Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
The Predictive Value of microRNA-134 and microRNA-1233 for the Early Diagnosis of Acute Exacerbation of Chronic Obstructive Pulmonary Disease with Acute Pulmonary Embolism
Authors Peng L , Han L , Li XN, Miao YF, Xue F , Zhou C
Received 26 June 2020
Accepted for publication 22 September 2020
Published 15 October 2020 Volume 2020:15 Pages 2495—2503
DOI https://doi.org/10.2147/COPD.S266021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Ling Peng,1,2 Li Han,2 Xiao-Ning Li,2 Ya-Fang Miao,2 Fei Xue,2 Chao Zhou1,3
1School of Clinical Medicine, Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China; 2Department of Respiratory Medicine, Zhoupu Hospital Affiliated to Shanghai University of Medicine and Health Sciences, Shanghai, People’s Republic of China; 3Department of Respiratory Medicine, Guangming Traditional Chinese Medicine Hospital of Pudong New Area, Shanghai, People’s Republic of China
Correspondence: Chao Zhou
Department of Respiratory Medicine, Guangming Traditional Chinese Medicine Hospital of Pudong New Area, No. 339 DongMen Street. Pudong New District, Shanghai 201399, People’s Republic of China
Tel +86-21-68019069
Email [email protected]
Background: The differential diagnosis of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) with acute pulmonary embolism (APE) complications are difficult because of the variability of clinical presentations and the shortage of an unfailing screening biomarkers or instruments.
Objective: Aimed to detect and compare the expression of serum microRNAs (miR-1233, miR-134) in AECOPD patients complicated with APE.
Patients/Methods: Blood samples were collected from 52 AECOPD patients (13 patients with APE complications, 39 patients without APE) and 10 patients with stable COPD. Serum miRNAs expression was detected with real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR). The levels of plasma D-dimers were determined by detection with an enzyme-linked immunosorbent assay (ELISA). The receiver-operator characteristic (ROC) curve was used for evaluating the diagnostic accuracy of the studied miRNAs.
Results: According to the Wells score, 42 of the 52 AECOPD patients were unlikely to have APE (≤ 4 points), whereas the remaining 10 (> 4 points) were likely to have APE. There were 4 cases (4/13 30.8%) in the AECOPD combined with APE group with a Wells score of > 4 points. The expression levels of miR-1233 and miR-134 in the serum were considerably upregulated in the AECOPD+APE group compared with the AECOPD group and the stable COPD group (P< 0.05). The areas under the curve (AUCs) for miR-134 and miR-1233 were, respectively, 0.931 (95% CI 0.863– 0.999) (P< 0.05) and 0.884 (95% CI 0.79– 0.978) (P< 0.05) and were higher compared with the AUC for D-dimer of 0.628 (95% CI 0.447– 0.809), the AUC for age-adjusted D-dimer of 0.705 (95% CI 0.525– 0.885) and the AUC for Wells score of 0.577 (95% CI 0.389– 0.765).
Conclusion: Our study indicated that serum miR-1233 and miR-134 have high clinical value in the early diagnosis of AECOPD patients combined with APE, or could be used as potential biomarkers for clinical identification of AECOPD with or without APE complication.
