001382624.1, Region: 161–442) forward primer: TCTGGTGAAGGAGGATCGCTA, reverse primer: TGGCAACCCAGGTAACCCTA, EGFR (Sequence ID: NM_001346897.2, Region: 2527–2540) forward primer: AAGGAAATCCTCGATGAAGCCT, reverse primer: TGTCTTTGTTCCCGGACATA, TNFα (Sequence ID: NG_007462.1, Region: 4993–7764) forward primer: ACAGATGTGGGGTGTGAGAAG, reverse primer: TCTTCTGTGTGCCAGACACC, and β-actin (Sequence ID: NM_001100.3, Region: 286–304) forward primer: CCAGAGCAAGAGAGGTATCC, reverse primer: CTGTGGTGGTGAAGCTGTAG. CT values were normalized to the housekeeping gene (β-actin) (2−∆Ct) in order to calculate the relative expression of each gene.
Statistical Analysis
Data were analyzed using the SPSS package (version 22; SPSS Inc., Chicago, IL, USA). It was tested for normalization using Shapiro test. Comparison of markers between patients and control group or before and after treatment was done using Mann-Whitney test. Associations between categorical variables were performed using Pearson’s chi-square. Spearman’s test was used to detect the strength of correlation between the tested markers. Kaplan–Meier analysis was used for comparing survival rates using log rank test. Disease-free survival (DFS) was calculated from date of primary treatment till the date of relapse or progressive disease, while overall survival (OS) was calculated from the date of diagnosis till the date of death. COX proportional-hazards model was used to determine the independent significant risk of individual factors. Multiple linear regression analysis was performed to detect the best fit equation for prediction of DFS rates of the patients. P-value ≤0.05 was considered statistically significant at two tailed test.
Results
Clinico-Pathological Features and Treatment Plan of the Assessed CRC Patients
The mean age of assessed CRC patients was 44.6±11.8 years, with a median of 44 (range=19–63 years). Males represented 42/90 (46.7%) and females were 48/90 (53.3%). There were 12 patients (13.3%) with a positive family history of CRC, and 22 (24.4%) were smokers. Most of the patients (95.6%) had good performance status (ECOG PS I), and only four (4.4%) had PS III. Almost half of the patients (53.3%) were diagnosed with rectum and recto-sigmoid cancers. More than two thirds of the patients were T3 and T4 tumors, with positive lymph nodes, and at stages III and IV of the disease. Distant metastasis was detected in 20 (22.2%) patients, and the remaining 70 (77.8%) patients were non-metastatic (Table 1). All patients received 5-FU based therapy either neoadjuvant (28.9%) or adjuvant (71.1%). Combination with oxaliplatin was prescribed and administered by 80 patients.
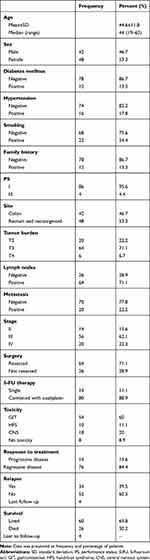 |
Table 1 Clinical Features of the Assessed CRC Patients |
There were 82 (91.1%) patients who experienced toxicity of 5-FU therapy, 54 (60.0%) of them had GIT toxicity in the form of nausea, vomiting, diarrhea, abdominal colic, mucositis, hepatomegaly, gall bladder calculi, severe constipation, and fatty liver. Eighteen (20.0%) patients had CNS toxicity in the form of peripheral neuropathy and back pain. While 10 (11.1%) patients had hand and foot syndrome (HFS), skin ulceration, dizziness, fatigue, knee edema, elevation of body temperature, and deteriorated physical activity. At the end of the study, 60 (69.8%) patients were live, and 26 (30.2%) died, while four patients were lost to follow-up (Table 1).
