Back to Journals » Infection and Drug Resistance » Volume 16
The Predictive Value of PCT and Other Infection Indicators in Postoperative Infection of Epithelial Ovarian Cancer
Received 30 November 2022
Accepted for publication 7 March 2023
Published 17 March 2023 Volume 2023:16 Pages 1521—1536
DOI https://doi.org/10.2147/IDR.S399666
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Xiangshu Kong, Kuiran Liu
Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, People’s Republic of China
Correspondence: Kuiran Liu, Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, 1100001, People’s Republic of China, Tel +86 18940251585, Email [email protected]
Purpose: To study the early predictive value of WBC, CRP and PCT on infectious complications after epithelial ovarian cancer surgery, draw ROC curves, and construct a nomogram prediction model.
Patients and Methods: The clinical data of patients with epithelial ovarian cancer in Shengjing Hospital from August 2019 to August 2022 were included. The levels of WBC, CRP and PCT were statistically analyzed on the first, third and fifth days after surgery, and the ROC was plotted. Multivariate logistic regression analysis determined independent influencing factors, individualized nomogram model for predicting the occurrence of postoperative infectious complications was constructed, and the correction curve was used for verification.
Results: A total of 116 patients were enrolled. The postoperative test levels of WBC, CRP and PCT were compared between two groups, and the differences on POD3 and POD5 were statistically significant. The ROC area on POD5 was 0.739, 0.838 and 0.804, respectively, better than that on POD3. Among them, CRP has the greatest value; The predicted value of the combined test of WBC, CRP and PCT on POD5 was greater than that of a single index on POD5. The nomogram model on POD5 was constructed, and the ROC analysis showed that it had a good degree of differentiation.
Conclusion: WBC, CRP and PCT can effectively predict the occurrence of postoperative infectious complications, among which CRP alone has the greatest diagnostic value on POD5, and the combined test value of the three indicators is higher than that of a single index. The nomogram model constructed by the combined indicators on POD5 can assess the risk individually.
Keywords: surgery, epithelial ovarian cancer, postoperative infectious complications, prediction
Introduction
The incidence of ovarian malignancies increased year by year, and the mortality rate ranked first in female genital malignancies. Epithelial ovarian cancer (EOC) is the most common pathological type of ovarian malignancies, accounting for about 70% of the ovarian malignancies, with high mortality and poor prognosis.1 Surgery and chemotherapy are the main means of treatment for ovarian cancer. Very few patients can be cured by surgery only, but the vast majority of patients need comprehensive treatment such as surgery combined with chemotherapy. The operation of ovarian cancer is complex and difficult, and most of the patients are middle-aged and elderly women, with many internal medicine complications, and often have problems such as low immunity.2 At the same time, due to the long operation time, wide surgical range, and excessive blood loss during surgery, the body’s anti-infection ability is reduced, and the chance of infectious complications after surgery is greatly increased.3
Infection is a common postoperative complication that typically occurs at the sites such as surgical incisions, the urinary system, and the respiratory system.4 Guidelines call for primary surgery for ovarian cancer to include full staging surgery and tumor cell reduction, which is designed to maximize the removal of all visible tumors. This procedure includes hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and appendectomy, para-aortic and pelvic lymphadenectomy.5 Most patients have advanced tumors, and in order to achieve satisfactory tumor reduction goals (that is, the diameter of the lesion visible to the naked eye is less than 1 cm), intraoperative bowel resection is highly likely. Anastomotic leakage is one of the most serious complications after intestinal resection, which will increase the incidence of secondary surgery and mortality rate of patients, and there is currently a lack of effective means to diagnose anastomotic leakages early. At the same time, general anesthesia requires the use of tracheal intubation during surgery, and the operation time and postoperative bed rest time are long, and the patient is not easy to discharge sputum, resulting in a high incidence of pulmonary infections, and the appearance of obvious pulmonary infection symptoms has a certain delay, so early diagnosis is difficult.6 In addition, preoperative urinary catheterization, tumor lesions eroding the bladder, surgical injury to bladder function nerves, and the application of anesthetic drugs can easily cause symptoms such as bladder paralysis or urinary retention, which may eventually lead to urinary tract infections.7 Patients with epithelial ovarian cancer are mostly middle-aged and elderly, and there are many high-risk factors such as diabetes and obesity before surgery, and the incidence of postoperative incision infections is higher than that of other surgeries.8 Therefore, it is of great clinical significance to effectively predict postoperative infectious complications and intervene as early as possible.
White blood cells (WBC) in blood routine are the most common biochemical indicators in clinical practice, which have the advantages of low price and high specificity, can effectively reflect body inflammation, and are widely used. C-reactive protein (CRP) and procalcitonin (PCT) are biochemical indicators closely related to infection. CRP has a half-life of 19 hours. When organ damage, infectious diseases, etc, occur, the serum level of CRP will rise rapidly, whereas when the condition is in remission, serum levels decrease rapidly.9 As a biochemical index that can indicate bacterial infection with high specificity, PCT has been used in many departments such as intensive medicine to predict sepsis and guide antibiotic therapy.
This study retrospectively analyzed the clinical data of 116 patients who underwent epithelial ovarian cancer surgery from August 2019 to August 2022, explored the predictive value of WBC, CRP and PCT on postoperative infectious complications, and constructed a nomogram prediction model to screen out early predictors with high sensitivity and specificity.