Keywords: acute exacerbation of chronic obstructive pulmonary disease, acute pulmonary embolism, D-dimer, microRNA, biomarker
Introduction
The total number of chronic obstructive pulmonary disease (COPD) cases in China is about 100 million. The prevalence of COPD in people over 40 years old was 13.7% and more than 27% in people over 60 years old. Among them, the number of men is 2.2 times than women.1 Acute pulmonary embolism (APE) is a frequent disease associated with high morbidity and mortality, requiring rapid diagnosis and treatment.2,3 APE is also the third cause of acute cardiovascular death, which causes approximately hundred thousand deaths per year in the United States, with a short-term mortality rate reaching up to 16%, second only to acute myocardial infarction (AMI) and stroke.4–7
COPD is regarded as an independent risk factor for APE.8,9 A population-based cohort study found that the incidence of APE in patients with COPD was four times higher than that in patients with non-COPD.10 However, because the clinical symptoms of APE are similar to AECOPD, when patients with known COPD present signs such as dyspnea, chest pain or cough, it is difficult to distinguish AECOPD caused by airway inflammation alone or caused by APE because both conditions can cause dyspnea, tachypnea, tachycardia, and cough, etc.11,12
Currently, the diagnosis of APE is primarily based on the amalgamation of blood tests with imaging analyses. Unfortunately, the D-dimer test is good for “ruling out” but not “ruling in” APE.13,14 In addition, many factors can cause the rise of D-dimer and even if the age-adjusted D-dimer test results are used to judge, but some patients can still be missed and delayed.15,16 Computed Tomography Pulmonary Angiography (CTPA) is reported as the “gold standard” for the diagnosis of APE.17 However, patients with renal insufficiency and iodine-containing contrast agents hypersensitivity often limit its use. Moreover, the use of CTPA also has the problem of radiation exposure.18–20 In addition, in clinical practice, many doctors overuse CTPA in order to reduce missed diagnosis, resulting in waste of medical supplies and excessive medical costs. Therefore, it is necessary to explore non-invasive, simple and reliable new biomarkers for the diagnosis of AECOPD complicated by APE.
MicroRNAs (miRNAs) belong to the class of endogenous, non-coding small RNA molecules that are only 21−25 nucleotides long, acting as posttranscriptional inhibitors of their target genes through binding to 3ʹ-untranslated regions (3ʹ-UTRs) of target mRNAs.21,22 MiRNAs play a vital role in biological processes such as cell differentiation, proliferation, metabolism, aging, and apoptosis, thereby participating in the pathogenesis of various diseases.23 In recent years, plasma and serum miRNAs have been widely studied as alternative biomarkers for diagnostic and prognostic purposes in various diseases, including myocardial infarction (MI), arteriosclerosis, heart failure and tumors.24,25
Few studies have examined the differential expression of serum miRNAs in AECOPD patients with APE complication. For example, The level of plasma miR−221 in APE was significantly higher than that of normal people, and it was positively correlated with BNP, troponin I, and D-dimer. At the same time, many miRNAs such as miR−28−3p, miR−1233, miR−134 also has an abnormal expression.4,26–28 The abnormal expression of miRNAs is directly or indirectly involved in the occurrence and development of the disease, but related further research is currently not documented. Among them, we found that miR-1233 and miR-134 have higher specificity in the diagnosis of APE, therefore, this study aimed to measure and compare the serum levels of miR-1233 and miR-134 in patients with APE and to evaluate the potential role of miR-1233 and miR-134 as a diagnostic biomarker for AECOPD complicated by APE.
Patients and Methods
Patients
Between June 2019 to May 2020, a total of 217 patients were hospitalized due to AECOPD (Patients with severe heart failure 23 cases, severe liver and kidney disease 17 cases, can not or refuse to complete CTPA 54 cases and repeated hospitalization 68 cases were excluded). There were 55 cases were enrolled after the initial evaluation, and all of them were examined by lower extremity compression ultrasound, and those with lower extremity deep vein thrombosis (DVT) were excluded (3 cases). Finally, we enrolled 52 AECOPD patients who meet all of the inclusion criteria in the Department of Respiratory Medicine, Zhoupu Hospital Affiliated to Shanghai University of Medicine and Health Sciences, Shanghai, China. The subjects were distributed into two groups according to the results of CTPA examination, including AECOPD with APE complication group (13 cases), AECOPD without APE group (39 cases). At the same time, 10 patients with stable COPD matching age and gender were included from outpatient follow-up.