The Baseline Expression of the Assessed Markers in CRC Patients
Before 5-FU therapy, there was a significant increase in the expression of EGFR in CRC patients compared to normal control subjects [9.6 (0–12.8) vs 7.9 (7.5–9.9); respectively, P=0.029]. COX2 increased significantly in CRC patients compared to normal control [4.4 (0.2–304.4) vs 0.3 (0.01–30.1); respectively, P=0.002]. Similarly, IL6 expression increased in CRC patients in comparison to control subjects [9.9 (0.15–100) vs 1.3 (0.5–18); respectively, P=0.007]. Also, TNFα increased significantly in CRC patients compared to normal control [0.1 (0.01–1.2) vs 0.005 (0–0.01); respectively, P<0.001]. While there was an insignificant decrease in the expression of IL1β in CRC patients compared to the control group [0.7 (0.1–27.7) vs 8.6 (0.5–57.7); respectively, P=0.056], also an insignificant difference between the expression of IL10 in CRC patients and control group was shown as well [59.6 (9–274) and 65.9 (47.1–143.5); respectively, P=0.295] (Figure 1).
 |
Figure 1 Levels of inflammation markers in 90 CRC patients in comparison to 30 healthy controls: (A) EGFR, (B) COX-2, (C) IL-1β, (D) IL-6, (E) TNFα, and (F) IL-10. |
The Impact of 5-FU Therapy on the Expression of the Assessed Markers
The expression of markers after treatment with 5-FU therapy showed a significant decrease in the expression of COX2 [baseline: 9.58 (0.1–2120); after 5-FU: 0.890 (0.17–1160), P=0.023], IL10 [baseline: 59.6 (9–274); after 5-FU: 47.06 (0.1–290), P=0.005], and CA19-9 [baseline: 21.3 (1–592); after 5-FU: 6.6 (1.8–800), P=0.027] in CRC patients after 5-FU therapy. However, there was an insignificant change in the expression of EGFR, IL6, IL1β, TNFα, and CEA after 5-FU therapy, as follows: EGFR [baseline: 9.48 (0–12.78); after 5-FU: 9.05 (0.16–12.78), P=0.791], IL6 [baseline: 14.03 (0.15–4482); after 5-FU: 1.56 (0.16–4513), P=0.455], IL1β [baseline: 0.65 (0–760); after 5-FU: 2.06 (0–8.4), P=0.106], TNFα [baseline: 0.09 (0.01–1.2); after 5-FU: 0.06 (0–2.5), P=0.599], and CEA [baseline: 2.65 (0.5–133); after 5-FU: 2.8 (0.9–2786), P=0.375], respectively, as demonstrated in Figure 2.
 |
Figure 2 Assessment of the serum levels of (A) EGFR, (B) COX-2, (C) IL-10, (D) IL-6, (E) IL-1β, (F) TNFα, (G) CEA, and (H) CA19-9 before and after 6 months of 5-FU therapy. |
Association Between the Effect of 5-FU Therapy on the Assessed Markers and Relevant Clinico-Pathological Features of the Patients
In Tables 2 and 3, the expression of EGFR, COX2, IL6, IL1B, TNFα, IL1β, and IL10 were assessed in the peripheral blood of the CRC patients before 5-FU treatment (baseline expression), and after 6 months of therapy. Accordingly, patients were categorized into two groups regarding the change of the markers’ expression whether decreased or increased after treatment.
 |
Table 2 Association Between the Change of COX2, IL6, and IL1β Expression with 5-FU Therapy and the Clinic-Pathological Features of the Assessed CRC Patients |
 |
Table 3 Association Between the Change of EGFR, TNFα, IL10, with 5-FU Therapy and Clinic-Pathological Features of the Assessed CRC Patients |
Out of the assessed 90 patients, 64 patients experienced a decrease in COX2 expression by 4.5% compared with 26 patients with an increased expression by 0.98%. COX2 expression increased significantly after 5-FU therapy in non- smoker patients [26/26 (100%), P=0.001], patients with T3 and T4 tumors (P=0.004), patients with positive lymph nodes (P=0.004), patients whose original site of the tumor is the colon rather than rectal or recto-sigmoid (P<0.001), and in patients who experienced GIT toxicity rather than CNS or HFS (P=0.048, Table 2).
Regarding IL6 expression, 53/90 patients experienced a decrease in IL6 expression by 5.35%, compared to 37/90 with an increased IL6 by 3.23%. IL6 expression increased significantly in patients who were diabetic, patients with PS III, distant metastasis, and with colonic cancer location (P=0.01, P<0.001, P=0.013, P<0.001; respectively, Table 2).
