Back to Journals » Clinical Ophthalmology » Volume 14
The Pro-Fibrotic Behavior of Human Tenon’s Capsule Fibroblasts in Medically Treated Glaucoma Patients
Authors Trelford CB , Denstedt JT, Armstrong JJ , Hutnik CML
Received 14 January 2020
Accepted for publication 17 April 2020
Published 22 May 2020 Volume 2020:14 Pages 1391—1402
DOI https://doi.org/10.2147/OPTH.S245915
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Charles B Trelford,1 James T Denstedt,2 James J Armstrong,2,3 Cindy ML Hutnik2–4
1Schulich School of Medicine and Dentistry, Department of Physiology and Pharmacology, Western University, London, Ontario, Canada; 2Schulich School of Medicine and Dentistry, Department of Ophthalmology, Western University, London, Ontario, Canada; 3Schulich School of Medicine and Dentistry, Department of Pathology and Laboratory Medicine, Western University, London, Ontario, Canada; 4Ivey Eye Institute, St. Joseph’s Healthcare, London, Ontario, Canada
Correspondence: Charles B Trelford
N6A 3K7
Tel +1 226-668-5778
Email [email protected]
Purpose: The aim of this study was to compare human Tenon’s capsule fibroblasts (HTCFs) obtained from patients who received medical therapy for glaucoma (glaucomatous patients) and patients not treated for glaucoma (non-glaucomatous patients) in terms of wound healing and fibrosis.
Patients and Methods: Bioartificial tissues (BATs) were generated using primary HTCF-populated collagen lattices. Pro-fibrotic gene expression within HTCFs was compared between glaucomatous patients and non-glaucomatous patients after BAT culture. The BATs were also assessed regarding fibroblast–myofibroblast transition, collagen remodeling and collagen contraction using alpha-smooth muscle actin immunohistochemistry, picrosirius red staining and collagen contraction assays, respectively.
Results: Pro-fibrotic gene expression in BAT-cultured HTCFs derived from glaucomatous patients was significantly increased compared to non-glaucomatous patients. BATs imbued with HTCFs collected from glaucomatous patients exhibited a greater proportion of myofibroblasts as well as increased collagen contraction/remodeling compared to HTCFs isolated from non-glaucomatous patients.
Conclusion: HTCFs from glaucomatous and non-glaucomatous patients differ in the expression of genes involved in fibrosis, proportion of fibroblasts undergoing transdifferentiation into myofibroblasts, contractile properties and collagen remodeling. These results suggest that for any number of reasons, at a cellular level, patients who received medical therapy for glaucoma have eyes primed for fibrosis.
Keywords: fibrosis, tenon’s capsule, bioartificial tissue, glaucoma, human Tenon’s capsule fibroblasts
Introduction
Glaucoma is defined as a group of chronic optic neuropathies characterized by progressive degeneration of the optic nerve and cells of higher cerebral structures such as the lateral geniculate nucleus and visual cortex.1 Different forms of glaucoma exist with the most common form being primary open-angle glaucoma (POAG).2 POAG is associated with functional abnormalities in the trabecular meshwork drainage system located at the junction of the cornea and iris in the anterior segment of the eye.3,4 This functional impairment in drainage of aqueous humor results in elevated intraocular pressure (IOP), which is an important modifiable risk factor for disease progression.1
Patients with glaucoma are initially managed with medications aimed at lowering IOP to prevent further loss of retinal ganglion cells, which are cells that comprise the optic nerve.1,2 Despite these efforts, some patients continue to lose their vision and require conjunctival-based surgical drainage procedures known as glaucoma filtration surgeries (GFS).1,5 Since the inception of conjunctival-based drainage procedures in 1968, excessive wound healing and fibrosis have been major risk factors that have contributed to the 35–43% surgical failure rate.6 According to the World Glaucoma Association, surgical failure is defined as an IOP above or below the upper or lower IOP limit, respectively, that leads to glaucoma-dependent vision loss or requires further surgical intervention.7 It has been reported that antiglaucoma medications taken perioperatively may increase the risk of surgical failure by increasing the number of inflammatory cells and inducing subconjunctival fibrosis.8–10 Furthermore, preservatives in glaucoma medications such as benzalkonium chloride (BAK) have been implicated in damaging cell membrane barriers and increasing the localization of pro-inflammatory cytokines in subconjunctival tissues.11–13
Experimental glaucoma surgery and associated in vitro work has suggested that human Tenon’s capsule fibroblasts (HTCFs) play a major role in wound healing after conjunctival-based drainage procedures and may contribute to fibrosis-related surgical failure.14,15 However, specific factors, intrinsic to HTCFs, that contribute to poor and excessive wound healing remain largely unknown. HTCFs reside beneath the conjunctiva in the Tenon’s capsule (TC), which functions as a fibrous connective tissue that fuses with the sclera anteriorly and extends back to the meninges of the optic nerve posteriorly.16 The TC is vital to the prognosis of GFS because it is the anatomical location where fibrosis can disrupt the therapeutic effects of filtration surgery.17 For this reason, there is a need for more precise modeling of TC-dependent wound healing and ocular fibrogenesis. This need has become more amplified recently with fibrosis being a major cause of failure of the newer minimally invasive glaucoma surgeries (MIGS).18
Previous studies investigating the fibrogenic processes following GFS have mainly been carried out using animal models and cell monolayer culture models.19,20 Modulating the healing response is the challenge for glaucoma surgery; however, both animal models and cell monolayer models have difficulty recreating the physiology of wound healing. This provided the motivation for recent advances in tissue engineering such as the development of bioartificial tissue (BAT) human-cell-based in vitro models of disease.21,22 BAT models such as fibroblast populated collagen lattices are created by embedding cells in a three-dimensional collagen medium. These models mitigate interspecies variability, a notorious confounding variable within animal models,23 while providing a more physiologically representative cell culture system that more accurately recreates the intricate cell to extracellular matrix (ECM) interactions lacking within cell monolayer culture models.24 As both the morphology and function of cells depend upon their environment, a BAT model more closely approximates the in vivo environment.25 BAT engineering has provided researchers with unprecedented levels of control over experimental variables such as cell type, ECM composition, exposure to therapeutics, temperature and biomechanical stress.24,26 In particular, in vitro BAT models appear to be ideally suited for studying the pathogenesis of human disease, modulating the wound healing response and serve as a revolutionary platform for the evaluation of both novel and existing therapeutics.