COPD and AECOPD Diagnosis
All included patients were clinically diagnosed with COPD according to the global chronic obstructive pulmonary disease initiative (GOLD) criteria stipulating that after bronchodilator, the forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) is lower than 70% (FEV1/FVC<70%).1 Stable COPD patients came from outpatient follow-up patients, which has been confirmed by pulmonary function tests but has not been exacerbated in the past six months. The included patients usually use long-acting beta2-agonists (LABA), Long-acting muscarinic antagonists (LAMA) or LABA+LAMA to control their condition. AECOPD is the deterioration of respiratory symptoms in patients with COPD characterized by increases in cough, wheezing, and sputum which appears as purulent or viscous sputum, even accompanied by fever and inflammation, and which exceeds the daily standard or needs to change the drug treatment plan. All included patients were given conventional anti-infection, anti-asthmatic, and expectorant treatments according to their condition.
Diagnosis of APE
CTPA is used as the gold standard to determine whether AECOPD is combined with APE.29 Direct signs of APE: pulmonary artery with a low-density filling defect, partly or fully enclosed by opaque blood flow, or a filling defect, and the distal vessels are not developed. Indirect signs of APE: lung field wedge with increased shadow density, banded high-density areas or discoid atelectasis, dilation of the central pulmonary artery and reduction or disappearance of distal blood vessel branches. All diagnoses were determined by at least two senior physicians in the radiology department.
Clinical Evaluation
The clinical data of all the research subjects were reviewed by two senior physicians in the respiratory department, and the clinical evaluation (two classifications) was conducted according to the Wells score standard.30,31 When the opinions are inconsistent, the superior doctor will participate in the scoring to avoid bias.
According to the 2014 ERS Guidelines for the management and diagnosis of APE,14 for the patients aged 50 and over, plasma D-dimer was corrected by age, and the age-adjusted critical D-dimer value is (age×10) μg/µL (plasma D-dimer ≥ (age×10) μg/µL is positive).
The study protocol conformed to the ethics guidelines of the Declaration of Helsinki and approval was obtained from the Research Ethics Committee of Zhoupu Hospital affiliated to Shanghai University of Medicine and Health Sciences, Shanghai, China. Written informed consent was obtained from all the participants before enrollment.
Blood and Serum Samples
Venous blood samples were collected in coagulation tubes. The samples were left at room temperature for half an hour and subsequently subjected to centrifugation for 10 mins at 3000 rpm. The supernatants were collected in tubes deprived of RNase/DNase and conserved at –80°C pending serum analysis.
RNA Isolation
RNA was extracted with a Quick-cfRNATM Serum & Plasma Kit (Zymo research, Irvine, CA, USA) combining with miRNeasy Serum/Plasma Spike-In Control kit (Qiagen, Hilden, German) and 100% pure ethanol (Merck Millipore, Germany), following to the protocol recommended by the manufacturers. Briefly, 600 µL of serum were homogeneously mixed with 600 µL of Quick-cfRNATM reagent Digestion Buffer (Zymo research, Irvine, CA, USA). After adding 20 µL Proteinase K (Zymo research, Irvine, CA, USA) the homogenate was incubated for two hours at 37°C, then 10 µL (4×109 ng/µL) synthetic C. elegans miR-39 (Qiagen, Hilden, German) was spiked-in the samples following the adjunction of denaturing solution as an external reference for the normalization of miRNA level. Finally, elution was achieved with a spin column and 100% ethanol and eluted RNA was collected RNase-free water.
qRT-PCR
The collected RNA was reverse-transcribed into cDNA template. The TaqMan™ microRNA Assay (Applied Biosystems™ ThermoFisher USA) and TaqMan™ MicroRNA Reverse Transcription Kit (Applied Biosystems™ ThermoFisher USA) were used in this experiment, and the related sequences and primers were shown in Table 1.