Meanwhile, 54/90 patients experienced an increase in IL1β expression by 2.24% compared with a decrease by 0.12% in 36/90 patients. Significant associations were shown between increased IL1β expression after 5-FU therapy and the presence of diabetes mellitus, blood hypertension (HTN), PS III, advanced tumor stage III or IV, colonic cancer location, and with GIT and CNS toxicities (P=0.02, P=0.001, P=0.02, P<0.001, P<0.001, and P=0.001; respectively, Table 2).
EGFR was found to be decreased after 5-FU therapy by 1.7% in 44 patients, while increased by 1.17% in 46 patients. A significant association was shown between the increased EGFR expression and the presence of diabetes mellitus (P=0.031), non-smoking (P=0.007), distant metastasis (P=0.01) and GIT toxicity (P=0.032). The expression of TNFα is also significantly increased by 20.32% in patients with diabetes mellitus (P=0.018), distant metastasis (P=0.001), GIT, and/or HFS toxicity (P=0.013). Similarly, IL10 increased by 0.18% in patients with distant metastasis (P<0.001), however it decreased by 0.06% in patients with family history of CRC, and with the presence of positive lymph nodes (P=0.034 and P=0.001; respectively), as demonstrated in Table 3.
Disease-Free (DFS) and Overall Survival (OS) Rates of the Patients
The mean DFS of all patients was 26.29 months. The mean DFS of the CRC patients associated significantly with the expression changes of COX2 expression (16.4 months in increased expression compared to 28.4 months in decreased expression, P<0.001), IL1β (21.9 months in increased expression compared to 16.1 months in decreased expression, P=0.041), IL6 (14.2 months in increased expression compared to 22.8 months in decreased expression, P<0.001), TNFα (16.7 months in increased expression compared to 29.7 months in decreased expression, P<0.001), IL10 (14.9 months in increased expression compared to 28.7 months in decreased expression, P<0.001), and CEA (18.7 months in increased level compared to 26.9 months in decreased level, P=0.003). However, there was no significant association between DFS rate and expression changes of EGFR (24.9 months in increased expression compared to 28.1 months in decreased expression, P=0.172) and CA19.9 (14.3 months in increased level compared to 24.3 months in decreased level, P=0.283, Figure 3).
 |
Figure 3 Disease-free survival (DFS) rates for the assessed CRC patients (A) EGFR, (B) COX-2, (C) IL-1β, (D) IL-6, (E) TNFα, (F) IL-10, (G) CEA, and (H) CA19.9. |
The mean OS rate of all patients was 29.06 months. The mean OS rates of the CRC patients were associated significantly with the changes of COX2 expression (23.7 months in increased expression compared to 30.4 months in decreased expression, P<0.001), IL6 (19.9 months in increased expression compared to 28.6 months in decreased expression, P<0.001), TNFα (20.2 months in increased expression compared to 32.9 months in decreased expression, P<0.001), IL10 (18.5 months in increased expression compared to 30.02 months in decreased expression, P<0.001), and CA19.9 (14.3 months in increased level compared to 30.9 months in decreased level, P=0.001). However, there was no significant association between OS rate and the expression changes of EGFR (27.3 months in increased expression compared to 31.1 months in decreased expression, P=0.094), IL1β (26.9 months in increased expression compared to 24.04 months in decreased expression, P=0.087), and CEA (27.5 months in increased level compared to 28.3 months in decreased level, P=0.492, Figure 4).
 |
Figure 4 Overall survival (OS) rates for the assessed CRC patients (A) EGFR, (B) COX-2, (C) IL-1β, (D) IL-6, (E) TNFα, (F) IL-10, (G) CEA, and (H) CA19.9. |
Univariate and Multivariate Survival Analyses
Univariate COX regression analysis revealed that patients’ gender, smoking, tumor stage, distant metastasis, GIT toxicity, CEA level, COX2, IL6, TNFα, and IL10 expression changes during the course of treatment had a significant impact on DFS. However, multivariate analysis showed that smoking, increased COX2, and IL6 expressions after 5-FU therapy were independent prognostic factors for poor DFS of our CRC patients (P=0.004, P=0.001, and P=0.001; respectively) (Figure 5).
 |
Figure 5 Multivariate COX regression for the hazard ratio of DFS and OS rates in CRC patients. |
On the other hand, Univariate COX regression analysis for OS demonstrated that smoking, distant metastasis, GIT toxicity, CA19.9 level, COX2, IL6, TNFα, and IL10 expression changes during the course of treatment had a significant impact on OS rates of the assessed patients. However, multivariate analysis showed that smoking, increased expressions of IL6, TNFα, and IL10 after 5-FU therapy are independent prognostic factors for OS in our CRC patients (P=0.003, P=0.003, P=0.002, and P=0.002; respectively) (Figure 5).
