Back to Journals » Clinical Interventions in Aging » Volume 17
The Prognostic Factors of Bloodstream Infection in Immunosuppressed Elderly Patients: A Retrospective, Single-center, Five-year Cohort Study
Authors Lin H , Gao Y, Qiu Y, Zhu H, Zhang S, Summah HD, Shi G, Cheng T, Yang Z , Feng Y
Received 19 August 2022
Accepted for publication 6 November 2022
Published 18 November 2022 Volume 2022:17 Pages 1647—1656
DOI https://doi.org/10.2147/CIA.S386922
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Hongxia Lin,1,2,* Yulian Gao,1,2,* Yanli Qiu,3,* Haixing Zhu,1,2 Shengxiong Zhang,1,2 Hanssa Dwarka Summah,4 Guochao Shi,1,2 Tingting Cheng,5,* Zhitao Yang,6 Yun Feng1,2
1Department of Respiratory and Critical Care Medicine, Ruijin Hospital Affiliated Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China; 2Institute of Respiratory Diseases, School of Medicine, Shanghai Jiao Tong University, Shanghai, 20025, People’s Republic of China; 3Department of Anesthesia, Ruijin Hospital Affiliated Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China; 4Poudre D’Or Chest Hospital, Rivière du Rempart, Mauritius; 5Department of Respiratory and Critical Care Medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, 20025, People’s Republic of China; 6Department of Emergency, Ruijin Hospital Affiliated Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhitao Yang; Yun Feng, Email [email protected]; [email protected]
Introduction: Elderly patients with immunosuppressive status may have increased risk of mortality. At present, few studies have explored the clinical characteristics of the elderly immunosuppressed population with bloodstream infection. Our objectives were to evaluate the prognostic factors in immunosuppressed elderly patients with bloodstream infection.
Methods: Three hundred and seventy-six elderly patients who were diagnosed with bloodstream infection in immunosuppressive status while receiving treatment in our hospital were selected from 2015 to 2019. The demographic data, underlying diseases, comorbidity, inducement, complications, pathogen sources, etiologies and the antibiotic therapy were analyzed between 90-day survival groups and 90-day mortality groups. The prognostic factors of 90-day mortality were evaluated by univariate logistic regression analysis and multivariate logistic regression analysis.
Results: The clinical characteristics of 376 immunosuppressed elderly people diagnosed with bloodstream infection were analyzed, and among those people about 111 were 90-day mortality. By univariate logistic regression analysis and multivariate logistic regression analysis, we found ICU admission (OR: 2.052, 95%CI: 1.088– 3.871, p=0.026), the decrease in BMI (OR: 0.307, 95%CI: 0.130– 0.723, p=0.007), coronary heart disease (OR: 2.028, 95%CI: 1.078– 3.816, p=0.028), biliary infection (OR: 4.406, 95%CI: 1.794– 10.821, p=0.001) and the use of tigecycline (OR: 2.480, 95%CI: 1.195– 5.147, p=0.015) were significantly different between the 90-day survival and 90-day mortality groups.
Conclusion: ICU admission, coronary heart disease, biliary infection, and the use of tigecycline were the independent prognostic risk factors of 90-day mortality in immunosuppressed elderly people, and the decrease in BMI was the protective factor, which would have the benefit of discriminating the prognostic factors in immunosuppressed elderly people with bloodstream infection.