It is known that the trabecular meshwork of glaucoma patients is associated with a build-up of ECM and upregulation of pro-fibrotic factors such as transforming growth factor β (TGFβ) and α-smooth muscle actin (αSMA).27 Indeed, pro-fibrotic factors induce fibroblast to myofibroblast transition, increase fibroblast contractility and excretion of extracellular matrix (ECM).28 Furthermore, common medications for glaucoma taken preoperatively and postoperatively may modulate wound healing and the risk of surgical failure.29 For instance, chronic glaucoma medication exposure has been associated with increasing fibrogenesis and inflammation, which are processes that contribute to surgical failure.30 Due to the excessive pro-fibrotic factors in the trabecular meshwork and glaucoma medical therapy, glaucoma patients could be at a greater risk of post-surgical fibrosis. Therefore, using BATs comprised of HTCF populated collagen lattices, the purpose of this study was to compare patients treated with medical therapy for glaucoma and patients not treated for glaucoma, hereafter referred to glaucomatous and non-glaucomatous patients, respectively, in terms of fibrogenic processes.
Patients and Methods
All reagents, chemicals and equipment used in this study were purchased from Thermo Fisher Scientific (Waltham, MA, U.S.A) unless otherwise specified.
Consent and Ethics for Tenon’s Capsule Sample Collection
Primary HTCF cell lines (Table 1) were derived from 0.2–0.4 cm surgically resected segments of TC. Patient charts were reviewed to document medical and demographic factors as shown in Table 1. Ophthalmic medications taken prior to surgery all contained BAK preservative, except for Moxifloxacin drops which were preservative free. Patient consent was written informed consent, and the ethics review boards of St. Joseph’s Hospital and the Ivey Eye Institute (London, Canada) approved this study. The TC specimens were extracted from male and female patients whom were medically treated for glaucoma (glaucomatous patients) or not medically treated for glaucoma (non-glaucomatous patients) during vitrectomy, seton implant or primary trabeculectomy surgeries. The specimens were immediately placed in primary culture growth media containing Dulbecco modified Eagle’s minimal essential medium (DMEM), 10% Fetal Bovine Serum (FBS), 1% penicillin/streptomycin (P/S), 0.1% gentamicin and 1% amphotericin.
 |
Table 1 Patient TC Donors That Created the HTCF Cell Lines Used for Experimentation |
Establishing Human Tenon’s Capsule Fibroblast Primary Cultures
At least 24 hours before sample collection, 6-well plates were coated with 5 μL/mL of fibronectin diluted in phosphate buffer saline (PBS). The 6-well plates were incubated in this fibronectin solution at 37°C 5% CO2 for 1 hour and stored at 4°C. Surgically dissected samples of TC were then cultured in the fibronectin coated wells and incubated at 37°C 5% CO2 with primary culture growth media that was changed every 3 days.
Cell Culture and Trypan Blue Assay
HTCFs cultured in DMEM supplemented with 10% FBS and 1% P/S were incubated at 37°C 5% CO2. Before the sixth passage, a 50:50 mixture of HTCFs suspended in serum free DMEM and trypan blue stain was inserted into an Invitrogen Automated Cell Counter (Invitrogen, Carlsbad, CA, U.S.A), which measured the number of cells/mL. The total HTCF number was used to determine how many BATs could be produced from each patient and to ensure all BATs contained 2x105 HTCFs.
Bioartificial Tissue Experimental Protocol
The BAT experiments were performed in 24-well plates with each well receiving 1.8 mg/mL rat-tail collagen, neutralizing solution, which was comprised of a 50:50 mixture of Waymouth media (Sigma-Aldrich, St. Louis, MO, U.S.A) and 0.28 M sodium hydroxide (Sigma-Aldrich), and HTCFs suspended in serum free DMEM. The proportion of each component of the BAT in percent volume is 80% collagen, 8% Waymouth media, 8% sodium hydroxide and 4% HTCF suspension. Each BAT contained 2x105 HTCFs that were incubated for 10 days and were nourished by DMEM containing 0.25 mmol/L ascorbic acid 2-phosphate (AA2P), 2% FBS, and 1% P/S. AA2P is an extended release vitamin C, which is essential for cellular collagen metabolism.31 The BATs were not disturbed for 3 days to allow the HTCFs to attach to the collagen lattices. The BATs were then incubated in this same media, which was changed twice a day, for 7 days prior to RNA collection, fixation or during a contraction assay time course.