Multiple Linear Regression Analysis for Disease-Free Survival (DFS)
Multiple linear regression analysis was performed to produce an equation or inflammation index that can be applied for CRC patients to help in the prediction of patients’ response and DFS rate. Different markers together were tested in all patients and then in patients with baseline over- and low- expression of EGFR (Table 4). The generated ROC curves were analyzed, and the best fit equation was elucidated in patients with baseline EGFR overexpression when COX2, IL6, IL1β, IL10, and TNFα were tested all together (AUC=0.883 and P-value<0.001). Also, a significant prediction of DFS was shown in patients with baseline EGFR overexpression when combining the assessed expressions of COX2, IL6, and IL1β in the peripheral blood of CRC patients (AUC=0.867 and P-value=0.001).
 |
Table 4 Multiple Linear Regression Analysis for DFS |
Discussion
5-FU based regimens (combined with oxaliplatin, irinotecan, and cetuximab) are still considered the main standard treatment for CRC.21 However, the incidence of chemoresistance is an emerging problem that affects the prognosis and the treatment outcome of CRC patients. Tumor microenvironment and body immune reaction including many inflammatory mediators and cytokines have an important role in the regulation and modulation of patients’ response to 5-FU therapy.22,23 Treatment with 5-FU even in non-toxic concentration induces immunogenic changes in CRC cells in- vitro.24 Accordingly, in this study, we evaluated the effect of the inflammatory and immunological changes which happened in the peripheral blood after treatment with 5-FU therapy, on the survival and treatment outcome of CRC patients.
Our data revealed significantly higher expressions of EGFR, COX2, IL6, and TNFα in CRC patients compared to healthy control, while there was an insignificant difference between the assessed two groups regarding IL10 and IL1β expressions. These results are in agreement with Szkaradkiewicz et al25 and Krzystek-Korpacka et al,26 who reported significant increases in the plasma levels of the pro-inflammatory cytokines TNFα, IL1β, and IL6 in CRC patients compared to healthy subjects, while there was an insignificant difference regarding the serum level of IL10. Similarly, Yamaguchi et al15 showed that TNFα was significantly increased in CRC patients compared to control healthy subjects, and the level of IL1β was insignificantly different between the two groups. However, in contrast to ours, they reported an insignificant difference between the assessed two groups regarding IL6 plasma level.15
The sensitivity of cancer patients to 5-FU therapy was found to be regulated by the tumor microenvironment and its associated M2 macrophages, which are the source of many inflammatory mediators and cytokines. This regulation was mediated through the programmed epithelial mesenchymal transition, PI3K/AKT pathway, and caspase-mediated apoptosis.23 So, by investigating the change in inflammation marker (COX2) and cytokines (TNFα, IL6, IL1β, and IL10) after 6 months of 5-FU therapy, we found that more than 50% of patients showed reduction in the levels of the assessed inflammatory markers, especially COX2, IL6, TNFα, and IL10. That reduction was averaged between 0.06–5.35%. However, IL1β was increased by 2.24% in 60% of patients after therapy. In spite of the reduction observed in our markers in the total group of patients, however, an increased inflammatory response (increased expression of the assessed markers) with 5-FU therapy was observed in patients with advanced tumor stage III or IV, distant metastasis, PS III, lymph node metastasis, and colon cancer location.