Keywords: bloodstream infection, elderly people, mortality, immunosuppressive states, prognosis
Introduction
Immune suppression generally refers to low immune response to inflammation, and it is widely thought that a counter-inflammatory response aiming to inhabit overzealous inflammation may result in immune suppression.1 This term is commonly used to qualify the immune status of patients with sepsis or systemic inflammatory response syndrome recently, which suggests that immune suppression indicates a status of immune failure making patients more vulnerable to the effects of diseases.2 In general, typical immunosuppressed patients are usually transplant recipients and those with hematological malignancy, while atypical immunosuppression status may be present in patients with diabetes mellitus, liver cirrhosis, burns, and other diseases.1 A study showed critical surgery could also lead to immunosuppression,3 and postoperative people are susceptible to opportunistic pathogen infections.4,5 Immunosuppressed people are more vulnerable to bacteria and viruses, which cause inflammatory and infection. Researchers discovered that microorganisms were particularly likely to colonize the respiratory tract to cause severe pneumonia, resulting in very high rates of infection and mortality during hospitalization for patients with immunosuppressive status.6–8
As the World Health Organization estimated, the populations over 65 years old are increasing roughly from five hundred and twenty-four million people in 2010 to nearly one point five billion people in 2050, the national public health system is facing huge challenges in elderly populations.9 Several investigations showed elderly people have a higher mortality or that age was the independent risk factor in immunosuppressive status. For instance, an eight-year retrospective study, which enrolled 42 esophageal cancer patients with photodynamic therapy in a Japanese cancer center, showed elderly people had a higher mortality compared with nonelderly people.10 A nine-year Spanish retrospective study, which enrolled 637 patients underwent peripheral blood haploidentical stem cell transplantation, also presented that patient age at transplantation was an independent prognostic factor for the first-year mortality (HR=1.05, p=0.002).11 What is more, a three-year prospective observational study, which enrolled 65 kidney transplant patients in the intensive care unit (ICU) of the transplant center of Prague, indicated that age had a close relationship with one-year mortality (HR=1.08, p=0.048).12 With China entering an aging society, high quality of health care for elderly patients has not been put into attention. In the process of aging, a body with deteriorating immunity is more prone to harmful diseases, causing high mortality and morbidity. Because of the weakened immunity, aging patients were more susceptible to infections.9 Previous investigations indicated that age could be used as a sign in severe infection.13 That is exactly why elderly immunosuppressed patients with bloodstream infection (BSI) should be paid more attention. However, there are few studies focusing on the prognostic factors of BSI in the elderly immunosuppressed population.
We collected the clinical data of 376 elderly people in Ruijin Hospital for the purpose of exploring the difference in the clinical features such as primary illness, complications and clinical treatment between 90-day survival and 90-day mortality groups, and to evaluate the independent prognostic factors of BSI in immunosuppressed elderly people.
Materials and Methods
Subjects
This was a single-center, retrospective study which enrolled totally 376 immunosuppressed elderly patients who developed BSI during their hospital admission in the Ruijin Hospital Affiliated to Shanghai Jiao Tong University School from 2015 to 2019.
Data Collection
We collected medical information about all enrolled patients to assess clinical features in immunosuppressed elderly people with BSI who received treatment in Ruijin Hospital from 2015 to 2019. Those medical records including demographic data, underlying disease, underlying conditions, inducements, complications, pathogen sources, etiology, and antibacterial therapy were collected.
Inclusion criteria: age ≥65 years and diagnosis of immunosuppression were two criteria for inclusion in the final study group. Immunosuppressed patients included the patients under immunosuppressive therapy (use of drugs like corticosteroids [prednisone equivalent > 20 mg/day] for at least 14 days, or methotrexate, cyclosporine, azathioprine, or biological modifiers within three months), posttransplantation includes solid-organ, stem cell, or bone marrow transplantation, cancer includes solid organ and hematological system tumors, burns, diabetes, liver cirrhosis, and critical postoperative condition.5,7–9,14–16
Exclusion criteria: patients age <65 years and incomplete data were excluded from the study.
Statistical Analysis
Continuous variables were shown as mean(X) ± standard deviation and analyzed by Student’s t-test. Categorical variables were presented as absolute numbers and percentages and compared by chi-squared (χ2) test or Fisher's exact test. Firstly, the factors, which indicated the p-value were less than 0.05, were selected after using chi-squared test or Fisher's exact test. Secondly, the factors obtained by using chi-squared test or Fisher's exact test were analyzed by univariate logistic regression analysis, the factors were acquired if the p-value was less than 0.05. Finally, the factors obtained by using univariate logistic regression analysis were analyzed by multivariate logistic regression analysis, the factors were acquired if the p-value was less than 0.05. The factors obtained by using multivariate logistic regression analysis were the prognostic factors. We used univariate logistic regression and multivariate logistic regression to analyze different clinical factors related to immunosuppressive patients with bloodstream infection. Patients were compared between 90-day survival and 90-day mortality groups, the data about underlying conditions, underlying disease, inducement, complications, pathogen sources, etiology and antibacterial therapy were analyzed. Clinical featured and prognostic factors of BSI were evaluated by operating SPSS version 26.0. P<0.05 was considered statistically significant.