RNA Isolation, Synthesis of Complementary DNA (cDNA) and Quantitative Polymerase Chain Reaction (qPCR)
RNA was isolated and purified from the HTCFs within BATs using Trizol buffer and a RNeasy mini kit (Qiagen, Venlo, Netherlands) as per manufacturers protocol. Quantity and purity of the RNA was assessed using a Nanodrop ND 1000 spectrophotometer. A 260/280 nm absorbance ratio between 1.8–2.0 was considered pure RNA with few confounding contaminants. Once the RNA purity and quantity were verified, the RNA was reverse transcribed to cDNA following the iSCRIPT reverse transcription supermix (Bio-Rad, Hercules, CA, U.S.A) protocol (Supp. Table 1). A C1000 Touch Thermal cycler (Bio-Rad), operated by Bio-Rad Real-Time PCR Detection System Software (Version 1.0.176), synthesized cDNA in a 3-step reaction (Supp. Table 2). After the reverse transcription reaction, qPCR was performed using the CFX96 Real-Time PCR system (Bio-Rad) operated by CFX manager version 3.0. SsoAdvanced Universal Probes Supermix (Bio-Rad) contained all the reagents needed for qPCR besides the cDNA, probes and primers (Supp. Table 3). The qPCR was set up according to the SsoAdvanced Universal Probes Supermix protocol (Supp. Table 4) and performed following the CFX96 qPCR reaction protocol (Supp. Table 5).
Contraction Assay for Bioartificial Tissues
After 3 days, the BATs not destined for RNA collection were released from the sides of the wells using a pipette tip. A laser scanner (HP Scanjet 8200) scanned the BATs every 24 hours until the 168-hour time course was complete, and the media was changed every 24 hours, just prior to imaging. The images were analyzed using ImageJ (version 1.52a, National Institute of Health, USA) to assess the cross-sectional area of the BAT. Contraction relative to baseline area was quantified for each BAT replicate as a measure of in vitro scarring activity.
Picrosirius Red Staining with Polarized Light Microscopy
Once the contraction assay concluded, BATs were fixed in 4% paraformaldehyde overnight. After fixation, BATs were dehydrated in ethanol, embedded in paraffin, sectioned into 5µM thick fragments, mounted on glass slides and stained with picrosirius red using standard protocols. Briefly, a solution of 0.1% Sirius red (Sigma-Aldrich) and saturated picric acid (Sigma-Aldrich) was applied for 1 hour and removed by washing with 0.5% acetic acid. Picrosirius red staining was imaged using an Abrio quantitative birefringence imaging system (Hinds Instruments, Portland, Oregon) mounted on an Olympus BX-51 microscope (Olympus Corporation, Tokyo, Japan). In order to facilitate consistency between samples, a constant light intensity, 45° angle to the polarizing filter and 20x objective was used for each sample.
Circular polarizing lenses were used to visualize Picrosirius red histology. Color changes that were dependent on collagen fiber thickness and spatial orientation were analyzed using ImageJ as previously described.32 ImageJ quantified the proportion of red, orange, yellow and green pixels, which represented very high, high, intermediate and low levels of collagen remodeling, respectively. Four patients were selected for each of the glaucomatous or non-glaucomatous treatment conditions. Three samples for each treatment condition were imaged using a 20x objective and ten images were taken for each sample. The number of pixels for each color was calculated and expressed as a proportion of the total number of pixels representing collagen within each image.
Determining Fibroblast-Myofibroblast Transition
After the 168-hour incubation period, the BATs were fixed in 4% paraformaldehyde and permeabilized for 30 minutes with 1% Triton X-100 (Sigma-Aldrich) in PBS. Non-specific sites were blocked using 1% bovine serum albumin in 1x PBS. Slides were stained with Alexa Fluor 488–conjugated primary antibody against the contractile protein αSMA (Abcam, Cambridge, United Kingdom) for 40 minutes. Finally, slides were stained with 4.6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich) for 10 minutes and imaged with a laser-scanning confocal microscope (A1R HD; Nikon Instruments Inc., Tokyo, Japan). ImageJ was used to count nuclei and areas greater than 30 pixels that stained positive for αSMA. These values were then normalized to the total area that enclosed the DAPI and αSMA. Ten random frames were taken with the 10x objective per BAT section, with three BATs imaged per patient cell line and treatment group. The laser intensity settings were kept constant between slides to facilitate consistent comparison between replicates.
Statistical Analysis
All data were expressed as mean ± standard error (SE). Data from qPCR, contraction assays and picrosirius red histology were analyzed using a one-way ANOVA, two-way ANOVA or an unpaired two-tailed t-test. Statistical analysis was performed in GraphPad Prism version 7 (GraphPad, San Diego, CA, U.S.A). P-values less than 0.05 (P<0.05) were considered statistically significant.
