Back to Journals » Journal of Asthma and Allergy » Volume 15
The Prevalence, Clinical Picture, and Triggers of Allergic Rhinitis in Saudi Population: A Systematic Review and Meta-Analysis
Authors Aburiziza A , Almatrafi MA, Alonazi AS, Zatari MH, Alqouzi SA, Mandili RA, Hawsawi WT, Aljohani RH
Received 24 September 2022
Accepted for publication 25 November 2022
Published 23 December 2022 Volume 2022:15 Pages 1831—1849
DOI https://doi.org/10.2147/JAA.S391142
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Abdullah Aburiziza,1 Mohammed A Almatrafi,1 Aishah Saud Alonazi,2 Mawaddah Hani Zatari,3 Samah Ali Alqouzi,3 Rasha Abdulaziz Mandili,3 Wedad Taher Hawsawi,3 Rehab Hejji Aljohani4
1Department of Pediatrics, College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia; 2College of Nursing, King Saud University, Riyadh, Saudi Arabia; 3College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia; 4College of Medicine, Taibah University, Medina, Saudi Arabia
Correspondence: Abdullah Aburiziza, Department of Pediatrics, College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia, Email [email protected]
Objective: To summarize the current evidence regarding the prevalence of Allergic rhinitis (AR) and its symptoms, triggers, and impact on the quality of life of the Saudi population.
Methods: A Computerized Search in MEDLINE via PubMed, MEDLINE Core database, Scopus, and Web of Science was conducted using relevant keywords. A two-stage screening process, data extraction, and quality assessment were conducted by four independent reviewers. Comprehensive Meta-analysis was used for all statistical analyses (CMA; USA: version 3.3.070).
Results: Sixteen articles (n= 31,990 patients) were included. The overall estimated prevalence of AR was 21.2%, 95% CI (12.8– 33.1%). Males had a higher prevalence of AR than females (31.7% vs 27.1%), although the difference was not significant (OR=1.24, 95% CI: 0.78– 1.953; p=0.356). Children and adolescents exhibited a lower prevalence of AR than adults (13.7% vs 31.1%). Urban AR prevalence was much greater than rural (38.4% vs 13.0%). Asthma, atopic dermatitis, and eczema are all associated with AR. The most common signs and symptoms of AR were headache 33.9%, watery discharge 28.6%, sneezing 24.6%, itchy nose, runny nose 22.2%, nasal obstruction or congestion 22.0%, loss of smell 21.9%, and wheezing 17.2%. The most prevalent triggers of AR were perfume 36.8%, dust 27.3%, air conditioning 23.4%, weather or temperature changes 17.8%, air pollution 14.5%, drugs or chemicals 13.8%, tobacco 10.8%, atopy 10.3%, and insects 10.2%.
Conclusion: The overall prevalence of AR in Saudi Arabia is 21.2%. The prevalence of AR was comparable in both males and females. However, it was higher in adults than in children and adolescents, and in urban areas than rural areas. Asthma, atopic dermatitis, and eczema co-occurrence with AR are common. AR has a negative impact on the quality of life of the patients in the form of interference with daily activities, sleep problems, difficulty of breath, and school absenteeism.
Keywords: allergic rhinitis, Saudi Arabia, prevalence, meta-analysis
Introduction
Allergic rhinitis (AR) is a hypersensitivity reaction that occurs when inhaled particles contact the nasal mucosa and trigger an immunoglobulin E (IgE)-mediated inflammatory reaction.1 Nasal blockage, rhinorrhea, sneezing, and nasal itching are among the most prevalent symptoms of AR.2 Fatigue, irritability, cough, and postnasal drip are also present.3 AR is influenced by a variety of elements, including environmental conditions, weather, and atopy. Seasonal allergens include spores of mildew and pollens from grasses and plants, whereas permanent allergens include home allergens, animal feces, mold, dust, and mites.4 However, neither a single gene nor a single environmental element can explain AR’s etiology.
The clinical symptoms of AR may be caused by a combination of many genes and particular environmental factors. AR is more likely to arise if there is a history of AR in the family.5 In the lack of family history, the probability of having AR was estimated to be 13%. This risk increased to 29% if one parent or sibling had AR, to 47% if both parents had AR, and to 72% if both parents had similar atopic appearance.6 A large number of genetic loci linked to an increased risk of AR have been determined using genetic linkage analysis.7 Patients’ socioeconomic status and quality of life are both affected negatively by the consequences of AR.8 In many cases, AR is associated with asthma, eczema, and atopic dermatitis. Diagnosis and management of AR require a multistage approach, which increases the burden on individuals and healthcare systems.9–11
A recent systematic review and meta-analysis showed that the pooled prevalence of AR in America was 9%, 95% CI (3.5–55%), Europe 19%, 95% CI (1–44%), Africa 10%, 95% CI (3.6–23%), Asia 15%, 95% CI (1–48%), and Oceania 38%, 95% CI (19–48%).12 However, Saudi Arabia was not included in the pooled analysis of Asia.
In Saudi Arabia and the Eastern Mediterranean region, there remains a limited number of epidemiological studies regarding the prevalence of AR.13 The prevalence of rhinitis among children under the age of 15 years increased from 20% in 1986 to 25% in 1995, according to Al Frayh et al.14 More than one-quarter of Saudi Arabian children between the ages of six and fifteen reported having symptoms of AR, according to another study.15 Another study found that AR was reported in 12.7% of children between the ages of 4 and 16 years old.16 In this systematic review and meta-analysis, we aimed to summarize the current evidence regarding the prevalence of AR and its symptoms, triggers, and impact on the quality of life of the Saudi population.