Statement of Ethics Compliance
Our research was based on previous clinical data and other personal information like the patient’s name, phone number, address were not involved. The patient informed consent exemption was approved and the ethical clearance had been acquired from the Medicine Ethics committee of Ruijin Hospital Affiliated to Shanghai Jiao Tong University School. We claim that we cover patient data confidentially and compliance with the Declaration of Helsinki.
Results
The Study Population
A total of 376 immunosuppressed elderly patients with bloodstream infection admitted to our hospital from 2015 to 2019 were included in our study. Two hundred and sixty-five patients were 90-day survival while 111 patients 90-day mortality. Among them about 261 patients were male and 115 were female. The characteristics of included patients were summarized in Table 1. Sex, smoking history, and drinking history made no difference between the two groups. In the 90-day survival group, 188 (70.94%) were male and 77 (29.06%) were female. In contrast, in the 90-day mortality group, 73 (65.77%) were male and 38 (34.23%) were female. BMI, PCT >0.5 µg/L, ICU admission, coronary heart disease, and cardiac dysfunction were evidently different between the two groups (p<0.05).
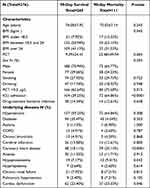 |
Table 1 Clinical Characteristics of Bloodstream Infection Immunosuppressive Patients |
The Underlying Condition
In the analysis of underlying conditions, acute myocardial infarction (1.51% vs 11.71%) and biliary infection (4.91% vs 12.61%) were obviously different between the two groups (p<0.05). The whole results were shown in Table 2. The other underlying diseases had no difference between the two groups.
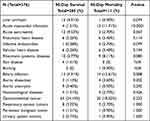 |
Table 2 Primary Illness Characteristics of Bloodstream Infection in Immunosuppressive Patients |
The Inducements and Complications
The inducements and complications were shown in Table 3, the inducements were mainly surgery (39.25% vs 36.04%), chemotherapy (3.02% vs 5.40%) had no significant difference between the two groups (p>0.05). In the analysis of complications, acute renal failure (9.43% vs 23.42%) and acute respiratory failure (7.55% vs 14.41%) were obviously different between two groups (p<0.05). The other complications had no difference between the two groups.
 |
Table 3 Clinical Characteristics of Bloodstream Infection in Immunosuppressive Patients |
The Pathogen Sources, Etiologies and Antibiotic Therapy
The pathogen sources of BSI were mainly the urinary system (3.77% vs 1.80%), digestive system (12.83% vs 19.82%) and respiratory system (19.25% vs 29.73%). The data indicated sources of pathogens from respiratory tract was statistically different between the two groups (Table 3).
The etiologies of pathogens isolating in the patients mainly included Acinetobacter baumannii (5.28% vs 8.11%), Escherichia coli (23.02% vs 17.12%), Enterococcus faecium (6.79% vs 8.11%), Klebsiella pneumoniae (17.36% vs 17.12%), Staphylococcus aureus (8.68% vs 9.01%), Staphylococcus epidermidis (7.55% vs 10.81%). The infectious microorganisms had no significant difference between the two groups. The results were shown in Table 4 and Supplementary Table 1.
 |
Table 4 The Pathogen Etiologies of Bloodstream Infection in Immunosuppressive Patients |
The antibiotic therapies were mainly carbapenems (60% vs 69.37%), tigecycline (8.68% vs 20.72%), third generation cephalosporins (19.25% vs 14.41%), vancomycin (21.13% vs 34.23%), linezolid (13.58% vs 13.51%). The data indicated the use of tigecycline and the use of vancomycin were statistically different between the two groups (Table 5).