Similarly, global decrease in cytokine levels correlated with the drop in white blood cell counts at the end of fluorouracil chemotherapeutic regimens was shown in the study done by Jabeen et al.27 They observed that a decrease in patients who are under treatment with 5-FU combination regimen with targeted biological therapy like bevacizumab, and also a more pronounced decrease was observed in patients with pathological complete response.27 Generally, Janelsins et al28 observed different immunological behavior of 5-FU relative to other DNA damaging agents (like doxorubicin). 5-FU based treatment caused a reduction in the level of IL6, IL8, and MCP1, while other DNA damaging agents (like doxorubicin) caused induction in the levels of the same cytokines.28 However, Pusztai et al29 recorded no change in the level of IL6 after treatment with cyclophosphamide, methotrexate, and 5-fluorouracil, while an increase in IL6 level was observed to be associated with the treatment with paclitaxel.29 For the effect of chemotherapy on IL-1β, an increase in IL-1β level was observed in patients under treatment for Hodgkin’s disease.30 Also, Di Caro et al31 reported a significant increase in IL1β level with 5-FU treatment in patients with advanced CRC tumor stage, however this association was not achieved in the other markers they assessed including IL6, IL10, or TNFα.
The new approach conducted in this study was through investigating the effect of the change in the inflammatory response during 5-FU therapy on the patients’ outcome. In the literature, the effect of baseline, or the effect of the preoperative levels of inflammatory markers on patients` survival have been extensively studied, although it is not enough for the precise prediction of patients` survival and treatment outcome.
The current study demonstrated a significant reduction in OS and DFS rates in CRC patients with increased expression of COX2, IL6, IL10, and TNFα with 5-FU therapy. Similarly, Olsen et al32 found a significant association between increased plasma levels of IL10 and TNFα at the time of surgery with the increase of CRC-specific mortality. Huang et al33 also reported that immune microenvironment conferred chemo-resistance in CRC patients through the IL6 receptor/STAT3 pathway. Rahman et al34 demonstrated that COX2 expression was associated with poor survival and 65% increased risk of mortality in CRC. Similarly, Hashemi Goradel et al35 concluded that COX2 is a marker of worse prognosis, and can induce CRC progression through its pro-inflammatory activity under the stimulation of IL1β and TNFα. Adding to that, deteriorated survival rates associated with the enhanced immunological response were also reported to be correlated with chemotherapy induced myelosuppression20 and cognitive (CNS-related) problems36 in many cancer patients. IL6 was found to be the key cytokine in the progression of chemotherapy induced myelosuppression, however IL-1, IL-3, and granulocyte macrophage CSF were rarely involved.20
Multivariate analysis showed that smoking, and the increased expression of COX2 and IL6 after 5-FU therapy; are independent prognostic factors for poor DFS in our CRC patients. In agreement with these data, Di Caro et al31 demonstrated that a preoperative increase of the plasma levels of IL6, IL10, TNFα, and IL1β associated significantly with poor outcome, and postoperative relapse. However, on multivariate analysis, none of these previously mentioned markers could independently and significantly predict patients’ outcome. Hence, determining the increased or decreased inflammation response associated with 5-FU treatment, as we did in this study, is an important step for achieving better prognostic prediction of our patients` survival.
Most of our patients (60%) experienced 5-FU induced-GIT toxicity especially with increased expression of COX2, IL6, IL1β, TNFα, and EGFR. In agreement with the present study, Lee et al37 demonstrated that 5-FU induced-GIT toxicity through the induction of TNFα, IL1β, IL6, and COX2 stimulated by NF-κB.38,39 Also, 5-FU therapy caused elevation of reactive oxygen species through inducing the myeloperoxidase enzymes, resulting in an increase in the intestinal wall thickness and crypt length, while causing a decrease in the villus height.38,39 In addition, the combination of 5-FU therapy with oxaliplatin was found to cause an aggravation of GI mucositis in animal models.40 So, the blocker of IL1 receptor was used to reduce apoptosis and protect against the mucositis induced by 5-FU therapy in Wu et al.41
In addition, we found a significant impact of smoking on patients’ outcome, as it increased the hazard of both progression and death in the COX regression analysis. The effect of smoking on the treatment response of CRC is related to the activity of the 5-FU-related metabolic enzymes. Indeed, smoking may reduce the anticancer activity of 5-FU, possibly through the induction of dihydropyrimidine dehydrogenase activity, which is the initial and rate-limiting enzyme in the catabolic pathway of 5-FU.42 Moreover, smoking contains thousands of different compounds, which have carcinogenic activity.43
Another interesting finding in the current study was shown in CRC patients with baseline over-expression with EGFR, in them the combination of COX2 with other cytokines expression levels generated a significant inflammation index for the prediction of DFS. As explanation to that result, the co-expression and the significant correlation was demonstrated between COX2 and HER-2 in CRC patients.44 Studies tried to combine the level of multiple cytokines into a composite score either for diagnostic or prognostic purposes. However, they were varied in the number, the selection of cytokines, and the way in which they were combined as well.31,45 From these studies, Yamaguchi et al15 constructed panels of inflammatory indices, composed of 13 plasma cytokines, and they found a significant association of the indices with the presence of CRC.