Methods
We have followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist and Cochrane handbook for systematic reviews of interventions in reporting this study.17,18
Eligibility Criteria
The used eligibility criteria were as follows (PECOs): Population: Studies that included data regarding Saudi patients; Exposure: AR and its associated factors; Comparator: Studies that compare between adults and children or report data for each separately; Outcomes: Studies reported data regarding the prevalence and incidence of AR; Study Design: Cross-sectional studies. We excluded case reports, conference abstracts, and non-English studies.
Information Sources and Search Strategy
On February 7, 2022, we have searched the following databases: MEDLINE via PubMed, MEDLINE Core database, Scopus, and Web of Science, using this search term “(Allergic rhinitis OR hay fever OR seasonal rhinitis OR perennial rhinitis) AND (Saudi Arabia OR Kingdom of Saudi Arabia OR Saudis Arabia OR Saudi Arab)” to identify the relevant citations. These databases were searched from inception to the date of search. Moreover, the reference lists of all included citations were searched. The retrieved citations were imported to EndNote X9 software and duplications were removed.
Selection Process
A screening sheet was developed using Microsoft Excel software. It contains the following information: Study ID, year of publication, title, abstract, keywords, DOI, and URL. Four independent reviewers conducted the selection process through a two-step screening approach. The first step was to screen the title and abstract of each study identified from the literature search to identify the studies that were eligible to be included in the second step (Full-text screening), where the reviewers read the full manuscript and decided if it fulfilled the eligibility criteria (included) or not (excluded). Any disagreement between the reviewers was solved by the judgment of the study supervisor.
Data Items and Collection Process
Four independent reviewers extracted the following data from the included studies to an offline pre-prepared Excel sheet: Demographic data of the included patients (Age, gender, education, marital status, economic status, and residency), study characteristics (used questionnaire, study duration, total sample size, city, and main findings), outcomes (prevalence of AR, the prevalence of associated allergic diseases, triggers of AR, symptoms of AR, and the impact of AR on the quality of life).
Risk of Bias and Quality Assessment
Using the National Institutes of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies, two authors independently evaluated the risk of bias and the quality of each included article. Reviewers can critically evaluate the internal validity of research using this tool. Studies were deemed “good”, “fair”, or “poor”. In the case when the authors disagreed on a rating, a third author resolved any disagreements.
Data Synthesis
The AR prevalence was calculated using the random-effects model with 95% CI. Using the I2 statistic, we calculated the percentage of heterogeneity and inconsistency between studies, with values of 25%, 50%, and 75% deemed low, moderate, and high, respectively. The random-effect model was employed if the heterogeneity was considerable and I2 > 50%; otherwise, the fixed-effect model was utilized. Comprehensive Meta-analysis was used for all statistical analyses (CMA; USA: version 3.3.070). To resolve heterogeneity, sensitivity analysis was performed by removing one study in each scenario, which is known as sequential sensitivity analysis. Furthermore, subgroup analysis was performed to minimize the risk of inconsistency.
Publication Bias Assessment
Publication bias was assessed based on the criteria of Egger’s test, and a funnel plot was generated for the forest plots that included 10 studies or more.
Results
Study Selection
Based on our literature search, we found a total of 404 relevant citations. After removing duplication, 260 articles underwent title/abstract screening. Then, 240 studies were deemed ineligible to our criteria. The full-text screening was performed on 20 articles, and only four studies were excluded. Finally, 16 articles (n= 31,990 patients) were included in the qualitative (systematic review) and quantitative synthesis (meta-analysis).5,8,15,16,19–30 Figure 1 shows the PRISMA flow diagram of included studies.
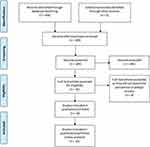 |
Figure 1 PRISMA flow diagram. |
Patients and Study Characteristics
All of the included studies were conducted in Saudi Arabia, except for Abdul Rahman et al, which was conducted in Egypt, Saudi Arabia, Lebanon, and United Arab Emirates.30 The study duration of all included studies ranges from 1 month to 12 months. Eight studies used ISAAC questionnaire,5,8,15,16,23,27–29 seven studies used self-administered, self-developed questionnaire,19,20,22,24–26,30 and one study used interview questionnaire.21 Regarding the age of the included population, seven studies included children and adolescents,15,16,22,23,27–29 six studies included only adults,5,8,20,21,25,26 and three studies included both children and adults.19,24,30 The percentage of females in all studies was 47.14%. Table 1 summarizes the characteristics of included studies and patients.
 |  |  |  |
Table 1 Summary of the Included Studies |
Quality Assessment of Included Studies
Based on the NIH quality assessment tool for observational cohort and cross-sectional studies, about 62.5% of the studies were deemed as “Good”, and 37.5% of the studies were deemed as “Fair”. There were no “Poor” studies. All studies reported their objectives clearly and defined their population, except for Abdul Rahman et al, where the objectives were not clearly presented. Only five studies (31.25%) reported the response rate, and eight studies (50%) justified their sample size.