Results
HTCFs Derived from Patients Treated with Medical Therapy for Glaucoma Had an Increased Expression of Pro-Fibrotic Genes
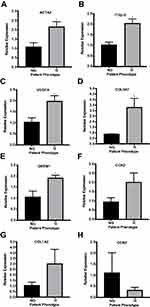
Real-time qPCR of HTCFs demonstrated that HTCFs from glaucomatous patients had an increased mRNA expression of genes that regulate or function in fibrosis compared to HTCFs derived from non-glaucomatous patients (Supp. Table 3). As seen in Figure 1A–E, HTCFs derived from glaucomatous patients had approximately a 2-fold increase in the expression of ACTA2 (αSMA), ITGβ-5, VEGFA, COL3A1 and GREM1, respectively. However, there was no significant difference in the expression of CCN2 (Figure 1F), COL1A2 (Figure 1G) and CCN3 (Figure 1H). As a result, HTCFs derived from glaucomatous patients had an increased expression of pro-fibrotic genes that could promote excessive wound healing. For this reason, we next assessed the phenotypes of HTCFs derived from glaucomatous and non-glaucomatous patients.
BATs Imbued with HTCFs from Patients Treated with Medical Therapy for Glaucoma Had a Greater Proportion of Contained Fibroblasts Exhibiting a Myofibroblast-Like Phenotype
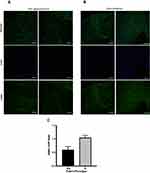
We demonstrated that HTCFs derived from glaucomatous patients, compared to HTCFs derived from non-glaucomatous patients, had a greater αSMA mRNA expression (Figure 1A) but the differences between HTCF-myofibroblast transition remained unknown. For this reason, we investigated the proportion of cells positive for αSMA protein, which serves as a marker for myofibroblasts.33 Figure 2 contains images acquired using a confocal microscope of HTCFs derived from non-glaucomatous (Figure 2A) and glaucomatous (Figure 2B) patients that were stained with DAPI (blue) and an antibody targeting αSMA (green). When the number of nuclei and amount of αSMA were standardized to the total area of BAT captured in an image, it was found that glaucomatous patients had a greater proportion of HTCFs positive for αSMA staining (Figure 2C).
HTCFs Derived from Patients Treated with Medical Therapy for Glaucoma Had a Greater Contractile Phenotype Than HTCFs Derived from Patients Not Treated for Glaucoma
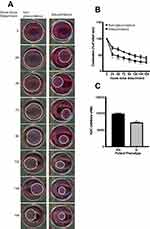
The contraction assay (Figure 3) assessed the relative differences in HTCF-mediated collagen contraction between cells derived from glaucomatous patients and those derived from non-glaucomatous patients. HTCFs derived from glaucomatous or non-glaucomatous patients were imbued in BATs and contraction was monitored every 24 hours for 168 hours. We found that HTCFs derived from glaucomatous patients exhibited significantly more collagen gel contraction than HTCFs from non-glaucomatous patients (Figure 3A). The difference in HTCF-mediated collagen contraction between glaucomatous and non-glaucomatous patients was significant for the first 144 hours after the BATs were released from the edges of the wells (Figure 3B). When the area under the curve (AUC) was calculated (Figure 3C), HTCFs from glaucomatous patients had a significantly lower AUC suggesting a greater rate of contraction during the 168-hour time course. Since HTCFs derived from glaucomatous patients were different transcriptionally and phenotypically from HTCFs from non-glaucomatous patients, we needed to measure if these differences could impact HTCF-mediated collagen remodeling activity.
BATs Containing HTCFs from Patients Treated with Medical Therapy for Glaucoma Exhibited a Greater Degree of Collagen Remodeling
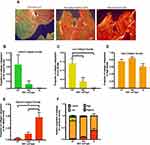
Cell free BATs were compared to BATs embedded with HTCFs derived from glaucomatous or non-glaucomatous patients. ImageJ quantified the pixel density of images acquired using polarized light that created red, orange, yellow and green pixels (Figure 4A), which corresponded to localized areas with greater or lesser degrees of collagen remodeling, respectively, based on changes in collagen fiber thickness and spatial orientation. The changes observed in green (Figure 4B), yellow (Figure 4C), orange (Figure 4D) and red staining (Figure 4E) between glaucomatous and non-glaucomatous treatment groups, compared to cell free control, is indicative of a self-evident remodeling process having occurred in glaucomatous and non-glaucomatous BATs. No statistical difference was observed in the proportion of orange staining between glaucomatous and non-glaucomatous treatment groups, compared to cell free control (Figure 4D). BATs imbued with HTCFs from glaucomatous patients had a significantly greater proportion of red staining when compared to non-glaucomatous treatment groups (Figure 4E). In conclusion, after examining the total proportion of each pixel color for all treatments (Figure 4F), we verified that HTCFs derived from glaucomatous patients increased collagen remodeling to a greater degree than cell free control and HTCFs derived from non-glaucomatous patients.
