Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
The Prognostic Role of Circulating FPR Before Operation in Patients with BCLC A-C Hepatocellular Carcinoma: A Retrospective Cohort Study
Authors Shen Y , Xu Y, Wei J, Li W
Received 8 April 2022
Accepted for publication 25 May 2022
Published 31 May 2022 Volume 2022:9 Pages 467—476
DOI https://doi.org/10.2147/JHC.S369168
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Yanjun Shen, Yawen Xu, Jianying Wei, Wendong Li
Department of Oncology, Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Wendong Li, Department of Oncology, Beijing Ditan Hospital, Capital Medical University, 8 Jingshun East Street, Chaoyang District, Beijing, People’s Republic of China, Tel +86-010-84322470, Email [email protected]
Background: This research aimed to comprehensively assess the prognostic role of fibrinogen to prealbumin ratio (FPR) in BCLC A-C HCC patients treated by TACE and RFA.
Methods: The research included 240 patients at stage BCLC A-C treated by TACE and RFA at Beijing Ditan Hospital of Capital Medical University from May 2011 to November 2018.
Results: The results showed that the size of the tumor, vascular invasion, α-foetoprotein, cirrhosis, NLR, LMR, and PLR showed prognostic value in predicting 5-year OS. Besides, FPR (95% confidence interval: 1.006– 1.013, hazard ratio: 1.009) was a prognostic factor for the prediction of 5-year OS in HCC.
Conclusion: Our research indicated that FPR was a potential indicator for patients with BCLC A-C hepatocellular carcinoma after treatment of RFA and TACE.
Keywords: FPR, hepatocellular carcinoma, prognosis, overall survival
Introduction
As the 6th most prevalent tumor in the world, hepatocellular carcinoma (HCC) ranks the 3rd among all the causes of malignancy-associated death in China.1,2 Only 30–40% of HCC patients could gain benefits from conventional therapies, including locoregional treatments, liver transplantation, and hepatic resection.3 It is of great necessity to discover novel prognostic markers for individualized treatment. Currently, serum alpha fetalprotein (AFP) is the most common serological diagnostic tumor marker for HCC. Serum markers could be used in the prediction of HCC survival and recurrence since they could be easily obtained at a low cost.4
Previous research demonstrated that fibrinogen (Fib) and its corresponding peptides could promote inflammation in malignancies.5 Furthermore, accumulating findings showed that increased Fib was related to worse OS and survival without tumor.6,7 Except for Fib, prealbumin (pAlb), a factor indicating nutritional status, can also be independently used in predicting prognosis. A decreased level of pAlb before operation indicates a poor result of survival.8,9 Therefore, combined use of FPR, Fib and pAlb could provide more reliable results on the nutrition and inflammation status of the patients and might be used as a prognostic factor.
To date, a limited number of research focused on the role of FPR in HCC prognosis after treated by TACE and RFA. In our research, the effects of FPR on overall survival in BCLC A-C HCC patients treated by TACE and RFA were explored.
Materials and Methods
Patient Selection
The experiment protocol was approved by the Ethics Committee of Beijing Ditan Hospital, Capital Medical University. Our research strictly abided by the Declaration of Helsinki. Informed consent was signed by all participants prior to the treatment.
In this study, 240 patients at stage BCLC A-C treated by TACE and RFA at Beijing Ditan Hospital of Capital Medical University from May 2011 to November 2018 were analyzed. The clinicopathological features were recorded according to the criteria of the American Association for the Study of Liver Disease.10
Inclusion criteria: (1) liver function before operation: Child-Pugh Class A or Class B; (2) without immunity-associated or hematological diseases; (3) without other kinds of malignancies; (4) without infectious diseases except for hepatitis B or C; and (5) preoperative FPR collected <1 week before treatment.
Exclusion criteria: (1) critical diseases, such as hepatic or heart failure; (2) gastric variceal or esophageal hemorrhage within 1 month; (3) severe coagulation disorders, (4) neoadjuvant/adjuvant chemoradiotherapy.
Clinical Parameters and Laboratory Results
Based on the medical record of the patient, the basic information was collected (number of tumors, the maximum diameter of the tumor (cm), sex, Child-Pugh classification, age, presence of liver cirrhosis, AFP concentration in serum, Fib, serum CEA, prealbumin (PA), count of platelets, lymphocytes, monocytes, and neutrophils, presence of thrombosis in portal vein tumor). We collected peripheral blood from the patients between 7:30–9:30 am in 1 week before combination treatment. FPR, PLR, LMR and NLR refer to fibrinogen/prealbumin, platelet/lymphocyte, lymphocyte/monocyte, and neutrophil/lymphocyte ratios, respectively. Prognostic nutritional index (PNI)= albumin (g/L)+ 5× lymphocyte (109 /L).