Meta-Analysis
The Overall Prevalence of AR
The pooled analysis of 15 studies showed that the overall estimated prevalence of AR was 21.2%, 95% CI (12.8–33.1%). The pooled data were heterogeneous (I2=99.74%; p<0.001; Figure 2). Sensitivity analysis by removing one study in each scenario demonstrated that no study affects the estimated prevalence (Figure 3); however, it could not solve the heterogeneity; therefore, subgroup analysis was performed. The funnel plot showed a risk of publication bias (Figure 4); however, egger’s test demonstrated that this risk of bias was not significant (p=0.44).
 |
Figure 2 Overall prevalence of AR; shows the forest plot of the random effect estimated prevalence of AR. |
 |
Figure 3 Sensitivity analysis of overall prevalence of AR; shows the sensitivity analysis of overall prevalence of AR. |
 |
Figure 4 Funnel plot; shows the funnel plot of the overall prevalence of AR. |
Subgroup Analysis
The prevalence of AR was slightly higher in males vs females [31.7%, 95% CI (24.1–40.4%) vs 27.1%, 95% CI (18.8–37.3%)], respectively; however, there was no significant difference (OR=1.24, 95% CI: 0.78–1.953; p=0.356) (Figure 5). Regarding age, adults were associated with higher prevalence of AR compared to children and adolescents [31.1%, 95% CI (11.6–60.9%) vs 12.7%, 95% CI (7.50–20.7%)], respectively. Three studies reported prevalence of AR for both adults and children 36.5%, 95% CI (24.8–50.1%).
 |
Figure 5 Prevalence of AR in females vs males; shows the forest plot of random-effect estimated odds ratio of the difference between males and females in terms of the prevalence of AR. |
In terms of the geographical area, Central region was associated with higher prevalence 29.2%, 95% CI (6.20–72.0%), followed by the Northern region 23.0%, 95% CI (2.50–77.6%), the Western region 18.1%, 95% CI (0.90–84.2%), and the Southern region 13.7%, 95% CI (6.20–27.7%). Only one study reported prevalence for the Eastern region 48.0%, 95% CI (44.5–51.4%). The pooled analysis of three studies showed that the prevalence of AR in urban areas was considerably higher than in rural areas [38.4%, 95% CI (7.20–83.4%) vs 13.0%, 95% CI (9.20–17.9%)]. Two studies showed that the prevalence of AR in patients who presents with a family history of allergy in all of the family members was 40%, 95% CI (20.2–63.7%).
The prevalence of AR was comparable in both Summer and Winter 17.6%, 95% CI (9.10–31.1%) and 17.3%, 95% CI (10.2–27.8%), while the prevalence in the Spring and Autumn was much lower 6.00%, 95% CI (1.60–20.6%) and 5.20%, 95% CI (1.50–16.5%), respectively. Based on the quality assessment, there was no substantial difference in terms of the prevalence obtained from good studies and fair studies 20.7%, 95% CI (8.30–43.1%) and 22.3%, 95% CI (13.3–34.8%), respectively. In terms of sample size, studies with a small sample size (≤500) showed a lower prevalence of 7.20%, 95% CI (5.20–9.80%) compared with larger (>2500) sample size 27.3%, 95% CI (7.80–62.5%) (Table 2).
 |
Table 2 Subgroup Analysis Based on the Demographic and Study Characteristics |
Based on the assessment tool, the prevalence of AR retrieved from validated tools such as ISAAC and SAQAD-143 questionnaires was substantially lower than the prevalence retrieved from self-developed tools 16.2%, 95% CI (10.5–24.2%) vs 35.1%, 95% CI (16.5–59.7%), respectively.
Prevalence of Associated Allergies
The pooled analysis of 13 studies showed that the estimated prevalence of asthma associated with AR was 16.8%, 95% CI (11.8–23.4%). The prevalence of associated atopic dermatitis was reported in four studies at 13.6%, 95% CI (4.40–34.8%). In addition, the overall prevalence of associated eczema was 8.50%, 95% CI (4.10–16.8%). Table 3 summarizes the subgroup analysis of associated allergies.
 |
Table 3 Associated Conditions, Including Asthma, Atopic Dermatitis, and Eczema |
Signs and Symptoms of AR
Table 4 demonstrates that the most common signs and symptoms of AR were headache 33.9%, 95% CI (14.2–61.4%), watery discharge 28.6%, 95% CI (5.70–72.7%), sneezing 24.6%, 95% CI (14.5–38.6%), itchy nose 24.2%, 95% CI (5.40–64.3%), runny nose 22.2%, 95% CI (4.40–63.8%), nasal obstruction or congestion 22.0%, 95% CI (3.20–70.7%), loss of smell 21.9%, 95% CI (6.80–52.0%), wheeze 17.2%, 95% CI (9.20–29.8%), cough 13.8%, 95% CI (6.10–28.5%), and itchy, redness or watery eyes 8.60%, 95% CI (5.50–13.1%).
 |
Table 4 Signs and Symptoms of AR |
Triggers of AR
Our analysis showed that the most prevalent triggers of AR were perfume 36.8%, 95% (14.8–66.1%), dust 27.3%, 95% CI (11.7–51.5%), air conditioning 23.4%, 95% CI (10.0–45.7%), weather or temperature changes 17.8%, 95% CI (6.80–39.3%), air pollution 14.5%, 95% CI (9.10–22.4%), drugs or chemicals 13.8%, 95% CI (7.30–24.4%), tobacco 10.8%, 95% CI (1.60–48.0%), atopy 10.3%, 95% CI (0.30–79.2), insects 10.2%, 95% CI (4.40–22.2%), grass or plant 9.20%, 95% CI (0.30–74.7%), animals like dogs and cats 8.50%, 95% CI (3.40–19.9%), respiratory infections 6.80%, 95% CI (3.40–13.2%), pollen 4.80%, 95% CI (0.50–34.1%) (Table 5).