In conclusion, the peripheral immunomodulatory effect of 5-FU therapy has an impact on the treatment outcome and survival of our CRC patients. As increased inflammatory response in the form of COX2, IL6, IL1β, IL10, and TNFα during the course of treatment could be considered a negative prognostic and predictive marker for DFS and OS rates, increased COX2 and IL6 levels after 5-FU are independent prognostic factors for poor DFS. However, increased levels of IL6, TNFα, and IL10 after 5-FU are independent negative prognostic factors for OS. This could help for opening a new avenue for research which could investigate the effect of these inflammatory reactions on the sensitivity of CRC patients to chemotherapy as conducted in this study, and also the sensitivity to targeted immunological therapies such as immune checkpoint inhibitors, vaccination, and adoptive T cell therapy. This will improve patient’s prognosis, survival, and treatment outcome. Furthermore, the combination of COX2 with interleukins in a predictive equation of DFS was significant in patients with baseline over-expression of EGFR. However, other studies are required to validate this predictive equation on the peripheral serum protein levels of these markers, as well as on a larger number of patients. One of the limitations in this study is the number of CRC patients who were followed up till the end of the study, and whose peripheral blood samples were available after 6 months of 5-FU therapy; there were only 90 patients compared to 30 healthy control subjects.
Compliance with Ethical Standard
This study was conducted according to Good Clinical Practice guidelines. The study was approved by the Institutional Human Research Ethics Committee of NCI, Egypt (number 00004025), which was in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient and healthy control individual before the enrollment in the study.
Author Contributions
MSA and MAF carried out the experimental work, bio-statistical analysis, and drafting the manuscript. MAF, SES, and DB worked on the eligibility testing, sample collection, and informed consent collection. SES and MH took over the management and follow-up of the patients. DMB extracted data of the patients, and shared in writing the manuscript. SS and AZ supervised the work and reviewed the manuscript. All authors contributed to data analysis, drafting, and revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors declare no conflicts of interest for this work.
References
1. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–732. doi:10.1038/s41575-019-0189-8
2. Akhtar R, Chandel S, Sarotra P, Medhi B. Current status of pharmacological treatment of colorectal cancer. World J Gastrointest Oncol. 2014;6(6):177–183. doi:10.4251/wjgo.v6.i6.177
3. Miura K, Kinouchi M, Ishida K, et al. 5-FU metabolism in cancer and orally-administrable 5-FU drugs. Cancers (Basel). 2010;2(3):
4. Jones JA, Avritscher EB, Cooksley CD, Michelet M, Bekele BN, Elting LS. Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer. 2006;14:505–515. doi:10.1007/s00520-006-0055-4
5. Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122:321–326. doi:10.7326/0003-4819-122-5-199503010-00001