The Follow-Ups and OS (Overall Survival)
The regular follow-ups were performed every 4 weeks through medical records, emails and telephone call until November 2021. The CT, MRI, or triphasic scanning technique was used to evaluate the therapeutic effects based on mRECIST (modified RECIST).11 The main end point in our research was OS, which was defined as the interval between diagnosis and the last follow-up or death.
Statistics
The data were analyzed using IBM SPSS 22.0 statistical software (SPSS Inc) and R version 3.2.3. Figures and survival curves were generated by using GraphPad Prism 6.0. The optimal threshold value for FPR was calculated based on the 5-year OS, and X-tile software version 3.6.1 was used. Chi-square or Student’s t-test was used to compare the differences. Survival rate differences were assessed by using Log rank test and Kaplan–Meier curve. Cox proportional hazards model was used to conduct survival analysis and identify possible prognosis-related factors. If p < 0.05, the difference was regarded as statistically significant.
Results
The Characteristics of the Patients
The characteristics of 240 patients at baseline are listed in Table 1. Two hundred (83.33%) cases were male and 40 (16.67%) female. They were aged 59 years on average (range, 35–87 years). A total of 200 (200%) cases were diagnosed with liver cirrhosis. According to Child‐Pugh classification, a total of 153 (63.75%) patients were scored as grade A and 87 (36.25%) patients as grade B prior to the treatment of RFA and TACE. Fifty-eight (24.17%) patients had thrombosis in the portal vein. Lung metastasis, metastasis into lymph nodes, metastasis to bones, and other types of metastases were observed in 10, 6, 1, and 3 cases, respectively.
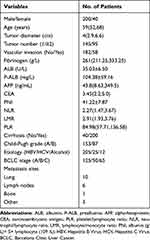 |
Table 1 Clinical and Pathological Features |
The results showed that the ideal cut-off value was 3.3 for FPR (Figure 1). According to the cut-off value, the patients included in his study were assigned into low and high subgroups according to each biomarker. FPR was markedly related to Child-Pugh grade (p = 0.001), tumor diameter (p = 0.048), BCLC stage (p = 0.026), and PNI (p = 0.011) (Table 2). FPR was remarkably related to Child-Pugh grade (p = 0.014), and PNI (p = 0.047) (Table 3). FPR was remarkably related to Child-Pugh grade (p = 0.043), and LMR (p = 0.013) (Table 4). FPR was markedly related to LMR (p=0.028) (Table 5).
 |
Table 2 Correlation Between Clinical Characteristics and FPR in 240 Subjects with HCC |
 |
Table 3 Correlation Between Clinical Characteristics and FPR in BCLC a |
 |
Table 4 Correlation Between Clinical Characteristics and FPR in BCLC B |
 |
Table 5 Correlation Between Clinical Characteristics and FPR in BCLC C |
Survival Curves Between High and Low FPR
Up to the last day of the follow-up, there were 203 deaths in this study. Kaplan–Meier method and Log rank test were used to compare the survival curve between high and low FPR. The cumulative overall survival at 10, 20, 30, 40, 50, and 60 months was 62.5%, 42.91%, 29.58%, 19.58%, 11.25%, and 6.25%, respectively (Figure 2A). In Figure 2B, the high FPR (>3.3) was related to poor 5-year OS. Additionally, we compared the survival curves according to pathological phases.
 |
Figure 2 5 years’ OS in 240 subjects with HCC. Abbreviation: FPR, fibrinogen/prealbumin ratio. Note: Cumulative overall survival curve (A) and Kaplan–Meier curves of FPR for 5-year OS (B). |
In BCLC C (Figure 3F), higher FPR was remarkably related to poorer 5-OS (p = 0.01) compared with lower FPR. The cumulative overall survival at 10, 20, 30, 40, 50, and 60 months was 41.53%, 21.53%, 12.31%, 6.15%, 1.54%, and 1.54%, respectively (Figure 3E). In BCLC A (Figure 3B) and B (Figure 3D), higher FPR showed a worse result in survival compared with lower FPR; however, statistically significant difference was not observed. The cumulative overall survival at 10, 20, 30, 40, 50, and 60 months was 74.4%, 55.2%, 37.6%, 24.8%, 16.8%, 8%, and 60%, 40%, 32%, 24%, 10%, 8%, respectively (Figure 3A, Figure 3C).
Prognostic Value of FPR for 5-Year OS
In this study, Cox proportion regression model was used to investigate the prognostic effects of the basic characteristics and FPR, PLR, LMR, NLR, and PNI in HCC. According to the univariate analysis, the tumor diameter (P = 0.001), vascular invasion (P = 0.004), AFP (P = 0.000), cirrhosis (P = 0.000), NLR (P = 0.000), LMR (P = 0.000), PLR (P = 0.00), and FPR (P = 0.000) were all associated with 5-year OS (Table 6).
 |
Table 6 Overall Survival Analysis |
Besides, multivariate logistic regression analysis showed that FPR (95% confidence interval: 1.006–1.013, hazard ratio: 1.009) was a prognostic factor for the prediction of 5-year OS in HCC. Moreover, the size of the tumor, vascular invasion, AFP, cirrhosis, NLR, LMR, and PLR also showed prognostic value in predicting 5-year OS (Table 6).