Materials and Methods
Study Design and Sample
The clinical data of 116 patients with epithelial ovarian cancer who underwent open surgery in the gynecological ward of Shengjing Hospital affiliated to China Medical University from August 2019 to August 2022 were collected by retrospective analysis. Inclusion criteria for this study were as follows: 1) Primary cytoreductive surgery for ovarian cancer; 2) Complete preservation of clinical data; 3) The results of various laboratory indicators after surgery are complete; 4) All diagnoses were confirmed pathologically; and 5) Sign the informed consent form. Exclusion criteria were as follows: 1) Preoperative infections, hematological diseases, rheumatism, rheumatoid and other connective tissue diseases; 2) Combined with cardiac, liver, kidney, and pulmonary insufficiency; 3) Neoadjuvant chemotherapy before surgery; 4) Have a mental illness; 5) Pregnant and lactating women; 6) History of other malignant tumors; 7) Postoperative reception of intraperitoneal heat perfusion chemotherapy; and 8) Anastomotic leakage occurs due to high tension or restricted blood flow. A total of 198 patients were included in this study, and 82 of them were not statistically analyzed because they met the exclusion criteria. This study (No. 2022PS1053K) was approved by the Hospital Ethics Committee.
The basic information and clinical data of patients are derived from the HIS system of Shengjing Hospital affiliated to China Medical University, including age, past history, surgical conditions, postoperative complications, etc. The pathological staging of epithelial ovarian cancer is carried out with reference to the 2014 criteria of the International Federation of Gynecology and Obstetrics (FIGO). The occurrence of infectious complications such as high fever, anastomotic leakages, urinary tract infections, pulmonary infections, surgical incision infections and septic shock was recorded from surgery to discharge. Complications are graded using the Common Terminology Criteria for Adverse Events 5.0 (CTCAE V5.0) criteria, as shown in Figure 1. If there are no complications after surgery, it is classified as grade 0.
 |
Figure 1 CTCAE V5.0 criteria. |
Instrument Reagents and Specimen Collection
WBC was determined by Coulter Gen. S System and supporting kit electrical impedance method, CRP was determined by Pumen PA990 and supporting kit immunoscattering turbidimetry, and PCT was determined by Roche E411 type and supporting kit electrochemiluminescence. All operations are carried out in strict accordance with the requirements of the department’s operation manual and kit instructions. The WBC count, PCT value and CRP value of the patient on the first, third and fifth postoperative days (hereinafter referred to as POD1, POD3 and POD5) were recorded. In the early morning, 3mL of venous blood from the patient’s elbow joint was drawn, stored in a vacuum collection tube, and gently reversed and mixed 5 times after collection to promote blood clotting. After the specimen is placed at room temperature for 10 minutes, it is sent to the Department of Clinical Laboratory, Shengjing Hospital affiliated to China Medical University for testing. After standing for 10min at room temperature, it was placed in a centrifuge tube, centrifuged at a speed of 3000r/min for 10min, and the supernatant was frozen in a −80°C refrigerator for reserve.
Diagnostic Criteria
The diagnostic criteria for colorectal anastomotic leakage: At present, there is no clear definition and classification of colorectal anastomosis leakages in academia. This article refers to the definition of colorectal anastomosis leakages by the International Study Group of Rectal Cancer (ISREC):10 interruption or absence of bowel wall integrity at the anastomosis site of colorectal or colonic canal lesions, resulting in the communication of the internal and external chambers of the intestinal canal and the appearance of a pelvic abscess next to the anastomotic site. ISREC classifies colorectal anastomotic leakages into three levels, see Table 1.
 |
Table 1 Colorectal Anastomotic Leak Classification, Clinical Manifestations and Management Principles |
The diagnostic criteria for small bowel anastomosis leakage: Tu et al define small bowel anastomosis leakage as the patient’s postoperative drainage tube outflow of small intestinal contents or saliva; At the same time, if the methylene blue is elicited or the upper gastrointestinal contrast agent leaks when oral methylene blue is taken orally, it can also be diagnosed as a small bowel anastomotic leak.11
The diagnostic criteria for urinary tract infection: urinalysis shows that WBC ≥10/high-magnification field of view, and the patient has irritating symptoms such as frequent urination, urgency, dysuria, or percussion pain in the renal area, lower abdominal pain, with or without fever and one of the following conditions: 1) Cleaning midstream urine culture suggests gram-positive bacteria or fungi >104 cfu/mL; 2) Bacterial culture of cleaning midstream urine showed gram-negative bacteria >105 cfu/mL; and 3) Cleaning midstream urine examination showed that WBC >5/high visual field.7
The diagnostic criteria for pulmonary infection: Pulmonary infection is diagnosed by one of the following: imaging findings of an infiltrative lesion in the lungs; Auscultation with a murmur in breathing, dyspnea, increased total number of leukocytes and/or proportion of neutrophils; Temperature ≥38°C, fever, and acute inflammation of the upper respiratory tract such as nasopharynx, paranasal sinuses and tonsils.12
The diagnostic criteria for surgical incision infection: Local redness and swelling with varying degrees of purulent exudation, patients with varying degrees of pain, purulent discharge found after puncture or suture removal.13
The diagnostic criteria for septic shock: Septic shock is defined as infection-induced sepsis with organ dysfunction or altered consciousness.14
Perioperative Management
The perioperative period implements the enhanced recovery after surgery (ERAS) management concept. Preoperative education and adaptive training were carried out, and the burden of mental stress and anxiety of patients was reduced through outpatient education and inpatient education. Relevant biochemical and imaging examinations before surgery, comprehensively assess the patient’s heart, lung, liver, kidney and other functions were improved, underlying diseases were screened, and the consultation of relevant departments weas improved and correction and targeted treatment were given according to the consultation opinions. Through testing and corresponding treatment, the patient’s hemoglobin was ensured to be greater than 100g/L before surgery. Antibiotics are given intravenously 30 minutes before surgical cuts, and once if the operation takes more than 3 hours, antibiotics are added. On the day of surgery and after surgery until discharge, the patient was given third-generation cephalosporin 2.5g twice daily. Postoperative individualized rehydration therapy was provided. The catheter was removed as soon as possible after surgery, encourage patients to get off the ground as soon as possible, and eat as soon as possible according to the actual situation. Discharge criteria were as follows: no fever, no nausea and vomiting, no abdominal distension, no cough and phlegm and other uncomfortable complaints; The surgical incision heals well and the drainage tube has been removed; tolerate liquid or semi-liquid eating; Have defecated and can move autonomously.