 |
Table 1 miRNA Related Sequences |
The 7500 Fast Real-Time PCR System purchased from Applied Biosystems (Foster City, CA) was used for amplification. The reverse transcription system was 7.5 µL at 16°C for 30 mins, 42°C for 30 mins and 85°C for 5 mins. Then, 17.5 µL DEPC water was added to each sample to obtain a total volume of 25 µL of cDNA. 20 µL qRT-PCR system consisted of 9 µL cDNA, 10 µL TaqMan™ Universal Master Mix II, NO UNG (Applied Biosystems™ ThermoFisher USA), and 1 µL RT-primer 20×(Applied Biosystems™ ThermoFisher USA). The reaction occurred at 50°C for 2 mins; 95°C for 10 mins; 95°C for 15 s and 60°C for one min, and the number of amplification cycles was 40 cycles. The Ct values were normalized to the computed mean Ct of each sample (ΔCt=CtmiRNA − Ctcel-miR-39) and the comparative Ct approach (2−ΔΔCt) was used for determining the relative miRNA expression.
Statistical Analysis
The statistical analysis was done using the freely available version 25.0 of the SPSS Statistics software (IBM, USA). The Kolmogorov–Smirnov normality test was performed on all data. The categorical variables were expressed as percentages and numbers while the continuous data were formulated as average ± SD or median with an interquartile range. The t-test and the Man-Whitney U-test were used for the comparison of continuous variables while the chi-square test was used for the comparison of categorical variables between two groups. The area under the curve (AUC) derived from the analysis of the ROC curve was used to assess the potential of the biomarkers in the distinction between the AECOPD group complicated by APE and the control group. Two-tailed P <0.05 was used for screening significant differences.
Results
The characteristics of individuals including 52 patients with AECOPD and 10 patients with stable COPD enrolled in the study were listed in Tables 2 and 3. Among them, 13 patients with AECOPD with APE complication (mean age 75.69±8.4, 11 (84.62%) male) had more serious dyspnea. The plasma D-dimer was also higher in AECOPD with APE complication patients than AECOPD patients without APE (P<0.05).
 |
Table 2 Characteristics of Individuals Enrolled in the Study |
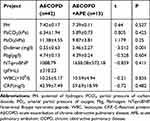 |
Table 3 Comparison of Laboratory Test Data Between the Two Groups [ |
Expression of microRNAs
miR-1233 (average fold change of 21.56) and miR-134 (average fold change of 14.59) were pointedly up-regulated in the serum of AECOPD patients with APE complications compared to AECOPD patients without APE (a 4-fold difference in serum concentration was considered to be significant) (P<0.05). As shown in Figure 1, no significant change was found between AECOPD patients without APE and patients with stable COPD (P>0.05).
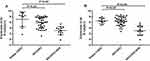 |
Figure 1 Expression level of miR-1233 (A) and miR-134 (B) in serum of 13 AECOPD+APE patients and matched AECOPD and COPD controls. |
Increased Serum Levels of miR-1233 and miR-134 Were Diagnostic Biomarkers for AECOPD with APE
The receiver operating characteristic (ROC) curve was employed to gauge the possible role of serum miR-1233 and miR-134 as a diagnostic biomarker for AECOPD complicated with APE. The AUC for serum miR-1233 was 0.884 (95% CI 0.79–0.978) (P<0.05) while the AUC for miR-134 was 0.931 (95% CI 0.863–0.999) (P<0.05) (Figure 2). In addition, the AUC for D-dimer was 0.628 (95% CI 0.447–0.809) while the AUC for age-adjusted plasma D-dimer was 0.705 (95% CI 0.525–0.885). The AUC for Wells score was 0.577 (95% CI 0.389–0.765).
Diagnosis Efficiency of microRNA Levels and Clinical Parameters of Patients
The efficiency of miR-1233, miR-134 with plasma D-dimer and Wells score were compared. As shown in Figure 3, The AUC of the ROC curve for miR-1233 combined with miR-134 was 0.957 (95% CI 0.908–1.000, P<0.05), the AUC for miR-1233+D-dimer was 0.88 (95% CI 0.783–0.977, P<0.05) whereas the AUC for miR-134+D-dimer was 0.936 (95% CI 0.87–1.000, P<0.05). In addition, the AUC of D-dimer+Wells was 0.684 (95% CI 0.492–0.876 P<0.05) while those of miR-1233+D-dimer+Wells and miR-134+D-dimer+Wells were respectively 0.908 (95% CI 0.815–1.000, P<0.05) and 0.947 (95% CI 0.884–1.000, P<0.05). No significant improvement in diagnostic efficiency was found in the combined analysis of miRNAs and age-adjusted plasma D-dimer (Figure 4).