Discussion
Due to high postoperative complication and failure rates of GFS, new strategies continue to be of interest to improve glaucoma surgical patient outcomes. This demand for novel strategies to effectively lower IOP while improving the risk profile has given rise to MIGS.34 MIGS procedures tend to be less invasive than traditional GFS because they minimize tissue manipulation.18,34 Despite this, the first generation of MIGS procedures have not escaped the risk of fibrosis and have been found to have failure rates similar to that of traditional GFS.18 For this reason, we developed a novel in vitro approach to study the behaviour of HTCFs in a tissue mimetic model.35 Our BATs are similar to the established fibroblast populated collagen lattice model in that they are appropriate for measuring fibroblast-collagen interactions.36 However, AA2P, which is vitamin C that is required for collagen metabolism,31 and imbued HTCFs allow this model to more closely approximate the TC compared to cell monolayer models.22,25 The characterization of this model involved examination of the expression of genes essential to wound healing and fibrogenic processes (Figure 1), assessing imbued HTCF phenotypes and how the HTCFs functionally interacted with the microenvironment.
A significant increase in relative mRNA expression of the ACTA2, ITGβ-5, VEGFA, COL3A1 and GREM1 genes by HTCFs donated from patients medically treated for glaucoma (glaucomatous patients) compared to those donated from patients not treated for glaucoma (non-glaucomatous patients). COL3A1 encodes type III collagen,37 which is the predominant collagen formed in early wound healing, is eventually replaced by type I collagen over 1–3 weeks.38 HTCFs from glaucomatous patients showed a greater propensity to produce type III collagen. Indeed, scar tissue is known to contain a greater proportion of type III collagen to type I collagen.39 Considering that the release of the BAT from the sides of the culture well simulates surgical tissue manipulation, it is likely that our BAT model captured this aspect of early scar formation. Furthermore, glaucomatous patient-derived HTCFs displayed greater expression of collagen genes in our model, suggesting that glaucoma patients may be more susceptible to scar formation.
Vascular endothelial growth factor (VEGF) is an important growth factor for fibrosis and the development of blood vessels in a process known as angiogenesis.40 VEGFA and its role in fibrosis are not well understood but anti-VEGF pharmacological agents are known to have anti-fibrotic activity.41 Lastly, GREM1 encodes the protein gremlin, which has been shown to increase myofibroblast formation from fibroblasts.42 In glaucomatous patients, gremlin is up-regulated in trabecular meshwork cells and has been shown to increase IOP directly.43 This is in keeping with our results, demonstrating increased mRNA expression of GREM1 in glaucomatous patient-donated HTCFs.
Several integrin genes, including ITGβ-5, have been shown to be involved in the activation of latent transforming growth factor-beta 1 (TGFβ1), an important pro-fibrotic growth factor.44 Since circulating TGFβ1 plays a role in wound healing but was absent in our BAT model, the question regarding how circulating and endogenously produced TGFβ1 regulate HTCF-dependent wound healing in glaucomatous and non-glaucomatous patients remains unknown. What is known is that latent TGFβ1 is activated by contracting myofibroblasts45 and may contribute to the increased fibrogenic response seen in BATs comprised of HTCFs from glaucomatous patients. An important measure of fibroblast activation is the degree of differentiation into the contractile myofibroblast, representing a key stage in wound healing and repair. Upon differentiation, myofibroblasts express αSMA, and the αSMA protein has been commonly used to measure fibroblast-to-myofibroblast differentiation.46 A greater myofibroblast-to-fibroblast ratio was observed in the BATs produced from glaucomatous patients (Figure 2), which was consistent with the increased ACTA2 mRNA expression in HTCFs collected from glaucomatous patients (Figure 1A). Therefore, HTCFs derived from glaucomatous patients had a greater propensity to differentiate into myofibroblasts, which would have implications for postoperative scar formation.
In comparison to HTCFs collected from non-glaucomatous patients, HTCFs collected from glaucomatous patients displayed an in vitro phenotype that would suggest they possessed a greater propensity for fibrogenic activity in vivo. The pro-fibrotic behaviours assessed in our study included increased collagen contractility, deposition and remodeling. The contraction assay (Figure 3) demonstrated that HTCFs collected from glaucomatous patients contracted the collagen constructs at a greater rate compared to HTCFs collected from non-glaucomatous patients. The increased contractility may have been mediated by the observed increased myofibroblast-to-fibroblast ratio within BATs containing glaucomatous HTCFs.45,47
Collagen remodeling, as measured by Sirius red staining, has been correlated to bleb function in a GFS animal model.48 Anti-fibrotic therapies, which reduced scarring and yielded better bleb function, were shown to decrease collagen remodeling.49 Interestingly, HTCFs derived from glaucomatous patients displayed the greatest levels of collagen remodeling. Therefore, HTCFs collected from glaucomatous patients required less stress and/or were primed to differentiate into myofibroblasts thus explaining the pro-fibrotic phenotype observed.