Statistical Analysis
SPSS 25.0 software was used for statistical analysis. The measurement data conforming to the normal distribution were expressed as mean ± standard deviation (X±s), the t-test was used for the comparison between groups of continuous variables, the analysis of variance was used, and the Turkey–Kramer test was used for multiple comparisons between groups. The measurement data conforming to the skewed distribution were expressed by the median with quartiles, and the Kruskal–Wallis test was used for the comparison between skewed distribution groups. The counting data were expressed by example (%), and the χ2 test was used for the comparison between groups; Directly plot the receiver operating characteristic curve (ROC) for WBC, CRP and PCT, and calculate its sensitivity, specificity, cut-off value and area under curve (AUC); In the joint test of multiple indicators, the regression equation and the joint prediction probability of the related joint index are first calculated, and then the ROC curve is plotted by the joint prediction probability to analyze the diagnostic value of the joint index. P < 0.05 is a statistically significant difference. Multivariate analysis was included when there were statistically different variables in univariate logistic regression analysis.
Results
Patient’s General Information
A total of 116 patients with epithelial ovarian cancer were included in this study. All patients underwent initial cytoreductive surgery for epithelial ovarian cancer and performed surgery, such as intestinal resection anastomosis, spleen surface lesion resection and diaphragmatic lesion dissection, according to the actual conditions seen during the operation, the wishes of the patient and her family, etc, and there were no residual lesions after surgery. The general information and clinical stage comparison of patients are shown in Table 2, and the classification is assigned. There were no significant differences between the infectious complications group (hereinafter referred to as the infected group) and the non-infectious complications group (hereinafter referred to as the non-infected group) in age (P=0.218), body mass index (BMI) (P=0.595), pelvic surgery history (hereinafter referred to as surgical history) (P=0.134), comorbidities such as hypertension (P=0.142), diabetes (P=0.551), and coronary heart disease (P=0.686).
 |
Table 2 Patient’s Clinical Characteristics [n (%)] |
A total of 28 patients underwent abdominal surgery, including epigastric and intestinal surgery. Twenty-three patients (82.1%) underwent bowelectomy and anastomosis, and 5 (17.9%) underwent only intestinal wall surface mass resection and repair. Among them, 15 cases (53.5%) were sigmoidectomy, 4 (14.3%) patients underwent sigmoidostomy during surgery, 4 cases (14.3%) were partial ileectomy, 2 cases (7.1%) were right hemicolectomy, 1 case (3.6%) was left hemicolectomy, and 1 case (3.6%) was rectal resection. Four patients (14.3%) underwent epigastric surgery, 2 cases of spleen surface lesion resection, 1 case of liver surface lesion resection, and 1 case of diaphragmatic surface peritoneal dissection.
Infectious Complications, CATCAE Grading and Treatment
A total of 46 cases (39.6%) developed infectious complications after surgery, and the median days for diagnosis were 4 days, of which the days for diagnosis of anastomotic leakages were 10–19 days. Among the postoperative patients, there were 43 cases (37.1%) with high fever, 11 cases (9.4%) with urinary tract infections, 7 cases (6.0%) with septic shock, 6 cases (5.1%) with incision infections, 5 cases (4.3%) with anastomotic leakages, 4 cases (3.4%) with pulmonary infections, and no perioperative deaths. A total of 2 cases of reopening of the abdomen due to anastomotic leakages were performed. The classification of complications, treatment modalities, and CTCAE complications are detailed in Table 3.
 |
Table 3 Infectious Complications, CTCAE Grading and Treatment |
Clinical Value of WBC, CRP and PCT in Diagnosing Postoperative Infectious Complications
Table 4 and Figure 2 compare the levels of WBC, CRP and PCT in patients between the infected group and non-infected groups. The levels of WBC, CRP and PCT in the infected group were significantly higher than those in the non-infected group. In the infection group, the median values of the first, third and fifth days after WBC were 11.42×109/L (P=0.747), 8.79×109/L (P<0.05), 7.74×109/L (P<0.05), and the median values on the first, third and fifth days after CRP were 88.85 mg/L (P=0.168), 121.50 mg/L (P<0.05) and 81.85 mg/L (P<0.05), the median values on the first, third and fifth postoperative days of PCT were 0.643 ng/mL (P=0.290), 0.543 ng/mL (P<0.05) and 0.248 ng/mL (P<0.05), respectively, and the differences between the infected group on the third and fifth postoperative days were statistically significant.
 |
Table 4 The Compared Median WBC, CRP, and PCT of the Patients |
 |
Figure 2 WBC, CRP and PCT on the third and fifth days after surgery. |
Table 5 shows the ROC analysis of the diagnostic value of WBC, CRP and PCT for postoperative infectious complications. According to the AUC and Youden indexes, WBC had the highest diagnosis accuracy on the fifth day after surgery, the cut-off value was 9.67×109/L, and the sensitivity and specificity were 73.91% and 78.59%, respectively. CRP on the fifth postoperative day had the highest diagnosis accuracy for postoperative infectious complications, with the cut-off value of 91.15 mg/L and sensitivity and specificity of 95.70% and 70.00%, respectively. PCT also had the highest diagnosis accuracy on the 5th postoperative day, with the cut-off value of 0.476 ng/mL, sensitivity and specificity of 93.51% and 68.59%, respectively.