Discussion
Acute pulmonary embolism (APE), a form of venous thromboembolism (VTE), is a life-threatening dangerous condition. The Diagnosis of APE at an early stage is very difficult due to the absence of specific symptoms and clinical signs. Studies have reported that the 3-year mortality rate for patients with stable APE was between 6% and 11% and that this rate may even increase to 30% or more in patients in shock or with hemodynamic instability.32 APE is one of the factors of acute exacerbation of unexplained COPD. Clinically, it is often difficult to distinguish AECOPD caused by airway inflammation alone or caused by APE, leading to missed and misdiagnosed diseases. Though the advent of D-dimer and chest CTPA have allowed advances in the discovery and elimination of APE, the half-life of D-dimer is long, contrary to its other counterparts including cardiac troponin and the thrombin-antithrombin complex, and despite its high sensitivity, it has poor specificity for confirming APE. Therefore, it is still urgent to develop reliable and simple biomarkers for APE diagnosis.19,33
The vital characteristics of an ideal biomarker are its reproducible measurability and its high specificity and sensitivity for the clinicopathological outcome of diseases.34,35 Peripheral venous blood samples are the preferred source for the detection of biomarkers of disease, and most established biomarkers evaluated by this method have shown consistent and repeatable results.36 miRNAs are regarded as promising biomarkers because they meet these criteria and are protected from endogenous RNase activity, which confers them high stability in the body. Thus, circulating miRNAs have been widely used as biomarkers in different types of human diseases such as liver diseases, tumors, diabetes, heart failure, coronary diseases and stable angina.37–40
The current research on the relationship between microRNAs and pulmonary embolism had been performed only on small samples, and only few studies were focused on AECOPD combined with pulmonary embolism. According to the current research data, the expression levels of plasma miR-134 (AUC 0.833), miR-28-3p (AUC 0.756) and miR-221 (AUC 0.823) in APE are significantly higher than those in healthy and non-APE patients.4,26,27 In addition, a study comparing 30 patients diagnosed with APE by CTPA and 12 healthy controls confirmed that miR-1233 distinguished between APE and non-ST-segment elevation cardioinfarction (NSTEMI) patients and healthy control with a sensitivity of 90%, a specificity of 100% and 92%, and the AUCs of 0.95 and 0.91.28 However, it was inconclusive whether microRNAs can effectively identify AECOPD complicated by APE. There is no specific research about the role and the specific mechanism they play in the disease, whether it is a protective factor or a pathogenic factor has not been specifically studied.
The results of this study shown that the levels of serum miR-1233 and miR-134 in AECOPD patients with APE complication were significantly increased (P<0.05), suggesting that these microRNAs may be used as early diagnostic markers for AECOPD with APE complications and, thus, could help reduce the missed diagnosis rate of patients.
Herein, we evaluated the levels of miR-1233 and miR-134 in 13 AECOPD patients with APE complications and 39 AECOPD patients without APE. We discovered that the miR-1233 and miR-134 expression levels were significantly increased compared with AECOPD individuals without APE. At the same time, 10 patients with stable COPD who were matched with gender and age were included and the comparison of the miRNA expression levels indicated no statistical difference in miR-1233 and miR-134 expression between patients with AECOPD and patients with stable COPD (P>0.05), but significant discrepancies between AECOPD patients with APE complications and patients with stable COPD (P< 0.05) were observed. These results confirmed that these microRNAs can effectively identify AECOPD combined with APE.