Based on the presented data, we know that, in comparison to HTCFs isolated from patients not treated for glaucoma, HTCFs isolated from patients treated medically for glaucoma had an increased expression of pro-fibrotic genes, increased proportion of myofibroblast differentiation and increased collagen contraction and collagen remodeling. The purpose of this study was to introduce a methodology that would allow an exploration of these factors with the first step, presented here, to establish if indeed any differences exist and if they could be measured. These differences could be due to the fact that glaucoma may impact HTCFs directly, which would make HTCFs important in wound healing and glaucoma pathogenesis; however, the causes for the differences observed are likely multifactorial. For instance, glaucomatous patient donors were older than the non-glaucomatous patient donors and were taking different topical medications prior to surgery. For example, most patients treated medically for glaucoma were taking prostaglandin analogues; non-glaucomatous patients were not (Table 1). Prostaglandin analogues are a common treatment for glaucoma because they reduce IOP.50 However, prostaglandin analogues and preservatives in glaucoma medications such as BAK have been implicated in chronic inflammation via macrophage accumulation and increase collagen gel contraction.13,51 Furthermore, BAK induces subconjunctival fibrosis and delays corneal wound healing post-transscleral surgeries.12,13,52 Therefore, the medications taken prior to their GFS and during recovery could modulate HTCF-mediated wound healing and result in fibrosis. To determine any causative role of the glaucomatous disease state and fibrotic outcomes, further studies controlling for such confounding variables would be required.
HTCFs are known to be essential in the postoperative wound healing and scarring response following GFS.35 Therefore, they have been used extensively in experiments to outline this wound healing response and test potential therapeutic interventions to modulate fibrosis.53,54 Using a BAT model, we demonstrated differing fibrogenic properties of HTCFs collected from patients medically treated for glaucoma compared to HTCFs isolated from patients not treated for glaucoma. Furthermore, we demonstrated the utility of this model in the measurement of functional fibrotic outcomes such as increased tissue contraction and matrix remodeling for HTCFs collected from glaucomatous patients. These findings show the advantages of using a BAT model when investigating tissue fibrosis in the context of subconjunctival wound healing.
The BAT model used in the present study has some limitations. Extraction of RNA from the collagen tissue required tissue homogenization, which may have led to some RNA degradation due to temperature changes experienced by the cells. Furthermore, it is possible that not all of the cellular content, and therefore RNA, was collected in this process. Careful experimental protocol was required to mitigate these effects, but they should be considered when interpreting results. In addition, while the BAT model provides a more physiological environment compared to a cell monolayer, one should still be cautious of other factors that may act on HTCFs in vivo when translating findings from in vitro BAT experiments to clinical scenarios. For example, this model does not take into account circulating immune cells and cytokines which would be present in vivo.
In conclusion, the utility of using BAT experimental models to characterize differences between HTCFs obtained from patients medically treated for glaucoma and those who were not was demonstrated in this study. Pro-fibrotic tendencies were observed in the HTCFs derived from patients medically treated for glaucoma using qPCR, immunohistochemistry to stain DAPI and αSMA, contraction assay time courses and picrosirius red staining. Although, several factors such as patient variability, disease state, medications, age and other comorbidities could be responsible for the differences observed, these findings may help explain why patients receiving antiglaucoma therapies may be particularly susceptible to fibrosis related surgical failure following bleb-forming procedures.
Acknowledgments
We would like to thank Dr. David Tingey and Dr. John Gonder for consent and collection of Tenon’s capsule specimens. We would also like to acknowledge the McGrath Family, the Department of Ophthalmology at Schulich School of Medicine and Dentistry, the Summer Research Opportunities Program of Schulich School of Medicine and Dentistry and the Lawson Health Research Institute Internal Research Fund for their generous financial support of this work. Special thanks to Dr. Gianni M. Di Guglielmo who served as a scientific advisor. This work was funded by the Canadian Glaucoma Society.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sharts-Hopko NC, Glynn-Milley C. Primary open-angle glaucoma. AJN. 2009;109(2):40–47. doi:10.1097/01.NAJ.0000345434.37734.ee
2. Beidoe G, Mousa S. Current primary open-angle glaucoma treatments and future directions. Clin Ophthalmol. 2012;6:1699–1707.
3. Kapetanakis VV, Chan MP, Foster PJ, Cook DG, Owen CG, Rudnicka AR. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol. 2015;100(1):86–93. doi:10.1136/bjophthalmol-2015-307223
4. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80(5):389–393. doi:10.1136/bjo.80.5.389
5. Nongpiur ME, Ku JYF, Aung T. Angle closure glaucoma: a mechanistic review. Curr Opin Ophthalmol. 2011;22(2):96–101. doi:10.1097/ICU.0b013e32834372b9
6. Landers J, Martin K, Sarkies N, Bourne R, Watson P. A twenty-year follow-up study of trabeculectomy: risk factors and outcomes. Ophthalmology. 2012;119(4):694–702. doi:10.1016/j.ophtha.2011.09.043
7. Heuer DK, Barton K, Grehn F, Shaarawy T, Sherwood M. Guidelines on Design and Reporting of Glaucoma Surgical Trials. Guidelines on Design And Reporting of Glaucoma Surgical Trials. Kugler Publications; 2009.