Moreover, a nomogram was established for predicting OS, and 8 significant variables proved by multivariate analysis were used (Figure 4A). C-index was 0.604 (95% CI: 0.521–0.756) as shown by the model (Figure 4B).
Discussion
HCC diagnosis at an early stage is highly related to the prognosis and could increase the 5-year survival rate.12 Nowadays, BCLC stage and cell differentiation classification are the most effective and important prognostic indicators for this disease. AFP, which regulates and monitors HCC, still has limitations in detecting HCC, with unsatisfactory diagnostic function.13 Therefore, it is urgent to discover new and useful markers to evaluate HCC development.
It was demonstrated that HCC development could be influenced by nutritional status and coagulation. Fibrinogen, a kind of reacting glycoprotein in the acute phase, is mainly generated in hepatocytes.14 A research by Zhu et al15 presented that mRNA expression of fibrinogen was upregulated in vivo and in vitro, and the elevated fibrinogen level in plasma was related to thrombosis in tumor. Additionally, it was suggested that an elevated fibrinogen level in plasma was associated with the prognosis of ovarian cancer.16,17 Thus, the FPR level might be increased in malignancies.
A high level of FPR before operation was remarkably related to a larger size of tumor and a more advanced HCC stage, indicating that FPR could indicate HCC phenotype. These results were consistent with previous research on HCC, CRC, and GC.18–20 Wang et al21 demonstrated that the ratio of prealbumin/fibrinogen was decreased in critical acute pancreatitis and negatively correlated with its development. Furthermore, a high level of FPR in circulating blood was remarkably related to worse OS of patients with HCC after treatment of TACE and RFA, implying that FPR could be independently used as a factor predicting the prognosis. Certain research also presented that FPR before operation could be used in predicting the prognosis of multiple solid tumors.16,22
The mechanisms underlying the relationship between FPR and HCC are still unclear. Some hypothesis may explain our results. Firstly, fibrinogen might influence the biological activities and function of cancer cells.23 The connection between VEGF, PGF, TGF-B and Fib could result in proliferation, metastasis, and angiogenesis of cancer cells and suppress cellular apoptosis.24 Secondly, platelet-fibrin microthrombi provided a barrier to separate tumor cells and natural killer cells to prevent their contact and enhance metastasis.25 Fib also provided a bridge between normal cells and tumor cells, and increased the adhesion between cancer cell emboli in the vessels.26 Thirdly, pAlb in circulating blood could indicate nutrition status and chronic inflammation in the patients with malignancy. Lack of nutrition is a common disorder in cancer,27 and nutritional status remarkably influenced the tolerance to chemotherapy and survival.28
However, there are some limitations in this study. Firstly, the study was retrospective in nature, and the sample size was relatively small, which might result in unavoidable bias. Secondly, there might be bias in the evaluation of the predictive effects of the markers since it was a single-center study. Thus, our findings remain to be further verified by prospective, multiple-center and large-scale studies.
Conclusion
Our research indicated that FPR was a potential indicator for patients with BCLC A-C hepatocellular carcinoma after treatment of RFA and TACE.