 |
Table 5 The Antibacterial Therapy of Bloodstream Infection in Immunosuppressive Patients |
Prognostic Factors of 90-day Mortality
The factors related to immunosuppressed elderly patients were assessed by univariate and multivariate logistic regression analysis. ICU admission (OR: 2.052, 95%CI: 1.088–3.871, p=0.026), the decrease in BMI (OR: 0.307, 95%CI: 0.130–0.723, p=0.007), coronary heart disease (OR: 2.028, 95%CI: 1.078–3.816, p=0.028), biliary infection (OR: 4.406, 95%CI: 1.794–10.821, p=0.001) and tigecycline (OR: 2.480, 95%CI: 1.195–5.147, p=0.015) were significantly different between the two groups. Thus, we concluded that ICU admission, coronary heart disease, biliary infection, and tigecycline were the independent risk factors of 90-day mortality for immunosuppressed elderly patients. However, the decrease in BMI was the protective factor for the immunosuppressed elderly patients. The results were shown in Table 6.
 |
Table 6 Univariate and Multivariate Analysis of Factors Related to 90-day Mortality in Immunosuppressive Patients |
Discussion
Many previous investigations evaluate prognostic factors with BSI in typical immune suppression. Yet the investigations of typical immunosuppressive states and atypical immunosuppressive status which are related with BSI are rare. Moreover, the evaluation of medical features of elderly patients in overall immunosuppressive status are scarce. The objectives of our study were to evaluate the prognostic factors of aging people not only in typical immunosuppressive status with solid organ tumor, hematological system tumor, transplantation status, immunosuppressive treatment, but also in atypical immunosuppressive status with diabetes, liver cirrhosis, critical postoperative condition, and burning status. We analyzed the prognostic factors not only in typical immunosuppressed elderly patients, but also in atypical immunosuppressed elderly patients. ICU admission, coronary heart disease, biliary infection, and the use of tigecycline were independent risk factors of 90-day mortality for immunosuppressed elderly patients. However, the decrease in BMI was the protective factor for immunosuppressed elderly patients.
Older patients are at a higher risk of infection-related mortality than are younger patients.17–19 Studies demonstrated that the patient’s immune state had a close link with their clinical prognosis.7,8 Previous investigations indicated that different immunosuppressive conditions had different prognostic factors. For instance, in the malignancies, the independent risk factors were age, comorbidities, ICU admission, pathogen sources, etiologies, antibiotic therapy, antibiotic-resistant organisms, the underlying diseases, hypoalbuminemia, and shock.20–26 Many investigations mainly showed the prognostic risk factors of many immunosuppressive diseases was age, the prognostic factors of death in overall BSI in immunosuppressed elderly people were still unclear. In our study, the prognostic factors in overall immunosuppressed elderly patients were analyzed.
Bloodstream infection is closely associated with high mortality in ICU.5 Immunosuppressed people were vulnerable to severe infections because of ICU admission. The high mortality in immunosuppressive status was also caused by infectious complications.16 Studies showed that diabetic patients, who had an experience of ICU admission, presented high mortality when developing bacterial infections, mostly depending on the age of the patients.15 Previous investigations also showed that ICU admission was an indicator in hematopoietic stem cell transplant patients who developed a bloodstream infection.16,20 This suggests that ICU admission was closely related with bloodstream infection. In our study, ICU admission was an independent prognostic risk factor of 90-day death for immunosuppressed elderly people (OR: 2.052, 95%CI: 1.088–3.871, p=0.026).
Moreover, rarely research demonstrated the association between BSI and coronary heart disease, the same applies to biliary tract infections. A previous study had evaluated that coronary heart disease was the risk factor for pneumonia when patients were in a postoperative critical condition.27 Our study got a similar result. In our study, coronary heart disease was an independent prognostic risk factor of 90-day death for immunosuppressed elderly people (OR: 2.028, 95%CI: 1.078–3.816, p=0.028).
Previous studies also showed that the infectious site was more prone to biliary duct infection in solid organ transplantation and biliary complication was the prognostic risk factor of carbapenem-resistant Enterobacteriaceae infection after liver transplantation.28,29 Our study acquired the similar conclusion. In our study, the biliary infection could be an independent prognostic risk factor of 90-day death for immunosuppressed elderly people (OR: 4.406, 95%CI: 1.794–10.821, p=0.001).