 |
Table 5 ROC Analysis of Indicators Predicting Infectious Complications After Surgery for Ovarian Cancer |
Figure 3 shows the ROC prediction curves of POD1, POD3, and POD5 after WBC, CRP and PCT. The AUCs for WBC were 0.723 (95% CI 0.630–0.815), 0.625 (95% CI 0.517–0.734), and 0.739 (95% CI 0.636–0.843) on POD1, POD3, and POD5, respectively; The AUC of CRP on postoperative day 1, 3 and 5 was 0.722 (95% CI 0.628–0.815), 0.717 (95% CI 0.618–0.816), and 0.838 (95% CI 0.764–0.912), respectively. The AUC for PCT on postoperative day 1, 3 and 5 was 0.725 (95% CI 0.625–0.826), 0.732 (95% CI 0.635–0.829), and 0.804 (95% CI 0.724–0.885), respectively. In the single-index test on POD1, POD3, and POD5, the AUC of CRP was greater than that of PCT, and the diagnosis accuracy of both was better than that of WBC.
 |
Figure 3 ROC curves of WBC, CRP and PCT alone and in combination in predicting infectious complications after epithelial ovarian cancer. |
At the same time, also as shown in Figure 3, the combined test of WBC, CRP and PCT on the first, third and fifth postoperative days had better diagnosis accuracy than that of the single index test, and the combined test of the three indicators had the highest accuracy. Among them, the combined test on the fifth postoperative day had the highest accuracy (AUC 0.860), sensitivity of 84.80%, and specificity of 80.00%.
We also noted that the median WBC level on the fifth postoperative day was 13.8 (7.2–33.4)×109/L, the median CRP level was 92.5 (28.1–250) mg/L, and the median PCT level was 8.47 (0.56–38.33) ng/mL on the fifth postoperative day. The median WBC level of the remaining 41 patients with postoperative infectious complications was 9.88 (3.73–16.37)×109/L, the median CRP level was 31.6 (6.06–333) mg/L, and the median PCT level was 0.251 (0.033–47.58) ng/mL on the fifth postoperative day.
Establishment and Validation of Nomogram Models
When POD5 WBC, POD5 CRP, and POD5 PCT are greater than or equal to their respective forecast cut-off values, the assignment is 2 and the rest of the case is assigned 1. The assignment of patient’s clinical data as variables, and the variables in univariate analysis that were significantly associated with postoperative infectious complications were included in multivariate analysis. There are three independent predictors of risk factors in multivariate logistic regression analysis, namely POD5 WBC (OR=2.219, P=0.045), POD5 CRP (OR=2.373, P=0.035), and POD5 PCT (OR=1.873, P=0.013). The univariate logistic regression analysis and the multivariate logistic regression analysis of each variable are shown in Table 6.
 |
Table 6 Univariate and Multivariate Logistic Regression Analyses of Each Variable |
Using the R Programming language software, based on the results of multivariate and tivariate logistic regression analyses of POD5 WBC, POD5 CRP and POD5 PCT, a nomogram model that can assess the risk of postoperative infectious complications was drawn, as shown in Figure 4. Nomogram shows that as the level of WBC, CRP and PCT increases, the higher sum of individual scores, the higher risk of postoperative infectious complications, and the sum of the three scores is on the Total Points axis. The calibration curve model test level of nomogram is shown in Figure 5, the corrected C-index of the graph is 0.858, and after 1000 internal verifications, the mean absolute error between the predicted risk of postoperative infectious complications and the actual risk is 0.034, and the adjusted C-index is 0.859, and the calibration of model is good. Figure 6 shows the ROC curve of nomogram plotted by Stata, AUC of 0.882, 95% confidence interval of 0.965–0.987, maximum Youden index of 0.689, sensitivity and specificity of 82.10% and 86.80%, respectively.
 |
Figure 4 Nomogram prediction model of POD5 WBC, CRP and PCT on postoperative infectious complications of epithelial ovarian cancer. |
 |
Figure 5 Nomogram’s calibration curve. |
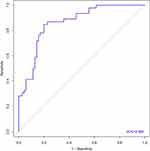 |
Figure 6 Nomogram’s ROC curve. |
Discussion
Epithelial ovarian cancer is more common in postmenopausal women. Due to the anatomical location of the ovary in the pelvic cavity, the early symptoms are not obvious, mostly non-specific symptoms, hidden onset, difficult to early diagnosis, about 75% of epithelial ovarian cancer patients have been diagnosed at the late stage.15 At present, the initial treatment of ovarian cancer is surgical treatment, the purpose is to excise the lesion, reduce the tumor load, determine the stage, improve the curative effect of chemotherapy and guide the follow-up treatment. Chemotherapies such as postoperative adjuvant chemotherapy and neoadjuvant chemotherapy, targeted therapy such as PARP inhibitors and anti-angiogenic drugs are all included in the follow-up treatment of ovarian cancer and can effectively improve the prognosis and survival of patients. Ovarian cancer is surgically excised, the intraoperative abdominal cavity is exposed for a long time, the postoperative recovery is slow, the body’s anti-infection ability is poor, and infectious complications are prone to occur after surgery.16,17 The infection will further aggravate the condition, delay treatment, affect the prognosis of patients, and cause a vicious circle.