6. Koorts AM, Levay PF, Becker PJ, Viljoen M. Pro- and anti-inflammatory cytokines during immune stimulation: modulation of iron status and red blood cell profile. Mediators Inflamm. 2011;2011:716301. doi:10.1155/2011/716301
7. Hung AL, Lim M, Doshi TL. Targeting cytokines for treatment of neuropathic pain. Scand J Pain. 2017;17:287–293. doi:10.1016/j.sjpain.2017.08.002
8. Hofheinz RD, Heinemann V, von Weikersthal LF, et al. Capecitabine-associated hand-foot-skin reaction is an independent clinical predictor of improved survival in patients with colorectal cancer. Br J Cancer. 2012;107(10):1678–1683. doi:10.1038/bjc.2012.434
9. Soveri LM, Hermunen K, de Gramont A, et al. Association of adverse events and survival in colorectal cancer patients treated with adjuvant 5-fluorouracil and leucovorin: is efficacy an impact of toxicity? Eur J Cancer. 2014;50(17):2966–2974. doi:10.1016/j.ejca.2014.08.017
10. Mager LF, Wasmer MH, Rau TT, Krebs P. Cytokine-induced modulation of colorectal cancer. Front Oncol. 2016;6:96. doi:10.3389/fonc.2016.00096
11. Pedrosa L, Esposito F, Thomson TM, Maurel J. The tumor microenvironment in colorectal cancer therapy. Cancers (Basel). 2019;11(8):1172. doi:10.3390/cancers11081172
12. Kantola T, Klintrup K, Väyrynen JP, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107(10):1729–1736. doi:10.1038/bjc.2012.456
13. Marszałek A, Szylberg L, Wiśniewska E, Janiczek M. Impact of COX-2, IL-1β, TNF-α, IL-4 and IL-10 on the process of carcinogenesis in the large bowel. Pol J Pathol. 2012;4(4):221–227. doi:10.5114/pjp.2012.32768
14. Väyrynen JP, Kantola T, Väyrynen SA, et al. The relationships between serum cytokine levels and tumor infiltrating immune cells and their clinical significance in colorectal cancer. Int J Cancer. 2016;139(1):112–121. doi:10.1002/ijc.30040
15. Yamaguchi M, Okamura S, Yamaji T, et al. Plasma cytokine levels and the presence of colorectal cancer. PLoS One. 2019;14(3):e0213602. doi:10.1371/journal.pone.0213602
16. Choi J, Maeng HG, Lee SJ, et al. Diagnostic value of peripheral blood immune profiling in colorectal cancer. Ann Surg Treat Res. 2018;94(6):312–321. doi:10.4174/astr.2018.94.6.312
17. Gunawardene A, Dennett E, Larsen P. Prognostic value of multiple cytokine analysis in colorectal cancer: a systematic review. J Gastrointest Oncol. 2019;10(1):134–143. doi:10.21037/jgo.2018.07.11
18. Nixon AB, Schalper KA, Jacobs I, et al. Peripheral immune-based biomarkers in cancer immunotherapy: can we realize their predictive potential? J ImmunoTher Cancer. 2019;7(1):325. doi:10.1186/s40425-019-0799-2
19. Lereclus E, Tout M, Girault A, et al. A possible association of baseline serum IL-17A concentrations with progression-free survival of metastatic colorectal cancer patients treated with a bevacizumab-based regimen. BMC Cancer. 2017;17(1):220. doi:10.1186/s12885-017-3210
20. Chen YM, Whang-Peng J, Liu JM, et al. Serum cytokine level fluctuations in chemotherapy-induced myelosuppression. Jpn J Clin Oncol. 1996;26(1):18–23. doi:10.1093/oxfordjournals.jjco.a023173
21. Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, et al. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer. 2015;77(3):441–451. doi:10.1002/(SICI)1097-0142(19960201)77:3<441::AID-CNCR4>3.0.CO;2-N
22. Mcmillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov. 2013;12(3):217–228.
23. Wei C, Yang C, Wang S, et al. M2 macrophages confer resistance to 5-fluorouracil in colorectal cancer through the activation of CCL22/PI3K/AKT signaling. Onco Targets Ther. 2019;12:3051. doi:10.2147/OTT.S198126
24. De Almeida CV, Zamame JA, Romagnoli GG, et al. Treatment of colon cancer cells with 5-fluorouracil can improve the effectiveness of RNA-transfected antitumor dendritic cell vaccine. Oncol Rep. 2017;38(1):561–568. doi:10.3892/or.2017.5692
25. Szkaradkiewicz A, Marciniak R, Chudzicka-Strugała I, et al. Proinflammatory cytokines and IL-10 in inflammatory bowel disease and colorectal cancer patients. Arch Immunol Ther Exp (Warsz). 2009;57(4):291. doi:10.1007/s00005-009-0031-z
26. Krzystek-Korpacka M, Diakowska D, Kapturkiewicz B, Bębenek M, Gamian A. Profiles of circulating inflammatory cytokines in colorectal cancer (CRC), high cancer risk conditions, and health are distinct. Possible implications for CRC screening and surveillance. Cancer Lett. 2013;337(1):107–114. doi:10.1016/j.canlet.2013.05.033
27. Jabeen S, Zucknick M, Nome M, et al. Serum cytokine levels in breast cancer patients during neoadjuvant treatment with bevacizumab. Oncoimmunology. 2018;7(11):e1457598. doi:10.1080/2162402X.2018.1457598
28. Janelsins MC, Mustian KM, Palesh OG, et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support Care Cancer. 2012;20(4):
29. Pusztai L, Mendoza TR, Reuben JM, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25(3):94–102. doi:10.1016/j.cyto.2003.10.004
30. Villani F, Busia A, Villani M, Vismara C, Viviani S, Bonfante V. Serum cytokine in response to chemo-radiotherapy for Hodgkin’s disease. Tumori. 2008;94(6):803–808. doi:10.1177/030089160809400605
31. Di Caro G, Carvello M, Pesce S, et al. Circulating inflammatory mediators as potential prognostic markers of human colorectal cancer. PLoS One. 2016;11(2).
32. Olsen RS, Nijm J, Andersson RE, Dimberg J, Wågsäter D. Circulating inflammatory factors associated with worse long-term prognosis in colorectal cancer. World J Gastroenterol. 2017;23(34):6212. doi:10.3748/wjg.v23.i34.6212
33. Huang Z, Yin Y, Yao S, et al. The immune-microenvironment confers Chemoresistance of colorectal Cancer through macrophage-derived IL-6. Clin Cancer Res. 2017;23(23):7375–7387. doi:10.1158/1078-0432.CCR-17-1283
34. Rahman M, Selvarajan K, Hasan MR, et al. Inhibition of COX-2 in colon cancer modulates tumor growth and MDR1 expression to enhance tumor regression in therapy-refractory cancers in vivo. Neoplasia. 2012;14(7):624–IN18. doi:10.1593/neo.12486
35. Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase‐2 in cancer: a review. J Cell Physiol. 2019;234(5):5683–5699. doi:10.1002/jcp.27411
36. Cheung YT, Ng T, Shwe M, et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol. 2015;26(7):1446–1451. doi:10.1093/annonc/mdv206
37. Lee CS, Ryan EJ, Doherty GA. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: the role of inflammation. World J Gastroenterol. 2014;20(14):3751. doi:10.3748/wjg.v20.i14.3751
38. Chang CT, Ho TY, Lin H, et al. 5-Fluorouracil induced intestinal mucositis via nuclear factor-κB activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS One. 2012;7(3):e31808. doi:10.1371/journal.pone.0031808
39. Yasuda M, Kato S, Yamanaka N, et al. Potential role of the NADPH oxidase NOX1 in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1133–G1142. doi:10.1152/ajpgi.00535.2011
40. Nukatsuka M, Saito H, Sakamoto K, et al. Efficacy of combination chemotherapy using oral fluoropyrimidine S-1 with oxaliplatin (SOX) against colorectal cancer in vivo. Anticancer Res. 2012;32:2807–2812.
41. Wu Z, Han X, Qin S, et al. Interleukin 1 receptor antagonist reduces lethality and intestinal toxicity of 5-Fluorouracil in a mouse mucositis model. Biomed Pharmacother. 2011;65(5):339–344. doi:10.1016/j.biopha.2011.04.013
42. Yamashita T, Kato K, Long NK, et al. Effects of smoking and alcohol consumption on 5-fluorouracil-related metabolic enzymes in oral squamous cell carcinoma. Mol Clin Oncol. 2014;2(3):
43. Zevin S, Benowitz NL. Drug interactions with tobacco smoking. Clin Pharmacokinet. 1999;36(6):425–438. doi:10.2165/00003088-199936060-00004
44. Wu QB, Sun GP. Expression of COX-2 and HER-2 in colorectal cancer and their correlation. World J Gastroenterol. 2015;21(20):
45. Chen ZY, He WZ, Peng LX, et al. A prognostic classifier consisting of 17 circulating cytokines is a novel predictor of overall survival for metastatic colorectal cancer patients. Int J Cancer. 2015;136(3):584–592.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