Regarding the link between the use of antibiotic and bloodstream infection, several investigations presented that the use of fluoroquinolones, broad-spectrum cephalosporins, and carbapenems increased the risk of resistant bacterial infection, which indicated poor prognosis.30 At present, tigecycline has been widely used for bloodstream infection. However, the use of tigecycline may not achieve the desired therapeutic effect. A meta-analysis showed the use of tigecycline presented fewer benefits compared with standard antimicrobial drugs in the treatment of serious infections, and the rate of treatment success was lower with tigecycline than with control antibiotic treatments.31 A study also found that the use of tigecycline was a risk indicator of 28-day mortality.30 Our study obtained a similar conclusion. In our study, the use of tigecycline could be an independent prognostic risk factor of 90-day death for immunosuppressed elderly people (OR: 2.480, 95%CI: 1.195–5.147, p=0.015).
In addition, we evaluated the association between BMI and mortality in the immunosuppressed elderly patients. Some investigations showed that different BMI ranges presented prognostic factor in bacterial infections. For instance, a study indicated that improved 90-day survival in obese (BMI >35 kg/m2) COVID-19 ECMO patients, obesity is not a risk factor for poor prognosis.32,33 The other study showed that BMI <24.9 kg/m2 was associated with increased mortality risk.34 This may indicate that BMI had an effect on the prognosis of infectious diseases. In our study, BMI <18.5 kg/m2 was a protective factor of 90-day death for immunosuppressed elderly people (OR: 0.307, 95%CI: 0.130–0.723, p=0.007). Our result was contrary to previous literature, this may be related to the baseline conditions of the enrolled patients, 90-day survival patients with poor nutrition due to infection and long hospitalization took up a certain percentage.
Some limitations of our study should be considered. Firstly, the collection of clinical data was in a single center, the results of other different medical centers may not be represented. Secondly, there might exist a bias of selection of clinical information. Thirdly, the analysis was based on the previous data, further confirmation by large sample cohort studies may be needed.
Conclusions
The most of clinical characteristics were similar in immunosuppressed elderly patients with bloodstream infection. ICU admission, coronary heart disease, biliary infection, the use of tigecycline were the independent prognostic risk factors of 90-day mortality in immunosuppressed elderly people and the decrease in BMI was the protective factor.
Abbreviations
BMI, body mass index; PCT, procalcitonin; ICU, intensive care unit; BSI, bloodstream infection; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethics Guideline
Our research was based on previous clinical data and other personal information like the patient’s name, phone number, address were not involved. The patient informed consent exemption was approved and the ethical clearance had been acquired from the Medicine Ethics committee of Ruijin Hospital Affiliated to Shanghai Jiao Tong University School. We claim that we cover patient data confidentially and compliance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was supported by the National Natural Science Foundation of China (No. 82170086, No. 81900077), Shanghai Shenkang Hospital Development Center Clinical Science and Technology Innovation Project (SHDC12018102), Shanghai Municipal Key Clinical Specialty (shslczdzk02202), Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Diseases (20dz2261100).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lin H, Yang L, Fang J, et al. Clinical characteristics of bloodstream infection in immunosuppressed patients: a 5-year retrospective cohort study. Front Cell Infect Microbiol. 2022;12:796656. doi:10.3389/fcimb.2022.796656