A total of 116 patients who underwent cytoreductive surgery for epithelial ovarian cancer were included, of whom 46 developed infectious complications such as high fever, urinary tract infections, incision infections, pulmonary infections, anastomotic leakages and septic shock after surgery. There were no statistical differences in age, BMI, surgical history, hypertension, diabetes, and coronary heart disease.
Patients with ovarian cancer are mostly middle-aged and elderly people, the respiratory system degenerates, the ability to resist infection is weak, surgical trauma and tracheal intubation anesthesia can further damage the respiratory mucosa, so they are more likely to develop pulmonary infections. This group is often complicated by underlying diseases, such as diabetes and hypertension, and the immune function will be further suppressed after surgery, increasing the chance of pulmonary infections after surgery. In the early stage of pulmonary infections, there are no obvious symptoms, and once it is aggravated, the condition will rapidly progress and deteriorate, causing complications and affecting the prognosis of patients. Early prediction, diagnosis, and treatment of pulmonary infections is important.18 Sputum culture, as the gold standard for diagnosing pulmonary infections, is time-consuming and rarely used in clinical practice.19 Existing diagnostic methods do not meet the diagnostic needs for early pulmonary infections. Therefore, biochemical indicators that can indicate pulmonary infections are urgently needed. It has been reported in the literature that about 5.43% of the patients after surgery for ovarian cancer develop pulmonary infections. In this study, a total of 4 patients developed pulmonary infections after monitoring vital signs, biochemical tests, and imaging after surgery, and the incidence rate was 3.40%, which was basically consistent with the above results.20
Female patients have a close anatomical relationship between the reproductive system and the urinary system, and are susceptible to urinary tract infections, which may cause bladder dysfunction and urinary retention due to tumor erosion of the urinary system and prolonged indwelling of urinary catheters, which can lead to urinary tract infections. At the same time, routine postoperative indwelling urinary catheters destroy the function of the urethral mucosa, affect the urine flushing, and increase the incidence of urinary tract infections.21 Previous studies have found that the postoperative incidence of urinary tract infections in patients with ovarian cancer can reach 28.4% to 34.8%, and the positive signs in many patients are not obvious.22 At the same time, the biochemical indicators at this stage indicate that the positive rate of urinary tract infections is generally not high. Therefore, we lack biochemical indicators that can suggest urinary tract infections in clinical practice. In this study, according to the postoperative urine routine and urine culture examinations, a total of 11 patients developed urinary tract infections with an incidence of 9.4%, which is lower than the above results, which may be related to the implementation of ERAS concept and early removal of urinary catheters after surgery.
According to literature statistics, about 60–70% of the patients with advanced ovarian cancer find that the intestine is eroded by tumors during ovarian tumor cytoreductive surgery, and doctors choose to perform enterostomy or intestinal anastomosis.23 Although enterostomy can reduce the pressure of the distal anastomosis by diversion and diversion of stool, it can effectively avoid the complications related to intestinal anastomosis, but it will seriously affect the physical and mental health and quality of life of patients after surgery. In addition, enterostomy has clinical problems such as loss of intestinal fluid, ionic disturbance, and intestinal obstruction.24 Therefore, intestinal resection and anastomosis are often performed in clinical practice. Anastomotic leakage is one of the most serious complications of bowel surgery, with an incidence of about 1.4–34.3% and a mortality rate of as high as 18%.25 Postoperative anastomotic leakage occurs in less than 4% of ovarian cancers, but diagnosis is difficult due to its insidious course.26 In this study, a total of 28 patients underwent bowel surgery, including 5 patients with anastomotic leakages after bowel resection, with an incidence of 4.3%, which was consistent with the above results. There were 2 cases of anastomotic leakages after sigmoidectomy and anastomosis. One anastomotic leakage each occurred after partial ileectomy, right hemicolectomy, and left hemicolectomy. A total of 2 patients with anastomotic leakages received surgical treatment, and the remaining 3 patients recovered after conservative treatment such as adequate abdominal irrigation and drainage, and there were no deaths. A total of 4 patients underwent epigastric surgery, including 2 cases of splenic lesion resection, 1 case of liver lesion resection, and 1 case of diaphragmatic lesion dissection, and no infectious complications occurred after surgery. The causes of anastomotic leakage are complex and may be related to factors such as the patient’s own nutritional status, surgical difficulty, tumor size and location. Mayer et al report that more than 30% of the anastomotic leakages occur after hospital discharge, with more than 25% occurring 20 days postoperatively.27 In this study, postoperative anastomotic leakage was detected at 10–19 days, consistent with these results. At the same time, early diagnosis of anastomotic leakage, even grade A anastomotic leakage, can reduce the morbidity, mortality, and recurrence of malignancy.28 Therefore, within 3 weeks after surgery, we should closely observe the patient’s biochemical indicators, drainage tube and abdominal signs, etc., and diagnose anastomotic leakage as early as possible. Common methods for diagnosing anastomotic leakage in clinical practice include CT scan, gastroenterography, and re-surgical exploration, but CT examination has high false-negative results, and the rest are mostly invasive procedures and are not the preferred clinical methods.
Therefore, there is an urgent need for reliable biochemical indicators to help diagnose postoperative infectious complications such as pulmonary infections, urinary tract infections, and anastomotic leakages, with as much sensitivity and specificity as possible. The clinical indicators commonly used to diagnose infection include blood routine, interleukin-6, erythrocyte sedimentation rate, serum amyloid A, etc. Although culture of pathogens is the gold standard for diagnosing infection, its role in early diagnosis of infection is limited by factors such as long culture times and low positive rates. In recent years, CRP, PCT and other infection-related biochemical indicators have been applied to clinical practice and played an important role.