8. Broadway D. Adverse effects of topical anti-glaucoma medication. Arch Ophthalmol. 1994;112(11):1446–1454. doi:10.1001/archopht.1994.01090230060021
9. Bell RWD, Habib NE, Brien CO. Long-term results and complications after trabeculectomy with single per-operative application of 5-fluorouracil. Eye. 1997;11(5):663–671. doi:10.1038/eye.1997.174
10. Armstrong JJ, Welk BK, Reid JNS, Kansal V, Hutnik CML. Secondary surgical intervention after primary glaucoma filtration surgery: an Ontario population-based study. Can J Ophthalmol. 2019;54(2):212–222. doi:10.1016/j.jcjo.2018.04.004
11. Baudouin C, de Lunardo C. Short term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol. 1998;82(1):39–42. doi:10.1136/bjo.82.1.39
12. Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi:10.1016/j.preteyeres.2010.03.001
13. Steven DW, Alaghband P, Lim KS. Preservatives in glaucoma medication. Br J Ophthalmol. 2018;102(11):1497–1503. doi:10.1136/bjophthalmol-2017-311544
14. Baig NB, Shields MB, Darr DJ, Buckley EG, Freedman SF. In vitro characteristics of Tenon’s fibroblast lines derived from pediatric and adult eyes do not fully explain pediatric glaucoma surgery failure: a preliminary report. J AAPOS. 2015;19(5):455–461. doi:10.1016/j.jaapos.2015.08.005
15. Schlunck G, Meyer-ter-Vehn T, Klink T, Grehn F. Conjunctival fibrosis following filtering glaucoma surgery. Exp Eye Res. 2016;142:76–82. doi:10.1016/j.exer.2015.03.021
16. Kakizaki H, Takahashi Y, Nakano T, et al. Anatomy of Tenon’s capsule. Clin Exp Ophthalmol. 2012;40(6):611–616. doi:10.1111/j.1442-9071.2011.02745.x
17. Tong J, Fu Y, Xu X, et al. TGF-β1 stimulates human Tenon’s capsule fibroblast proliferation by miR-200b and its targeting of p27/kip1 and RND3. Investig Ophthalmol Vis Sci. 2014;55(4):2747–2756. doi:10.1167/iovs.13-13422
18. Agrawal P, Bradshaw SE. Systematic literature review of clinical and economic outcomes of micro-invasive glaucoma surgery (MIGS) in primary open-angle glaucoma. Ophthalmol Ther. 2018;7(1):49–73. doi:10.1007/s40123-018-0131-0
19. Laurell C. Effects of dexamethasone, diclofenac, or placebo on the inflammatory response after cataract surgery. Br J Ophthalmol. 2002;86(12):1380–1384. doi:10.1136/bjo.86.12.1380
20. Bergeron MG, Bergeron Y, Tardif M, Marchand S, Beauchamp D. Influence of indomethacin on the intrarenal uptake of gentamicin in endotoxemic rats. Antimicrob Agents Chemother. 1989;33:1342–1345.
21. Liu CJ, Huang Y-L, Ju J-P, Lu C-L, Chiu AW. Altered transcripts expression of matrix metalloproteinases and their tissue inhibitors in tenon capsule of patients with glaucoma. J Glaucoma. 2004;13(6):486–491. doi:10.1097/01.ijg.0000137871.19942.14
22. Benam KH, Dauth S, Hassell B, et al. Engineered in vitro disease models. Annu Rev Pathol. 2015;10(1):195–262. doi:10.1146/annurev-pathol-012414-040418
23. Perel P, Roberts I, Sena E, et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ. 2007;334(7586):197. doi:10.1136/bmj.39048.407928.BE
24. Steve P. Make mouse studies work. Nature. 2014;507:7–9.
25. Minuth WW, Sittinger M, Kloth S. Tissue engineering: generation of differentiated artificial tissues for biomedical applications. Cell Tissue Res. 1998;1–11.
26. Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9(5):967–979. doi:10.1089/107632703322495619
27. McDonnell F, O’Brien C, Wallace D. The role of epigenetics in the fibrotic processes associated with glaucoma. J Ophthalmol. 2014;2014.