2. Cavaillon JM, Giamarellos-Bourboulis EJ. Immunosuppression is inappropriately qualifying the immune status of septic and SIRS patients. Shock. 2019;52(3):307–317. doi:10.1097/SHK.0000000000001266
3. Si X, Ji G, Ma S, et al. In-situ-sprayed dual-functional immunotherapeutic gel for colorectal cancer postsurgical treatment. Adv Healthc Mater. 2021;10(20):e2100862. doi:10.1002/adhm.202100862
4. Shipman P, Highland J, Witt B, Alt J. Non-invasive fungal sinusitis as a complication of a steroid-eluting stent following endoscopic sinus surgery: a case report. Ann Otol Rhinol Laryngol. 2022;131(6):678–682. doi:10.1177/00034894211036844
5. Mada PK, Saldaña Koppel DA, Al Shaarani M, Joel Chandranesan AS. Primary cutaneous Aspergillus fumigatus infection in immunocompetent host. BMJ Case Rep. 2020;13(2):e233020. doi:10.1136/bcr-2019-233020
6. Li L, Hsu SH, Wang C, et al. Characteristics of viral pneumonia in non-HIV immunocompromised and immunocompetent patients: a retrospective cohort study. BMC Infect Dis. 2021;21(1):767. doi:10.1186/s12879-021-06437-5
7. Lindell RB, Nishisaki A, Weiss SL, Traynor DM, Fitzgerald JC. Risk of mortality in immunocompromised children with severe sepsis and septic shock. Crit Care Med. 2020;48(7):1026–1033. doi:10.1097/CCM.0000000000004329
8. McCann S, Byrne JL, Rovira M, et al. Outbreaks of infectious diseases in stem cell transplant units: a silent cause of death for patients and transplant programmes. Bone Marrow Transplant. 2004;33(5):519–529. doi:10.1038/sj.bmt.1704380
9. Zhang J, Du Z, Bi J, et al. Comparison of clinical characteristics and outcomes of pyogenic liver abscess patients < 65 years of age versus ≥ 65 years of age. BMC Infect Dis. 2019;19(1):233. doi:10.1186/s12879-019-3837-2
10. Nishikawa M, Yamamoto Y, Kushida S, et al. Assessment of photodynamic therapy as a salvage treatment for local failure after chemoradiotherapy or radiotherapy for esophageal cancer in patients aged 80 years or older. DEN Open. 2022;3(1):e167. doi:10.1002/deo2.167
11. Hernández-Sánchez A, Cabero A, Fonseca M, et al. CT-169 cytokine release syndrome after peripheral blood haploidentical stem cell transplantation with post-transplant cyclophosphamide: time of onset matters. Clin Lymphoma Myeloma Leuk. 2022;22(Suppl 2):S437–S438. doi:10.1016/S2152-2650(22)01653-6
12. Protus M, Uchytilova E, Indrova V, et al. Sepsis affects kidney graft function and one-year mortality of the recipients in contrast with systemic inflammatory response. Front Med. 2022;9:923524. doi:10.3389/fmed.2022.923524
13. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi:10.1097/01.ccm.0000194535.82812.ba
14. Turkkan S, Beyoglu MA, Sahin MF, Yazicioglu A, Tezer Tekce Y, Yekeler E. COVID-19 in lung transplant recipients: a single-center experience. Transpl Infect Dis. 2021;23(5):e13700. doi:10.1111/tid.13700
15. Blanchard F, Charbit J, Van der Meersch G, et al. Early sepsis markers in patients admitted to intensive care unit with moderate-to-severe diabetic ketoacidosis. Ann Intensive Care. 2020;10(1):58. doi:10.1186/s13613-020-00676-6
16. Barlas T, Inci K, Aygencel G, et al. Infections in hematopoietic stem cell transplant patients admitted to Hematology intensive care unit: a single-center study. Hematology. 2021;26(1):328–339. doi:10.1080/16078454.2021.1905355
17. Howlader N, Mariotto AB, Besson C, et al. Cancer-specific mortality, cure fraction, and noncancer causes of death among diffuse large B-cell lymphoma patients in the immunochemotherapy era. Cancer. 2017;123(17):3326–3334. doi:10.1002/cncr.30739
18. Lugtenburg P, Silvestre AS, Rossi FG, et al. Impact of age group on febrile neutropenia risk assessment and management in patients with diffuse large B-cell lymphoma treated with R-CHOP regimens. Clin Lymphoma Myeloma Leuk. 2012;12(5):297–305. doi:10.1016/j.clml.2012.06.004