As the most commonly used biochemical index, WBC has the highest specificity, which is important to exclude postoperative infectious complications. It has been suggested that, compared with CRP and PCT, WBC does not have the ability to sensitively distinguish between infectious and non-infectious complications.29 In this study, there was a statistically significant difference in WBC counts between the infected and non-infected groups on POD3 and POD5 postoperatively (P<0.05), and the AUC was 0.625 (95% CI 0.517–0.734) and 0.739 (95% CI 0.636–0.843), respectively. WBC on the fifth postoperative day had the cut-off value of 9.67×109/L, which was also an independent risk factor for diagnosing postoperative infectious complications of epithelial ovarian cancer (OR=2.219, P=0.045). However, patients with malignant tumors are mostly malnourished, which inhibits immune function and weakens the chemotaxis of immune cells, which affects the diagnostic value of WBC. Therefore, we recommend combining WBC with other indicators to diagnosis postoperative infectious complications.
CRP is an acute-phase protein produced primarily by hepatocytes in the presence of inflammation and is often used to demonstrate the presence of inflammation. As an acute reactive protein, CRP is usually present in the body in trace form at a concentration of less than 10 mg/L. When infection is present, CRP in serum concentrations begins to rise 6–8 hours after infection, peaking at 24–48 hours as the disease progresses, and their duration and degree of increase are related to the severity of infection. The association between CRP and postoperative infectious complications has been demonstrated in several studies, and routine testing of CRP on POD3 to POD5 postoperatively is recommended.30,31 This study found that CRP was slightly better than PCT in diagnosing infectious complications after surgery for epithelial ovarian cancer, especially with the highest AUC value of 0.838 (95% CI 0.764 to 0.912) and the cut-off value of 91.15 mg/L on postoperative day 5. POD5 CRP is an independent risk factor for diagnosing infectious complications after epithelial ovarian cancer (OR=2.373, P=0.035). Compared with PCT, CRP is slightly more sensitive and specific than PCT.
PCT, as a precursor substance of calcitonin, has very low levels (less than 0.05 ng/mL) in circulating blood.32 When the body is infected with a pathogen, procalcitonin is produced in large quantities in epithelial cells and enters the blood circulation, and the degree of increase in concentration correlates with the severity of the infection.33 Studies have shown that PCT can be detected within 3–4 hours after infection, and the concentration will peak 6–12 hours after infection.34 The half-life of PCT is about 24h and will maintain or further increase PCT concentration if infection persists or worsens.35 Once the infection is controlled, PCT levels in the body will decrease.36 PCT is of high clinical significance. PCT has been widely used in the diagnosis of infectious diseases such as upper respiratory tract infections, sepsis, and systemic inflammatory response syndrome (SIRS).37,38 Simon et al concluded that PCT diagnostic accuracy was highest on the fifth postoperative day, with the cut-off value of 0.66 ng/mL, a negative diagnostic value of 99%, and a positive diagnostic value of 44%.39 Plesko et al concluded that PCT had a higher diagnostic value for infectious complications than CRP on POD3 (AUC 0.873, sensitivity and specificity of 84% and 98.6%).29 This study found that PCT had the cut-off value of 0.476 ng/mL (AUC=0.804) on postoperative day 5, which was an independent risk factor for diagnosing postoperative infectious complications of epithelial ovarian cancer (OR=1.873, P=0.013). Although the AUC of postoperative PCT is slightly lower than CRP, which is inferior to the predictive value of CRP, we believe that PCT has high sensitivity and specificity, and postoperative testing is necessary to exclude the risk of postoperative infectious complications as much as possible.
This study found that the combined test for postoperative infectious complications had a higher prediction accuracy than that of the single index test, and the combined WBC, CRP and PCT test was the highest on the fifth postoperative day (AUC=0.860). We also found that WBC, CRP, and PCT levels were significantly higher in patients with anastomotic leakages than in other infectious complications, consistent with findings reported in previous studies.40
Although the above three risk factors are independent of each other, there is a certain correlation and may affect each other in the clinic. We built a nomogram model based on the relative risk of the factors. The internally validated corrected C-index is 0.858, which has good compliance. Timely diagnosis and clinical intervention of infectious complications after ovarian cancer are of great clinical significance but are often difficult. The above three risk factors are postoperative biochemical indicators, and the specimens are easy to obtain, simple to operate, and practical. For patients at higher risk predicted by nomogram plots, clinicians can perform early intervention, such as upgrading antibiotic levels, adding antibiotic doses, improving other relevant tests, delaying drainage removal and symptomatic treatment, etc, which may reduce or avoid postoperative infectious complications and improve prognosis. In addition, this study also provides digital reference value for clinical work, which is helpful for preoperative and postoperative doctor-patient communication.
There are still some limitations in this study, all patients were from a single center, and due to strict data collection according to the inclusion criteria, resulting in a small sample size, and lack of data from other centers for validation, data from other centers can be added for further prospective validation to improve the efficacy of this model. Therefore, the prediction of infectious complications after ovarian cancers still needs long-term prospective studies of multi-center and large samples to obtain more meaningful and authoritative results.