 |
Table 5 Allergy Triggers |
Impact of AR on Daily Activities
The overall prevalence of “AR affects the daily activities” was 10.6%, 95% CI (3.10–30.6%), stuffy nose affects daily activities 7.70%, 95% CI (6.50–9.10%), stuffy nose caused sleep problems 5.80%, 95% CI (3.70–9.10%), difficulty of breath due to stuffy nose 8.80%, 95% CI (4.30–17.1%), nasal obstructions led emergency department 7.90%, 95% CI (1.20–37.8%), AR caused school absenteeism 6.50%, 95% CI (5.40–7.80%), and hospital admission due to AR 1.50%, 95% CI (0.10–16.90%) (Table 6).
 |
Table 6 Effect of AR on Life |
Discussion
To the best of our knowledge, this is the first and most comprehensive meta-analysis that evaluates the prevalence of AR in the Saudi population. In Saudi Arabia, there are a variety of climatic and topographical characteristics, making it a unique country. Our findings showed that the overall prevalence of AR was 21.2%, 95% CI (12.8–33.1%). Adults were associated with a higher prevalence of AR compared to children and adolescents (31.1% vs 12.7%), respectively. In children and adolescents, the lowest reported prevalence was 4.20% and the highest prevalence was 26.51%. In adults, the lowest reported prevalence was 3% and the highest prevalence was 82.40%. AR prevalence ranged from 0.8 to 14.9% in 6–7-year-olds and from 1.4 to 39.7% in 13–14-year-olds in global studies.31 Asia has a significant population affected by this condition, ranging from 27% in South Korea to 32% in the UAE.32,33 In a survey of secondary school students, the overall prevalence of AR was 19.3%, of which 52.6% were female and 47.4% were male.34 Another study reported that seasonal AR was 47.8% and permanent AR was 32.7% with an overall prevalence of 40.8%.35 The prevalence of AR in children (aged 6–7) in Iranian research varied from 14% to 31.9%.36,37 These estimated prevalence rates were substantially higher than those reported in Spain and Croatia from the same age group.38–40 AR was estimated to affect 10–30% of the global population, which is lower than estimates from previous population-based studies in the Netherlands, Finland, Australia, and the United States.41–44 Surabaya’s rates were determined to be higher than in Kota Bahru (Malaysia) or Taoyuan (Taiwan), but equivalent to big cities such as Bangkok (Thailand) or Metro Manila (Republic of The Philippines).45–48 Regarding adults, around 10% to 30% of individuals in Europe and the United States are affected by AR.49,50 Approximately 27% of South Koreans and 53% of Malaysians are affected by AR, making Asia one of the most affected regions in the world.32,51
Both genders had a comparable prevalence of AR; however, it was slightly higher in males. It was reported that males during childhood are more likely to experience persistent moderate to severe forms of AR as they are more vulnerable to being affected by pollen as a trigger of AR compared to females.25,52 In addition, Alqahtani J. mentioned that Male and female students were found to have similar levels of diagnosed AR; however, males were significantly associated with atopy and polysensitization.8 A meta-analysis reported that the prevalence of AR showed a clear male predominance in childhood, while after puberty, it seems that the prevalence switch to be more predominant in females.53 It was suggested that sex hormones play a role in the homeostasis of immunity; higher levels of sex hormones such as estrogen and progesterone enhance type 2 and suppress type 1 responses in females, whereas testosterone suppresses type 2 responses in males.54,55 Furthermore, some studies reported that estrogens can enhance antibody synthesis and humoral immunity, while androgens seem to suppress inflammation and immunity.56,57 In a rat model, estradiol was shown to increase mast cell activation and allergy sensitization; this effect was likely degranulator-selective or allergen-specific. However, progesterone has the opposite effect, increasing IgE production while decreasing histamine release.58,59
Based on the geographical regions, the Central region was associated with higher prevalence, followed by the Northern region, the Western region, and the Southern region. In Arar city, the reported prevalence of AR was 7.80% in female students ranging from 15 to 18 years.22 In the Al-Qassim region, the prevalence of AR in adults was 13.5%.20 Other cities reported higher prevalence such as Jazan (27.10%),23 Aseer (30.20%),5 Al-Ahssa (48%),24 Taif (52.81%),19 and Hail (74%).29 On the other hand, other cities had a lower prevalence of AR such as Madinah (4.20%),29 and Najran (6.30%).27
Moreover, the prevalence of AR in urban areas was considerably higher than in rural areas. A growing body of evidence demonstrates that urbanization is frequently associated with an increase in the incidence of respiratory allergy diseases, such as hay fever (AR) and asthma in children and adults.60–63 An epidemiological study in Mongolia found that the prevalence of allergic sensitization rose steadily from 13.6% in villages to 25.3% in rural towns to 31.0% in cities, indicating that the degree of socioeconomic development and urbanization may have a direct impact on the prevalence rate of allergic sensitization.64 An Iranian study showed that the prevalence of AR in urban areas was 21.7% compared to 18.1% in the rural area.37 An investigation showed that air pollution, smoking, and urban living are considered independent predictors of the relatively high prevalence of AR in Saudi Arabia.25
Our findings showed that the prevalence of AR was higher in the summer and winter than spring and autumn. Saudi Arabia is known for its frequent and periodic sandstorms in all seasons. Sandstorms transport many types of microorganisms and dust particles that can trigger or exacerbate respiratory diseases such as AR and asthma.65 Meo et al, showed that the four-season sandstorm in Saudi Arabia was associated with increased incidence of wheeze, runny nose, cough, and acute asthmatic attack.65
The prevalence of AR in patients who presents with a family history of allergy in all of the family members was 40%, 95% CI (20.2–63.7%). The family history was reported only in three studies.21,22,25 Furthermore, Alruwaili et al showed that patients who had a sibling with asthma were associated with a higher prevalence of AR (4.3%) compared to 3% who had a mother with allergic rhinitis, 2.3% had a sibling with eczema, 1.6% had father and sibling with AR, and 1% in mother with asthma.22 Alanazy et al found that 65.7% of the included patients had a family history of AR.21 Another study by Almehizia et al showed that 64.1% of the patients had a family history of AR.21 Alqahtani J. admitted that his study has some limitations, including the absence of data regarding social and environmental risk factors, family history of atopic disorders, and the presence of pets or smokers in the family.8
Our findings showed that the prevalence of AR retrieved from validated questionnaires was substantially lower than the prevalence of non-validated tools. This finding suggests that the self-developing tools may overestimate the prevalence of AR. Therefore, we recommend employing the validated and commonly used questionnaires, including ISAAC, to accurately estimate the prevalence of AR.