19. Morrison VA, Weller EA, Habermann TM, et al. Patterns of growth factor usage and febrile neutropenia among older patients with diffuse large B-cell non-Hodgkin lymphoma treated with CHOP or R-CHOP: the Intergroup experience (CALGB 9793; ECOG-SWOG 4494). Leuk Lymphoma. 2017;58(8):1814–1822. doi:10.1080/10428194.2016.1265111
20. Torres VB, Azevedo LC, Silva UV, et al. Sepsis-associated outcomes in critically ill patients with malignancies. Ann Am Thorac Soc. 2015;12(8):1185–1192. doi:10.1513/AnnalsATS.201501-046OC
21. Marín M, Gudiol C, Castet F, et al. Bloodstream infection in patients with head and neck cancer: a major challenge in the cetuximab era. Clin Transl Oncol. 2019;21(2):187–196. doi:10.1007/s12094-018-1905-5
22. Gudiol C, Tubau F, Calatayud L, et al. Bacteraemia due to multidrug-resistant Gram-negative bacilli in cancer patients: risk factors, antibiotic therapy and outcomes. J Antimicrob Chemother. 2011;66(3):657–663. doi:10.1093/jac/dkq494
23. Marín M, Gudiol C, Ardanuy C, et al. Factors influencing mortality in neutropenic patients with haematologic malignancies or solid tumours with bloodstream infection. Clin Microbiol Infect. 2015;21(6):583–590. doi:10.1016/j.cmi.2015.01.029
24. Martinez-Nadal G, Puerta-Alcalde P, Gudiol C, et al. Inappropriate empirical antibiotic treatment in high-risk neutropenic patients with bacteremia in the era of multidrug resistance. Clin Infect Dis. 2020;70(6):1068–1074. doi:10.1093/cid/ciz319
25. Satlin MJ, Cohen N, Ma KC, et al. Bacteremia due to carbapenem-resistant Enterobacteriaceae in neutropenic patients with hematologic malignancies. J Infect. 2016;73(4):336–345. doi:10.1016/j.jinf.2016.07.002
26. Gudiol C, Albasanz-Puig A, Cuervo G, Carratalà J. Understanding and managing sepsis in patients with cancer in the era of antimicrobial resistance. Front Med. 2021;8:636547. doi:10.3389/fmed.2021.636547
27. Han SB, Kim SB, Shin KH. Risk factors for postoperative pneumonia in patients undergoing Hip fracture surgery: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2022;23(1):553. doi:10.1186/s12891-022-05497-1
28. Chen Y, Wang WL, Zhang W, et al. Risk factors and outcomes of carbapenem-resistant Enterobacteriaceae infection after liver transplantation: a retrospective study in a Chinese population. Infect Drug Resist. 2020;13:4039–4045. doi:10.2147/IDR.S278084
29. Lanternier F, Amazzough K, Favennec L, et al. Infection in solid organ transplantation: the nationwide “TRANSCRYPTO” study. Transplantation. 2017;101(4):826–830. doi:10.1097/TP.0000000000001503
30. Niu T, Xiao T, Guo L, et al. Retrospective comparative analysis of risk factors and outcomes in patients with carbapenem-resistant Acinetobacter baumannii bloodstream infections: cefoperazone-sulbactam associated with resistance and tigecycline increased the mortality. Infect Drug Resist. 2018;11:2021–2030. doi:10.2147/IDR.S169432
31. Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis. 2011;11(11):834–844. doi:10.1016/S1473-3099(11)70177-3
32. Daviet F, Guilloux P, Hraiech S, et al. Impact of obesity on survival in COVID-19 ARDS patients receiving ECMO: results from an ambispective observational cohort. Ann Intensive Care. 2021;11(1):157. doi:10.1186/s13613-021-00943-0
33. Galvagno SM, Pelekhaty S, Cornachione CR, et al. Does weight matter? Outcomes in adult patients on venovenous extracorporeal membrane oxygenation when stratified by obesity class. Anesth Analg. 2020;131(3):754–761. doi:10.1213/ANE.0000000000004454
34. Herrmann J, Lotz C, Karagiannidis C, et al. Key characteristics impacting survival of COVID-19 extracorporeal membrane oxygenation. Crit Care. 2022;26(1):190. doi:10.1186/s13054-022-04053-6
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