We conducted a joint analysis of each biomarker and compared their diagnostic efficiencies. The findings hinted that the combined diagnostic efficiency of miR-1233 and miR-134 was higher than the single diagnosis efficiency (AUC 0.957, 95% CI 0.908–1.000, P<0.05). The diagnostic efficiency of age-adjusted plasma D-dimer levels was also higher than the previous 500 µg/µL. Similarly, the combined evaluation of microRNA and clinical indicator plasma D-dimer and Wells score can also effectively improve the clinical diagnosis efficiency. Therefore, in clinics, patients should not be evaluated and diagnosed with a single index, but be analyzed and evaluated on a multi-level and multi-index basis to reduce clinical missed diagnosis or overdiagnosis.
The results of this study support the potential of miR-134 and miR-1233 as serum biomarkers for the diagnosis of AECOPD with or without APE complications. Moreover, there are few studies on miR-1233 and miR-134 in renal cell carcinoma, myocardial infarction, esophageal squamous cell carcinoma.41–43 For example, extracellular vesicles (EVs) are known as crucial regulators of the maintenance of homeostasis in the human airway via intercellular communication, and studies have even conveyed that lung epithelial cells represent the major producers of EVs in the lung.44,45 Specific miRNAs are known to be loaded in EVs, while others are expelled.46 However, the research on the mechanism of action and the target of action is rarely mentioned, so exploring the relationship between the upstream and downstream of the body’s microenvironment and the role of miRNAs packaged in EVs may be advantageous for clinical work is the handling of various diseases.
It was undeniable that this study was a single-center study with small sample size. It is well known that in different studies, due to differences in race, sample size, design, environment, and enrollment, the prevalence of APE in AECOPD patients varies greatly.8 Therefore, the interpretation of the results in this study is limited, mainly because (1) the sample size was small; (2) the uniqueness of the department, the patients treated with AECOPD are all patients with moderate to severe acute exacerbation, long-term hypoxia and hypoactivity, and are all high-risk patients with thrombosis. We recognize that our small sample size limits universality, and due to the complexity of miRNAs in biological systems, as well as different genetic, social, and therapeutic characteristics, differences in patient demographics may also affect these findings. Therefore, larger and more diverse studies are needed in the future to verify the reliability of our conclusions.
Conclusion
The findings of this study have shown that serum miR-1233, miR-134 levels were significantly increased in AECOPD patients with APE complications compared with AECOPD without APE and stable COPD. Therefore, the results of this study support miR-1233 and miR-134 as potential diagnostic biomarkers for AECOPD combined with APE or unconsolidated APE. At the same time, much more effort should be undertaken to explore the mechanism and the role of the miRNAs identified in our study.
Acknowledgments
This work was supported by the Key Discipline of Pudong New Area, Shanghai (No: PWZxk2017-22) and supported by Scientific Research Foundation of Shanghai Municipal Commission of Health and Family Planning (No.201740310).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interest.
References
1. GOLD. Global strategy for the diagnosis, management and prevention of COPD, global initiative for Chronic Obstructive Lung Disease (GOLD); 2019. Available from: http://guide.medlive.cn/guideline/19229.