Acknowledgment
The current research was funded by Capital Health development Scientific Research project (Shou fa 2022-2-2175).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
2. Feng R-M, Zong Y-N, Cao S-M, et al. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun. 2019;39:22. doi:10.1186/s40880-019-0368-6
3. Tada T, Kumada T, Hiraoka A, et al. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int. 2020;40:968–976. doi:10.1111/liv.14405
4. Ji F, Liang Y, Fu SJ, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC Cancer. 2016;16:137. doi:10.1186/s12885-016-2189-1
5. Göbel K, Eichler S, Wiendl H, et al. The coagulation factors fibrinogen, thrombin, and factor XII in inflammatory disorders-a systematic review. Front Immunol. 2018;9:1731. doi:10.3389/fimmu.2018.01731
6. Gu L, Ma X, Li H, et al. Prognostic value of preoperative inflammatory response biomarkers in patients with sarcomatoid renal cell carcinoma and the establishment of a nomogram. Sci Rep. 2016;6(1):23846. doi:10.1038/srep23846
7. Jiang HG, Li J, Shi SB, et al. Value of fibrinogen and D-dimer in predicting recurrence and metastasis after radical surgery for non-small cell lung cancer. Med Oncol. 2014;31:22. doi:10.1007/s12032-014-0022-8
8. Sato S, Shiozawa M, Nukada S, et al. Preoperative pre-albumin concentration as a predictor of short-term outcomes in elderly patients with colorectal cancer. Anticancer Res. 2021;41:5195–5202. doi:10.21873/anticanres.15338
9. Jermihov A, Tsalatsanis A, Kulkarni S, et al. Effect of lowest postoperative pre-albumin on outcomes after robotic-assisted pulmonary lobectomy. JSLS. 2021;25:
10. Bruix J, Sherman M. American association for the study of liver diseases. management of hepatocellular carcinoma: an update. Hepatology. 2011;53:
11. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi:10.1055/s-0030-1247132
12. Chen H, Zhang Y, Siwen L, et al. Direct comparison of five serum biomarkers in early diagnosis of hepatocellular carcinoma. Cancer Manag Res. 2018;10:1947–1958. doi:10.2147/CMAR.S167036
13. Erstad DJ, Tanabe KK. Hepatocellular carcinoma: early-stage management challenges. J Hepatocell Carcinoma. 2017;4:81–92. doi:10.2147/JHC.S107370
14. Tiscia GL, Margaglione M. Human fibrinogen: molecular and genetic aspects of congenital disorders. Int J Mol Sci. 2018;19:1597. doi:10.3390/ijms19061597
15. Zhu WL, Fan BL, Liu DL, et al. Abnormal expression of fibrinogen gamma (FGG) and plasma level of fibrinogen in patients with hepatocellular carcinoma. Anticancer Res. 2009;29:2531–2534.
16. Chen W, Shan B, Zhou S, et al. Fibrinogen/albumin ratio as a promising predictor of platinum response and survival in ovarian clear cell carcinoma. BMC Cancer. 2022;22:92. doi:10.1186/s12885-022-09204-0
17. McKendry K, Duff S, Huang Y, et al. The value of human epididymis 4, D-dimer, and fibrinogen compared with CA-125 alone in triaging women presenting with pelvic masses: a retrospective cohort study. Acta Obstet Gynecol Scand. 2021;100:1239–1247. doi:10.1111/aogs.14126
18. Huang L, Zhuning M, Zuojian H, et al. Diagnostic value of fibrinogen to prealbumin ratio and gamma-glutamyl transpeptidase to platelet ratio in the progression of AFP-negative hepatocellular carcinoma. Cancer Cell Int. 2020;20:77. doi:10.1186/s12935-020-1161-y
19. Ying HQ, Sun F, Liao YC, et al. The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer. Ther Adv Med Oncol. 2021;13:17588359211022886. doi:10.1177/17588359211022886
20. Zhang X, Zhao W, Chen X, et al. Combining the Fibrinogen-to-Pre-Albumin Ratio and Prognostic Nutritional Index (FPR-PNI) predicts the survival in elderly gastric cancer patients after gastrectomy. Onco Targets Ther. 2020;13:8845–8859. doi:10.2147/OTT.S264199
21. Yue W, Liu Y, Ding W, et al. The predictive value of the prealbumin-to-fibrinogen ratio in patients with acute pancreatitis. Int J Clin Pract. 2015;69:1121–1128. doi:10.1111/ijcp.12682
22. Baibei L, Deng H, Zhou Z, et al. The Prognostic value of the Fibrinogen to pre-albumin ratio in malignant tumors of the digestive system: a systematic review and meta-analysis. Cancer Cell Int. 2022;22:22. doi:10.1186/s12935-022-02445-w
23. Rybarczyk BJ, Simpson-Haidaris PJ. Fibrinogen assembly, secretion, and deposition into extracellular matrix by MCF-7 human breast carcinoma cells. Cancer Res. 2000;60:2033–2039.
24. Martino MM, Hubbell JA. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010;24:4711–4721. doi:10.1096/fj.09-151282
25. Budnik I, Shenkman B, Savion N. Role of G protein signaling in the formation of the fibrin (ogen)-integrin αIIbβ3-actin cytoskeleton complex in platelets. Platelets. 2016;27:563–575. doi:10.3109/09537104.2016.1147544
26. Nakayama H, Kitayama J, Nagawa H. Rat gastric adenocarcinoma cell line BV9 avidly adheres to lymphatic endothelium under lymphatic flow condition. J Exp Clin Cancer Res. 2002;21:289–294.
27. Shao J, Jing L, Zhang XL, et al. Prognostic significance of the preoperative controlled nutritional status score in lung cancer patients undergoing surgical resection. Nutr Cancer. 2021;73:2211–2218. doi:10.1080/01635581.2020.1850814
28. Okugawa Y, Shirai Y, Toiyama Y, et al. Clinical burden of modified Glasgow prognostic scale in colorectal cancer. Anticancer Res. 2018;38:1599–1610. doi:10.21873/anticanres.12390
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