The estimated prevalence of asthma, atopic dermatitis, and eczema associated with AR was 16.8%, 95% CI (11.8–23.4%), 13.6%, 95% CI (4.40–34.8%), and 8.50%, 95% CI (4.10–16.8%), respectively. Several scientific articles supported the link between AR and asthma by showing the similarity of upper and lower respiratory tract anatomy, as well as pathophysiological mechanisms in these two respiratory tracts. Similar inflammatory mediators, immunocompetent cells, and triggers are involved in the inflammation that occurs in both AR and asthma.66 Patients with allergic rhinitis are three times more likely to develop asthma than those without the condition. Asthma symptoms seem to improve at the same time as the rhinitis symptoms do. Patients with more severe and chronic rhinitis have a greater chance of acquiring asthma.67 A recent meta-analysis showed a significant association between AR and atopic dermatitis (OR 3.25, 95% CI 2.26–4.66).68 A large observational study demonstrated that eczema is the second most common condition to be associated with AR after asthma.69
Regarding the interference of AR with daily activity and quality of life, a recent meta-analysis showed that patients with AR were associated with a morning headache, daytime sleepiness, difficulty waking up, snoring, obstructive sleep apnea, sleep-disordered breathing, restless sleep, nocturnal enuresis, and insomnia.70 Nasal congestion, itchy nose, runny nose, and sneezing are all characteristics of AR. The most common and most unpleasant symptom of both adults and children is nasal congestion.71 Nasal allergies have a significant effect on how a patient feels about their overall health. When compared with other conditions, a Spanish study showed that the negative impact of AR on daily activities was higher than type 2 diabetes mellitus (T2DM) and hypertension.72 According to a review of relevant literature, there is a strong link between nasal allergies and anxiety/depression.73 Parent-educator-physician teamwork is essential to ensure good quality of life and maximal school performance in this population.74
We acknowledge that our study has some limitations, including the severe, unresolved heterogeneity, which could be attributed to the significant variation between the included studies in terms of assessment tools, population age, geographical area, and season and year of conduction. However, we conducted a sensitivity analysis and subgroup analysis to resolve it, with no significant change. Moreover, the prevalence of AR in the included studies relied mainly on the assessment tools (questionnaires), without confirming the diagnosis with allergic sensitization (IgE test). Another limitation, was the use of self-developing questionnaires and interviews rather than the validated and commonly used questionnaires such as ISAAC, which may overestimate the prevalence of AR.
In conclusion, the overall prevalence of AR in Saudi Arabia is 21.2%. The prevalence of AR was comparable in both males and females. Adults were associated with a higher prevalence of AR compared to children and adolescents. The central region was associated with a higher prevalence of AR compared with other regions. Urban areas had a considerably higher prevalence of AR than rural areas. Asthma, atopic dermatitis, and eczema co-occurrence with AR are common. AR has a negative impact on the quality of life of the patients in the form of interference with daily activities, sleep problems, difficulty of breath, and school absenteeism. Further studies are required to investigate predictors of increased prevalence of AR in Saudi Arabia and the role of mass screening programs on this prevalence. Moreover, studies that investigate the prevalence of AR based on confirmed laboratory tests are required to highlight the accurate prevalence of AR.
Acknowledgment
We would like to thank Dr. Noha Tashkandi and her program “Research Platform” for their efforts in facilitating the process of this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang Y, Zhang L. Prevalence of allergic rhinitis in China. Allergy Asthma Immunol Res. 2014;6(2):105–113. doi:10.4168/aair.2014.6.2.105
2. Kim DH, Lim DH, Samra M, Kim EH, Kim JH. How accurate are the ISAAC questions for diagnosis of allergic rhinitis in Korean children? Int J Environ Res Public Health. 2018;15(7). doi:10.3390/ijerph15071527
3. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(1 Suppl):S2–8. doi:10.1067/mai.2001.115569
4. Zamani M, Esfahani MN, Joumaa I, Heydari F. Accuracy of real-time intratracheal bedside ultrasonography and waveform capnography for confirmation of intubation in multiple trauma patients. Adv Biomed Res. 2018;7:95. doi:10.4103/abr.abr_179_17
5. Al-Ghamdi B. Adult allergic rhinitis in aseer, southwestern region of Saudi Arabia: prevalence and its concomitant aspects. World Fam Med Journal. 2020;18(5):20–25. doi:10.5742/mewfm.2020.93805
6. Wang DY. Risk factors of allergic rhinitis: genetic or environmental? Ther Clin Risk Manag. 2005;1(2):115–123. doi:10.2147/tcrm.1.2.115.62907
7. Barnes KC, Marsh DG. The genetics and complexity of allergy and asthma. Immunol Today. 1998;19(7):325–332. doi:10.1016/s0167-5699(97)01241-3
8. Alqahtani JM. Atopy and allergic diseases among Saudi young adults: a cross-sectional study. J Int Med Res. 2020;48(1):030006051989976. doi:10.1177/0300060519899760