2. Deng HY, Li G, Luo J, et al. MicroRNAs are novel non-invasive diagnostic biomarkers for pulmonary embolism: a meta-analysis. J Thorac Dis. 2016;(12):
3. Rivera-Lebron B, McDaniel M, Ahrar K, et al. Diagnosis, treatment and follow up of acute pulmonary embolism: consensus practice from the PERT consortium. Clin Appl Thromb Hemost. 2019;25:1076029619853037. doi:10.1177/1076029619853037
4. Zhou X, Wen W, Shan X, et al. MiR-28-3p as a potential plasma marker in diagnosis of pulmonary embolism. Thromb Res. 2016;138:
5. Douma RA, Kamphuisen PW, Büller HR. Acute pulmonary embolism. Part 1: epidemiology and diagnosis. Nat Rev Cardiol. 2010;7(10):
6. Furlan A, Aghayev A, Chang CC, et al. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology. 2012;265(1):
7. Jiménez D, Bikdeli B, Quezada A, et al. Hospital volume and outcomes for acute pulmonary embolism: multinational population based cohort study. BMJ. 2019;366:l4416. doi:10.1136/bmj.l4416
8. Cao YQ, Dong LX, Cao J. Pulmonary embolism in patients with acute exacerbation of chronic obstructive pulmonary disease. Chin Med J (Engl). 2018;131(14):
9. Bertoletti L, Quenet S, Mismetti P, et al. Clinical presentation and outcome of venous thromboembolism in COPD. Eur Respir J. 2012;39(4):
10. Chen WJ, Lin CC, Lin CY, et al. Pulmonary embolism in chronic obstructive pulmonary disease: a population-based cohort study. COPD. 2014;11(4):
11. Pourmand A, Robinson H, Mazer-Amirshahi M, Pines JM. Pulmonary embolism among patients with acute exacerbation of chronic obstructive pulmonary disease: implications for emergency medicine. J Emerg Med. 2018;55(3):
12. Morrone D, Morrone V. Acute pulmonary embolism: focus on the clinical picture [published correction appears in Korean Circ J. 2018 Jul;48(7):661–663]. Korean Circ J. 2018;48(5):
13. Kearon C. Diagnosis of pulmonary embolism. CMAJ. 2003;168(2):
14. Tak T, Karturi S, Sharma U, Eckstein L, Poterucha JT, Sandoval Y. Acute pulmonary embolism: contemporary approach to diagnosis, risk-stratification, and management. Int J Angiol. 2019;28(2):
15. Woller SC, Stevens SM, Adams DM, et al. Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest. 2014;146:1444–1451. doi:10.1378/chest.13-2386
16. Akpinar EE, Hoşgün D, Doğanay B, Ataç GK, Gülhan M. Should the cut-off value of D-dimer be elevated to exclude pulmonary embolism in acute exacerbation of COPD? J Thorac Dis. 2013;5(4):
17. Ma Y, Yan S, Zhou L, Yuan DT. Competitive assessments of pulmonary embolism: noninvasiveness versus the golden standard. Vascular. 2016;24(2):
18. den Exter PL, van der Hulle T, Klok FA, Huisman MV. Advances in the diagnosis and management of acute pulmonary embolism. Thromb Res. 2014;133(Suppl 2):
19. Chien CH, Shih FC, Chen CY, Chen CH, Wu WL, Mak CW. Unenhanced multidetector computed tomography findings in acute central pulmonary embolism. BMC Med Imaging. 2019;19(1):65. doi:10.1186/s12880-019-0364-y
20. Luk L, Steinman J, Newhouse JH. Intravenous contrast-induced nephropathy-the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):
21. He F, Lv P, Zhao X, et al. Predictive value of circulating miR-328 and miR-134 for acute myocardial infarction. Mol Cell Biochem. 2014;394(1–2):
22. Thibord F, Munsch G, Perret C, et al. Bayesian network analysis of plasma microRNA sequencing data in patients with venous thrombosis. Eur Heart J Suppl. 2020;22(SupplC):
23. Szymczak I, Wieczfinska J, Pawliczak R. Molecular background of miRNA role in asthma and COPD: an updated insight. Biomed Res Int. 2016;2016:7802521. doi:10.1155/2016/7802521
24. Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91(3):