28. Lichtman MK, Otero-Vinas M, Falanga V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016;24(2):215–222. doi:10.1111/wrr.12398
29. Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: the PESO study. J Glaucoma. 2013;22(9):730–735. doi:10.1097/IJG.0b013e31825af67d
30. Yang Y, Huang C, Lin X, et al. 0.005% preservative-free latanoprost induces dry eye-like ocular surface damage via promotion of inflammation in mice. Investig Ophthalmol Vis Sci. 2018;59(8):3375–3384. doi:10.1167/iovs.18-24013
31. Hata R‐I, Senoo H. L‐ascorbic acid 2‐phosphate stimulates collagen accumulation, cell proliferation, and formation of a three‐dimensional tissuelike substance by skin fibroblasts. J Cell Physiol. 1989;138(1):8–16. doi:10.1002/jcp.1041380103
32. Eberth JF, Gresham VC, Reddy AK, et al. Importance of pulsatility in hypertensive carotid artery growth and remodeling. J Hypertens. 2009;27(10):2010–2021. doi:10.1097/HJH.0b013e32832e8dc8
33. Reeves SR, Kolstad T, Lien T, Herrington-shaner S, Debley JS. Fibroblast-myofibroblast transition is differentially regulated by bronchial epithelial cells from asthmatic children. Respir Res. 2015;16(1). doi:10.1186/s12931-015-0185-7
34. Brandão LM, Grieshaber MC. Update on minimally invasive glaucoma surgery (MIGS) and new implants. J Ophthalmol. 2013;2013:1–12. doi:10.1155/2013/705915
35. Armstrong JJ, Denstedt JT, Trelford CB, Li EA, Hutnik CML. Differential effects of dexamethasone and indomethacin on Tenon’s capsule fibroblasts: implications for glaucoma surgery. Exp Eye Res. 2019;182:65–73. doi:10.1016/j.exer.2019.03.015
36. Dallon JC, Ehrlich HP. A review of fibroblast-populated collagen lattices. Wound Repair Regen. 2008;16(4):472–479. doi:10.1111/j.1524-475X.2008.00392.x
37. Vuorio E, Crombrugghe B. The family of collagen genes. Annu Rev Biochem. 1990;59(1):837–872. doi:10.1146/annurev.bi.59.070190.004201
38. Clore J, Cohen K, Diegelmann R. Quantitation of collagen types I and III during wound healing in rat skin. Exp Biol Med. 1979;161(3):337–340. doi:10.3181/00379727-161-40548
39. Bailey AJ, Bazin S, Sims TJ, et al. Characterization of the collagen of human hypertrophic and normal scars. Biochim Biophys Acta. 1975;405(2):412–421. doi:10.1016/0005-2795(75)90106-3
40. Chaudhary NI, Roth GJ, Hilberg F, et al. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007;29(5):976–985. doi:10.1183/09031936.00152106
41. Gaskin JCF, Nguyen DQ, Ang GS, Connor JO, Crowston JG. Wound healing modulation in glaucoma filtration surgery — conventional practices and new perspectives: the role of antifibrotic agents (part I). J Curr Glaucoma Pract. 2014;8(2):37–45. doi:10.5005/jp-journals-10008-1159
42. Koli K, Myllärniemi M, Vuorinen K, et al. Bone morphogenetic protein-4 inhibitor Gremlin is overexpressed in idiopathic pulmonary fibrosis. Am J Pathol. 2006;169(1):61–71. doi:10.2353/ajpath.2006.051263
43. Sethi A, Wordinger RJ, Clark AF. Gremlin utilizes canonical and non-canonical TGFβ signaling to induce lysyl oxidase (LOX) genes in human trabecular meshwork cells. Exp Eye Res. 2013;113:1–20. doi:10.1016/j.exer.2013.05.011
44. Sarrazy V, Koehler A, Chow ML, et al. Integrins αvβ5 and αvβ3 promote latent TGF-β1 activation by human cardiac fibroblast contraction. Cardiovasc Res. 2014;102(3):407–417. doi:10.1093/cvr/cvu053
45. Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol. 2007;179(6):1311–1323. doi:10.1083/jcb.200704042
46. Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127(3):526–537. doi:10.1038/sj.jid.5700613
47. Paul Ehrlich H, Sun B, Kainth KS, Kromah F. Elucidating the mechanism of wound contraction: rapid versus sustained myosin ATPase activity in attached-delayed-released compared with free-floating fibroblast-populated collagen lattices. Wound Repair Regen. 2006;14(5):625–632. doi:10.1111/j.1743-6109.2006.00170.x
48. How A, Chua JLL, Charlton A, et al. Combined treatment with bevacizumab and 5-fluorouracil attenuates the postoperative scarring response after experimental glaucoma filtration surgery. Investig Ophthalmol Vis Sci. 2010;51(2):928–932. doi:10.1167/iovs.09-3949
49. Esson DW, Popp MP, Liu L, Schultz GS, Sherwood MB. Microarray analysis of the failure of filtering blebs in a rat model of glaucoma filtering surgery. Investig Ophthalmol Vis Sci J. 2004;45(12):4450–4462. doi:10.1167/iovs.04-0375
50. Honrubia F, García-Sánchez J, Polo V, Martínez De La Casa JM, Soto J. Conjunctival hyperaemia with the use of latanoprost versus other prostaglandin analogues in patients with ocular hypertension or glaucoma: a meta-analysis of randomised clinical trials. Br J Ophthalmol. 2009;93(3):316–321. doi:10.1136/bjo.2007.135111
51. Jung KI, Woo JE, Park CK. Effects of aqueous suppressants and prostaglandin analogues on early wound healing after glaucoma implant surgery. Sci Rep. 2019;9(1):1–10. doi:10.1038/s41598-018-37186-2
52. Pinheiro R, Panfil C, Schrage N, Michael Dutescu R. The impact of glaucoma medications on corneal wound healing. J Glaucoma. 2016;25(1):122–127. doi:10.1097/IJG.0000000000000279
53. Jampel HD. Ascorbic acid is cytotoxic to Tenon ’ s capsule fibroblasts: a possible contributing factor in glaucoma filtration surgery success. Arch Ophthalmol. 2016;108(9):1323–1325.
54. Tripathi RC, Li J, Chalam KV, Tripathi BJ. Expression of growth factor mRNAs by human Tenon’s capsule fibroblasts. Exp Eye Res. 1996;63(3):339–346. doi:10.1006/exer.1996.0123
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.