9. Network GA. The global asthma report 2014; 2014. Available from: http://globalasthmareport.org/burden/burden.php.
10. Zuberbier T, Lötvall J, Simoens S, Subramanian SV, Church MK. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69(10):1275–1279. doi:10.1111/all.12470
11. Ha EK, Baek JH, Lee SY, et al. Association of polysensitization, allergic multimorbidity, and allergy severity: a cross-sectional study of school children. Int Arch Allergy Immunol. 2016;171(3–4):251–260. doi:10.1159/000453034
12. Savouré M, Bousquet J, Jaakkola JJK, Jaakkola MS, Jacquemin B, Nadif R. Worldwide prevalence of rhinitis in adults: a review of definitions and temporal evolution. Clin Transl Allergy. 2022;12(3):e12130. doi:10.1002/clt2.12130
13. Aït-Khaled N, Enarson DA, Ottmani S, El Sony A, Eltigani M, Sepulveda R. Chronic airflow limitation in developing countries: burden and priorities. Int J Chron Obstruct Pulmon Dis. 2007;2(2):141–150.
14. Al Frayh AR, Shakoor Z, Gad El Rab MO, Hasnain SM. Increased prevalence of asthma in Saudi Arabia. Ann Allergy Asthma Immunol. 2001;86(3):292–296. doi:10.1016/s1081-1206(10)63301-7
15. Sobki SH, Zakzouk SM. Point prevalence of allergic rhinitis among Saudi children. Rhinology. 2004;42(3):137–140.
16. Harfi H, Al Abbad K, Alsaeed A. Decreased prevalence of allergic rhinitis, asthma and eczema in Riyadh City, Saudi Arabia. Trends Med Res. 2010;5(2):57–62. doi:10.3923/tmr.2010.57.62
17. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol. Version 5. Wiley-Blackwell; 2008. doi:10.1002/9780470712184
18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. doi:10.1136/bmj.n71
19. Sabry EY. Prevalence of allergic diseases in a sample of Taif citizens assessed by an original Arabic questionnaire (phase I). A pioneer study in Saudi Arabia. Allergol Immunopathol. 2011;39(2):96–105. doi:10.1016/j.aller.2010.05.009
20. Almatroudi A, Mousa AM, Vinnakota D, et al. Prevalence and associated factors of respiratory allergies in the Kingdom of Saudi Arabia: a cross-sectional investigation, September–December 2020. PLoS One. 2021;16(6):1–12. doi:10.1371/journal.pone.0253558
21. Alanazy S, Alenezi M, Al-Quniabut I, et al. Patterns of allergic rhinitis among adults in Qassim region, Saudi Arabia: a cross sectional study. Pan Afr Med J. 2021;40. doi:10.11604/pamj.2021.40.70.30719
22. Alruwaili YS, Hammad SM, Elwan A. Prevalence of allergic rhinitis among female secondary school students, in Arar city, Saudi Arabia. 2021.
23. Mahnashi T, Faqihi M, Moafa A, et al. Severity and prevalence of allergic rhinitis among school children, Jazan Region Saudi Arabia. J Fam Med Prim Care. 2019;8(2):663. doi:10.4103/jfmpc.jfmpc_294_18
24. Albaloushi N, Alyahya K. The prevalence of allergic rhinitis and its complications: a survey from Al-Ahssa, Saudi Arabia. J Nat Sci Med. 2019;2(2):57. doi:10.4103/JNSM.JNSM_46_18
25. Almehizia AA, AlEssa RK, Alwusaidi KM, et al. Allergic rhinitis: disease characteristics and coping measures in Saudi Arabia. PLoS One. 2018;14(6):1–16. doi:10.1371/journal.pone.0217182
26. Alotaibi AD, Alshammari MS, Alkhalaf AA, et al. Prevalence of allergic rhinitis among students of University of Hail, Saudi Arabia. Int J Med Res Heal Sci. 2018;7(4):75–81.