25. Hobbs BD, Tantisira KG. MicroRNAs in COPD: small molecules with big potential. Eur Respir J. 2019;53(4):1900515. doi:10.1183/13993003.00515-2019
26. Xiao J, Jing ZC, Ellinor PT, et al. MicroRNA-134 as a potential plasma biomarker for the diagnosis of acute pulmonary embolism. J Transl Med. 2011;9:159. doi:10.1186/1479-5876-9-159
27. Liu T, Kang J, Liu F. Plasma levels of microRNA-221 (miR-221) are increased in patients with acute pulmonary embolism. Med Sci Monit. 2018;24:
28. Kessler T, Erdmann J, Vilne B, et al. Serum microRNA-1233 is a specific biomarker for diagnosing acute pulmonary embolism. J Transl Med. 2016;14(1):120. doi:10.1186/s12967-016-0886-9
29. Konstantinides SV, Torbicki A, Agnelli G, et al. Corrigendum to: 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2015;36. doi:2642.doi:10.1093/eurheartj/ehu479
30. Ishaaya E, Tapson VF. Advances in the diagnosis of acute pulmonary embolism. F1000Res. 2020;9:F1000Faculty Rev–44. doi:10.12688/f1000research.21347.1
31. Hargett CW, Tapson VF. Clinical probability and D-dimer testing: how should we use them in clinical practice? Semin Respir Crit Care Med. 2008;29(1):
32. Qin J, Liang H, Shi D, et al. A panel of microRNAs as a new biomarkers for the detection of deep vein thrombosis. J Thromb Thrombolysis. 2015;39(2):
33. Hembrom AA, Srivastava S, Garg I, Kumar B. MicroRNAs in venous thrombo-embolism. Clin Chim Acta. 2020;504:
34. Chen YW, Leung JM, Sin DD, Eickelberg O. A systematic review of diagnostic biomarkers of COPD exacerbation. PLoS One. 2016;11(7):e0158843. doi:10.1371/journal.pone.0158843
35. Xu J, Zhao J, Evan G, Xiao C, Cheng Y, Xiao J. Circulating microRNAs: novel biomarkers for cardiovascular diseases. J Mol Med (Berl). 2012;90(8):
36. Carmona P, Molina M, Toledano A. Blood-based biomarkers of Alzheimer’s disease: diagnostic algorithms and new technologies. Curr Alzheimer Res. 2016;13(4):
37. Danjuma MI, Sajid J, Fatima H, Elzouki AN. Novel biomarkers for potential risk stratification of drug induced liver injury (DILI): a narrative perspective on current trends. Medicine (Baltimore). 2019;98(50):e18322. doi:10.1097/MD.0000000000018322
38. Müller S, Janke F, Dietz S, Sültmann H. Circulating MicroRNAs as potential biomarkers for lung cancer. Recent Results Cancer Res. 2020;215:
39. Yao ZY, Chen WB, Shao SS, et al. Role of exosome-associated microRNA in diagnostic and therapeutic applications to metabolic disorders. J Zhejiang Univ Sci B. 2018;19(3):
40. Fung EC, Butt AN, Eastwood J, Swaminathan R, Sodi R. Circulating microRNA in cardiovascular disease. Adv Clin Chem. 2019;91:
41. Wulfken LM, Moritz R, Ohlmann C, et al. MicroRNAs in renal cell carcinoma: diagnostic implications of serum miR-1233 levels. PLoS One. 2011;6(9):e25787. doi:10.1371/journal.pone.0025787
42. Pan JY, Zhang F, Sun CC, et al. miR-134: a human cancer suppressor? Mol Ther Nucleic Acids. 2017;6:
43. Wang WW, Zhao ZH, Wang L, et al. MicroRNA-134 prevents the progression of esophageal squamous cell carcinoma via the PLXNA1-mediated MAPK signalling pathway [published correction appears in EBioMedicine. 2020 May;55:102772]. EBioMedicine. 2019;46:
44. Fujita Y, Kosaka N, Araya J, Kuwano K, Ochiya T. Extracellular vesicles in lung microenvironment and pathogenesis. Trends Mol Med. 2015;21(9):
45. Kulshreshtha A, Ahmad T, Agrawal A, Ghosh B. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J Allergy Clin Immunol. 2013;131(4):1194–1203.e14. doi:10.1016/j.jaci.2012.12.1565
46. Nolte-’t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, ‘t Hoen PA. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40(18):
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.