Conclusion
In the clinical management of postoperative patients with epithelial ovarian cancer under the ERAS pathway, testing WBC, CRP and PCT can effectively predict the occurrence of postoperative infectious complications. The cut-off values of WBC, CRP and PCT on the fifth postoperative day were 9.67×109/L, 91.15 mg/L and 0.476 ng/mL, respectively, among which the prediction effect of CRP alone was better, the AUC was 0.838 (95% CI 0.764–0.912), and the sensitivity and specificity were 95.70% and 70.00%. On the fifth postoperative day, the combined test of WBC, CRP and PCT had the greatest diagnostic value, with AUC of 0.860 (95% CI 0.793–0.927), sensitivity and specificity of 84.80% and 80.00%. At the same time, this study established a nomogram model that can predict postoperative infectious complications of epithelial ovarian cancer, which reflects a good calibration degree in internal verification, which is helpful to the individualized treatment of postoperative patients and the effective use of medical resources. When WBC, CRP, and PCT are greater than or equal to the cut-off of the best prediction or the risk of model assessment is high, it indicates that the patient has a greater chance of infectious complications, even if there are no obvious clinical signs or symptoms, and the patient is recommended to stay in the hospital for continued observation, and giving them symptomatic treatment.
Data Sharing Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Ethics Approval
In accordance with ethical guidelines of the 1975 Declaration of Helsinki, this study was approved by the Medical Ethics Committee of Shengjing Hospital Affiliated to China Medical University (2022PS240K).
Consent to Participate
The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received the support from Scientific research funding project of Liaoning Provincial Department of Science and Technology (No.2020JH2/10300050).
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. PMID: 33538338. doi:10.3322/caac.21660
2. Moschetta M, Boussios S, Rassy E, et al. Neoadjuvant treatment for newly diagnosed advanced ovarian cancer: where do we stand and where are we going? Ann Transl Med. 2020;8(24):1710. doi:10.21037/atm-20-1683
3. Kalogera E, Dowdy SC. Enhanced recovery pathway in gynecologic surgery: improving outcomes through evidence-based medicine. Obstet Gynecol Clin North Am. 2016;43(3):551–573. PMID: 27521884. doi:10.1016/j.ogc.2016.04.006
4. Kosuga T, Ichikawa D, Komatsu S, et al. Clinical and surgical factors associated with organ/space surgical site infection after laparoscopic gastrectomy for gastric cancer. Surg Endosc. 2017;31(4):1667–1674. PMID: 27506433. doi:10.1007/s00464-016-5156-7
5. Armstrong DK, Alvarez RD, Backes FJ, et al. NCCN guidelines® insights: ovarian cancer, version 3.2022. J Natl Compr Canc Netw. 2022;20(9):972–980. PMID: 36075393. doi:10.6004/jnccn.2022.0047
6. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–e1143. PMID: 34605781. doi:10.1097/CCM.0000000000005337
7. Baenas DF, Saad EJ, Diehl FA, et al. Epidemiología de las infecciones urinarias asociadas a catéter y no asociadas a catéter en un hospital universitario de tercer nivel [Nosocomial urinary tract infection: an analysis beyond urinary catheterization]. Rev Chilena Infectol. 2018;35(3):246–252. Spanish. PMID: 30534903. doi:10.4067/s0716-10182018000300246
8. Govindaraj C, Scalzo-Inguanti K, Madondo M, et al. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ Tregs within the tumor microenvironment. Clin Immunol. 2013;149(1):97–110. PMID: 23948613. doi:10.1016/j.clim.2013.07.003
9. Guirao X, Juvany M, Franch G, Navinés J, Amador S, Badía JM. Value of C-reactive protein in the assessment of organ-space surgical site infections after elective open and laparoscopic colorectal surgery. Surg Infect. 2013;14(2):209–215. PMID: 23544798. doi:10.1089/sur.2012.042
10. Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the international study group of rectal cancer. Surgery. 2010;147(3):339–351. PMID: 20004450. doi:10.1016/j.surg.2009.10.012
11. Tu RH, Lin JX, Zheng CH, et al. Complications and failure to rescue following laparoscopic or open gastrectomy for gastric cancer: a propensity-matched analysis. Surg Endosc. 2017;31(5):2325–2337. PMID: 27620911. doi:10.1007/s00464-016-5235-9
12. Liu D, Su LX, Guan W, Xiao K, Xie LX. Prognostic value of procalcitonin in pneumonia: a systematic review and meta-analysis. Respirology. 2016;21(2):280–288. PMID: 26662169; PMCID: PMC4738441. doi:10.1111/resp.12704
13. Dubory A, Giorgi H, Walter A, et al. Surgical-site infection in spinal injury: incidence and risk factors in a prospective cohort of 518 patients. Eur Spine J. 2015;24(3):543–554. PMID: 25148864. doi:10.1007/s00586-014-3523-4
14. Sganga G. Sepsi in chirurgia [Surgical sepsis]. Urologia. 2015. 82(2):75–83. Italian. PMID: 25754409. doi:10.5301/uro.5000113
15. Mah M, Mack LA, Hurton S, Bouchard-Fortier A. Cytoreductive surgery and heated intraperitoneal chemotherapy for peritoneal carcinomatosis from rare etiologies. Am J Surg. 2019;217(5):923–927. PMID: 30760409. doi:10.1016/j.amjsurg.2019.01.011
16. Brand AH, DiSilvestro PA, Sehouli J, Berek JS. Cytoreductive surgery for ovarian cancer: quality assessment. Ann Oncol. 2017;28(suppl_8):viii25–viii29. PMID: 29232471. doi:10.1093/annonc/mdx448
17. Rausei S, Uccella S, D’Alessandro V, et al. Aggressive surgery for advanced ovarian cancer performed by a multidisciplinary team: a retrospective analysis on a large series of patients. Surg Open Sci. 2019;1(1):43–47. PMID: 32754692; PMCID: PMC7391894. doi:10.1016/j.sopen.2019.05.005
18. Giuliano KK, Baker D, Quinn B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am J Infect Control. 2018;46(3):322–327. PMID: 29050905. doi:10.1016/j.ajic.2017.09.005
19. Hsu CD, Cohn I, Caban R. Reduction and sustainability of cesarean section surgical site infection: an evidence-based, innovative, and multidisciplinary quality improvement intervention bundle program. Am J Infect Control. 2016;44(11):1315–1320. PMID: 27317407. doi:10.1016/j.ajic.2016.04.217
20. Okeahialam NA, Thakar R, Sultan AH. Early secondary repair of obstetric anal sphincter injuries (OASIs): experience and a review of the literature. Int Urogynecol J. 2021;32(7):1611–1622. PMID: 33991222. doi:10.1007/s00192-021-04822-x
21. Kirmusaoglu S, Yurdugül S, Metin A, Vehid S. The effect of urinary catheters on microbial biofilms and catheter associated urinary tract infections. Urol J. 2017;14(2):3028–3034. PMID: 28299769.