27. Alqahtani JM. Asthma and other allergic diseases among Saudi schoolchildren in Najran: the need for a comprehensive intervention program. Ann Saudi Med. 2016;36(6):379–385. doi:10.5144/0256-4947.2016.379
28. Al-Ghobain MO, Al-Moamary MS, Al-Hajjaj MS, Al-Fayez AI, Basha SI. Prevalence of rhinitis symptoms among 16 to 18 years old adolescents in Saudi Arabia. Indian J Chest Dis Allied Sci. 2012;55(1):11–14. doi:10.5005/ijcdas-55-1-11
29. Nahhas M, Bhopal R, Anandan C, Elton R, Sheikh A. Prevalence of allergic disorders among primary school-aged children in Madinah, Saudi Arabia: two-stage cross-sectional survey. PLoS One. 2012;7(5):1–9. doi:10.1371/journal.pone.0036848
30. Abdul Rahman H, Hadi U, Tarraf H, et al. Nasal allergies in the Middle Eastern population: results from the “Allergies in Middle East Survey”. Am J Rhinol Allergy. 2012;26(SUPPL.1):3–23. doi:10.2500/ajra.2012.26.3836
31. Strachan D, Sibbald B, Weiland S, et al. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC). Pediatr Allerg Immunol. 1997;8(4):161–176. doi:10.1111/j.1399-3038.1997.tb00156.x
32. An SY, Choi HG, Kim SW, et al. Analysis of various risk factors predisposing subjects to allergic rhinitis. Asian Pacific J Allergy Immunol. 2015;33(2):143–151. doi:10.12932/AP0554.33.2.2015
33. Alsowaidi S, Abdulle A, Shehab A, Zuberbier T, Bernsen R. Allergic rhinitis: prevalence and possible risk factors in a Gulf Arab population. Allergy. 2010;65(2):208–212. doi:10.1111/j.1398-9995.2009.02123.x
34. Amizadeh M, Safizadeh H, Bazargan N, Farrokhdoost Z. Survey on the prevalence of allergic rhinitis and its effect on the quality of high school students’ life. Iran J Otorhinolaryngol. 2013;25(71):79–84.
35. Salarnia S, Momen T, Jari M. Prevalence and risk factors of allergic rhinitis in primary school students of Isfahan, Iran. Adv Biomed Res. 2018;7:157. doi:10.4103/abr.abr_194_18
36. Ghaffari J, Mohammadzadeh I, Khalilian A, Rafatpanah H, Mohammadjafari H, Davoudi A. Prevalence of asthma, allergic rhinitis and eczema in elementary schools in Sari (Iran). Casp J Intern Med. 2012;3(1):372–376.
37. Mohammadzadeh I, Barari-Savadkoohi R, Alizadeh-Navaei R. The prevalence of allergic rhinitis in Iranian children: a systematic review and descriptive meta-analysis TT. JPR. 2013;1(2):19–24.
38. Stipić-Marković A, Pevec B, Pevec MR, Custović A. [Prevalence of symptoms of asthma, allergic rhinitis, conjunctivitis and atopic eczema: ISAAC (International Study of Asthma and Allergies in Childhood) in a population of schoolchildren in Zagreb]. Acta Med Croatica. 2003;57(4):281–285. Croatian.
39. Banac S, Tomulić KL, Ahel V, et al. Prevalence of asthma and allergic diseases in Croatian children is increasing: survey study. Croat Med J. 2004;45(6):721–726.
40. Arnedo-Pena A, García-Marcos L, García Hernández G, et al. Tendencia temporal y variaciones geográficas de la prevalencia de síntomas de rinitis alérgica en escolares de 6-7 años de ocho áreas españolas, según el ISAAC [Time trends and geographical variations in the prevalence of symptoms of allergic rhinitis in 6–7-year-old children from eight areas of Spain according to the ISAAC]. An Pediatr. 2005;62(3):229–236. Spanish. doi:10.1157/13071837
41. Huurre TM, Aro HM, Jaakkola JJK. Incidence and prevalence of asthma and allergic rhinitis: a cohort study of Finnish adolescents. J Asthma. 2004;41(3):311–317. doi:10.1081/jas-120026088
42. Robertson CF, Dalton MF, Peat JK, et al. Asthma and other atopic diseases in Australian children. Australian arm of the international study of asthma and allergy in childhood. Med J Aust. 1998;168(9):434–438. doi:10.5694/j.1326-5377.1998.tb139022.x
43. van de Ven MOM, van den Eijnden RJ, Engels RC. Atopic diseases and related risk factors among Dutch adolescents. Eur J Public Health. 2006;16(5):549–558. doi:10.1093/eurpub/ckl022
44. Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief. 2013;121:1–8.
45. Vichyanond P, Jirapongsananuruk O, Visitsuntorn N, Tuchinda M. Prevalence of asthma, rhinitis and eczema in children from the Bangkok area using the ISAAC (International Study for Asthma and Allergy in Children) questionnaires. J Med Assoc Thai. 1998;81(3):175–184.
46. Kao CC, Huang JL, Ou LS, See LC. The prevalence, severity and seasonal variations of asthma, rhinitis and eczema in Taiwanese schoolchildren. Pediatr Allerg Immunol. 2005;16(5):408–415. doi:10.1111/j.1399-3038.2005.00268.x
47. Quah BS, Wan-Pauzi I, Ariffin N, Mazidah AR. Prevalence of asthma, eczema and allergic rhinitis: two surveys, 6 years apart, in Kota Bharu, Malaysia. Respirology. 2005;10(2):244–249. doi:10.1111/j.1440-1843.2005.00645.x
48. Abong JM, Kwong SL, Alava HDA, Castor MAR, De Leon JC. Prevalence of allergic rhinitis in Filipino adults based on the National Nutrition and Health Survey 2008. Asia Pac Allergy. 2012;2(2):129–135. doi:10.5415/apallergy.2012.2.2.129
49. Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24(5):758–764. doi:10.1183/09031936.04.00013904
50. Nathan RA, Meltzer EO, Derebery J, et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008;29(6):600–608. doi:10.2500/aap.2008.29.3179
51. Lim FL, Hashim Z, Than LTL, Md Said S, Hisham Hashim J, Norbäck D. Asthma, airway symptoms and rhinitis in office workers in Malaysia: associations with House Dust Mite (HDM) allergy, cat allergy and levels of house dust mite allergens in office dust. PLoS One. 2015;10(4):e0124905. doi:10.1371/journal.pone.0124905
52. Ivanova JI, Kelkar S, King S, et al. Budget impact model of a 5-grass sublingual immunotherapy tablet for the treatment of grass pollen-induced allergic rhinitis. J Med Econ. 2015;18(11):909–918. doi:10.3111/13696998.2015.1061533
53. Fröhlich M, Pinart M, Keller T, et al. Is there a sex-shift in prevalence of allergic rhinitis and comorbid asthma from childhood to adulthood? A meta-analysis. Clin Transl Allergy. 2017;7:44. doi:10.1186/s13601-017-0176-5
54. Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol. 2013;13(1):92–99. doi:10.1097/ACI.0b013e32835a6dd6
55. Chen W, Mempel M, Schober W, Behrendt H, Ring J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy. 2008;63(11):1418–1427. doi:10.1111/j.1398-9995.2008.01880.x
56. Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS. Testosterone as a protective factor against atherosclerosis--immunomodulation and influence upon plaque development and stability. J Endocrinol. 2003;178(3):373–380. doi:10.1677/joe.0.1780373
57. Cutolo M, Sulli A, Capellino S, et al. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus. 2004;13(9):635–638. doi:10.1191/0961203304lu1094oa
58. Melgert BN, Postma DS, Kuipers I, et al. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allerg. 2005;35(11):1496–1503. doi:10.1111/j.1365-2222.2005.02362.x
59. Yamatomo T, Okano M, Ono T, et al. Sex-related differences in the initiation of allergic rhinitis in mice. Allergy. 2001;56(6):525–531. doi:10.1034/j.1398-9995.2001.056006525.x
60. Liu Z, Albanese E, Li S, et al. Chronic disease prevalence and care among the elderly in urban and rural Beijing, China - A 10/66 Dementia Research Group cross-sectional survey. BMC Public Health. 2009;9:394. doi:10.1186/1471-2458-9-394
61. Soto-Quiros ME, Silverman EK, Hanson LA, Weiss ST, Celedón JC. Maternal history, sensitization to allergens, and current wheezing, rhinitis, and eczema among children in Costa Rica. Pediatr Pulmonol. 2002;33(4):237–243. doi:10.1002/ppul.10070
62. Crockett AJ, Cranston JM, Alpers JH. The changing prevalence of asthma-like respiratory symptoms in South Australian rural schoolchildren. J Paediatr Child Health. 1995;31(3):213–217. doi:10.1111/j.1440-1754.1995.tb00788.x
63. Nicolaou N, Siddique N, Custovic A. Allergic disease in urban and rural populations: increasing prevalence with increasing urbanization. Allergy. 2005;60(11):1357–1360. doi:10.1111/j.1398-9995.2005.00961.x
64. Viinanen A, Munhbayarlah S, Zevgee T, et al. Prevalence of asthma, allergic rhinoconjunctivitis and allergic sensitization in Mongolia. Allergy. 2005;60(11):1370–1377. doi:10.1111/j.1398-9995.2005.00877.x
65. Meo SA, Al-Kheraiji MFA, Alfaraj ZF, Alwehaibi NA, Aldereihim AA. Respiratory and general health complaints in subjects exposed to sandstorm at Riyadh, Saudi Arabia. Pakistan J Med Sci. 2013;29(2):642–646. doi:10.12669/pjms.292.3065
66. Obimbo EM, Levin ME. Allergic rhinitis and asthma - Evidence for an association. Curr Allergy Clin Immunol. 2013;26:4–7.
67. Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol. 2002;109(3):419–425. doi:10.1067/mai.2002.121701
68. Knudgaard MH, Andreasen TH, Ravnborg N, et al. Rhinitis prevalence and association with atopic dermatitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2021;127(1):49–56.e1. doi:10.1016/j.anai.2021.02.026
69. Steiner UC, Bachmann LM, Soyka MB, Regenass S, Steinegger L, Probst E. Relationship between rhinitis, asthma, and eczema and the presence of sensitization in young Swiss adults. Allergy Rhinol. 2018;9:2152656718773606. doi:10.1177/2152656718773606
70. Liu J, Zhang X, Zhao Y, Wang Y. The association between allergic rhinitis and sleep: a systematic review and meta-analysis of observational studies. PLoS One. 2020;15(2):e0228533. doi:10.1371/journal.pone.0228533
71. Meltzer EO, Blaiss MS, Derebery MJ, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124(3 Suppl):S43–70. doi:10.1016/j.jaci.2009.05.013
72. de la Hoz Caballer B, Rodríguez M, Fraj J, Cerecedo I, Antolín-Amérigo D, Colás C. Allergic rhinitis and its impact on work productivity in primary care practice and a comparison with other common diseases: the Cross-sectional study to evAluate work Productivity in allergic Rhinitis compared with other common dIseases (CAPRI) study. Am J Rhinol Allergy. 2012;26(5):390–394. doi:10.2500/ajra.2012.26.3799
73. Sansone RA, Sansone LA. Allergic rhinitis: relationships with anxiety and mood syndromes. Innov Clin Neurosci. 2011;8(7):12–17.
74. Blaiss MS. Allergic rhinitis and impairment issues in schoolchildren: a consensus report. Curr Med Res Opin. 2004;20(12):1937–1952. doi:10.1185/030079904x13266
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