22. Chan JK, Gardner AB, Mann AK, Kapp DS. Hospital-acquired conditions after surgery for gynecologic cancer - An analysis of 82,304 patients. Gynecol Oncol. 2018;150(3):515–520. PMID: 30037490. doi:10.1016/j.ygyno.2018.07.009
23. Philip CA, Pelissier A, Bonneau C, Hequet D, Rouzier R, Pouget N. Impact of neoadjuvant chemotherapy on the rate of bowel resection in advanced epithelial ovarian cancer. Anticancer Res. 2016;36(9):4865–4871. PMID: 27630342. doi:10.21873/anticanres.11050
24. Phatak UR, Kao LS, You YN, et al. Impact of ileostomy-related complications on the multidisciplinary treatment of rectal cancer. Ann Surg Oncol. 2014;21(2):507–512. PMID: 24085329; PMCID: PMC4026258. doi:10.1245/s10434-013-3287-9
25. Koscielny A, Ko A, Egger EK, Kuhn W, Kalff JC, Keyver-Paik MD. Prevention of anastomotic leakage in ovarian cancer debulking surgery and its impact on overall survival. Anticancer Res. 2019;39(9):5209–5218. PMID: 31519635. doi:10.21873/anticanres.13718
26. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253(5):890–899. PMID: 21394013. doi:10.1097/SLA.0b013e3182128929
27. Meyer J, Naiken S, Christou N, et al. Reducing anastomotic leak in colorectal surgery: the old dogmas and the new challenges. World J Gastroenterol. 2019;25(34):5017–5025. PMID: 31558854; PMCID: PMC6747296. doi:10.3748/wjg.v25.i34.5017
28. Warschkow R, Beutner U, Steffen T, et al. Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann Surg. 2012;256(2):245–250. PMID: 22735714. doi:10.1097/SLA.0b013e31825b60f0
29. Plesko M, Suvada J, Makohusova M, et al. The role of CRP, PCT, IL-6 and presepsin in early diagnosis of bacterial infectious complications in paediatric haemato-oncological patients. Neoplasma. 2016;63(5):752–760. PMID: 27468879. doi:10.4149/neo_2016_512
30. Muñoz JL, Alvarez MO, Cuquerella V, et al. Procalcitonin and C-reactive protein as early markers of anastomotic leak after laparoscopic colorectal surgery within an enhanced recovery after surgery (ERAS) program. Surg Endosc. 2018;32(9):4003–4010. PMID: 29520440. doi:10.1007/s00464-018-6144-x
31. Messias BA, Botelho RV, Saad SS, Mocchetti ER, Turke KC, Waisberg J. Serum C-reactive protein is a useful marker to exclude anastomotic leakage after colorectal surgery. Sci Rep. 2020;10(1):1687. PMID: 32015374; PMCID: PMC6997159. doi:10.1038/s41598-020-58780-3
32. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51. PMID: 28794881; PMCID: PMC5543591. doi:10.1186/s40560-017-0246-8
33. Broyles MR. Impact of procalcitonin-guided antibiotic management on antibiotic exposure and outcomes: real-world evidence. Open Forum Infect Dis. 2017;4(4):ofx213. PMID: 29164170; PMCID: PMC5695623. doi:10.1093/ofid/ofx213
34. Samsudin I, Vasikaran SD. Clinical utility and measurement of procalcitonin. Clin Biochem Rev. 2017;38(2):59–68. PMID: 29332972; PMCID: PMC5759088.
35. Conlon JM, Grimelius L, Thim L. Structural characterization of a high-molecular-mass form of calcitonin [procalcitonin-(60-116)-peptide] and its corresponding N-terminal flanking peptide [procalcitonin-(1-57)-peptide] in a human medullary thyroid carcinoma. Biochem J. 1988;256(1):245–250. PMID: 3265620; PMCID: PMC1135394. doi:10.1042/bj2560245
36. Lippi G, Sanchis-Gomar F. Procalcitonin in inflammatory bowel disease: drawbacks and opportunities. World J Gastroenterol. 2017;23(47):8283–8290. PMID: 29307988; PMCID: PMC5743499. doi:10.3748/wjg.v23.i47.8283
37. Knowles SJ, O’Sullivan NP, Meenan AM, Hanniffy R, Robson M. Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study. BJOG. 2015;122(5):663–671. PMID: 24862293. doi:10.1111/1471-0528.12892
38. Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med. 2011;9:107. PMID: 21936959; PMCID: PMC3186747. doi:10.1186/1741-7015-9-107
39. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39(2):206–217. PMID: 15307030. doi:10.1086/421997
40. Huang C, Yao H, Huang Q, Lu H, Xu M, Wu J. A novel nomogram to predict the risk of anastomotic leakage in patients after oesophagectomy. BMC Surg. 2020;20(1):64. PMID: 32252738; PMCID: PMC7137293. doi:10.1186/s12893-020-00726-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
