Back to Journals » Cancer Management and Research » Volume 13
The Predictive Significance of the Advanced Lung Cancer Inflammation Index (ALI) in Patients with Melanoma Treated with Immunotherapy as Second-Line Therapy
Authors Cheng X, Dong Y, Lou F
Received 12 October 2020
Accepted for publication 16 December 2020
Published 11 January 2021 Volume 2021:13 Pages 173—180
DOI https://doi.org/10.2147/CMAR.S286453
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Xi Cheng,* Yong Dong,* Fang Lou
Department of Medical Oncology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fang Lou
Department of Medical Oncology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, 3 East Qingchun Road, Hangzhou, People’s Republic of China
Email [email protected]
Purpose: The advanced lung cancer inflammation index (ALI) is a useful tool to predict the clinical outcome in several malignancies. The ALI not only contains indices related to inflammation but also the body mass index (BMI), which was reported to correlate with the sarcopenic status. However, to date, its predictive significance in metastatic melanoma patients treated with second-line immunotherapy has not been evaluated.
Methods: We retrospectively analyzed data from patients who were diagnosed with metastatic melanoma and treated with immunotherapy as second-line therapy between 2016 and 2019. Weight, height, neutrophil, lymphocyte and serum albumin were collected at baseline prior to receiving immunotherapy. The BMI was calculated by dividing the weight by height squared. The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. The ALI was defined as follows: ALI=BMI×serum albumin/NLR. The receiver operator curve (ROC) was used to determine the best cutoff value for ALI in predicting disease control (consisting of complete response, partial response and stable disease). The aim of this study was to investigate whether the ALI is a predictive indicator for progression-free survival in melanoma patients.
Results: Forty-three patients were included in this retrospective cohort study. By ROC, ALI> 50.98 before immunotherapy was predictive of disease control. Baseline continuous variables, such as BMI, NLR, C-reactive protein and C-reactive protein-to-albumin ratio, had significantly worse scores in patients of the low-ALI group (n=24) than high-ALI group (n=19). The median progression-free survival was significantly worse in the patients with ALI< 50.98 than the patients with ALI> 50.98 (2.60 months vs 11.17 months, P = 0.023, hazard ratio: 2.241, 95% confidence interval: 1.167– 5.097).
Conclusion: The advanced lung cancer inflammation index (ALI) > 50.98 before immunotherapy is a strong predictor for disease control. The ALI also provides great predictive value for metastatic melanoma patients treated with immunotherapy as second-line therapy.
Keywords: inflammatory marker, advanced lung cancer inflammation index, melanoma, immunotherapy, prediction
Introduction
Melanoma is one of the most aggressive cancers with estimated 287,723 new cases of melanoma of skin and 60,712 deaths worldwide in 2018.1 In the past, chemotherapy and interferon were the main treatments for metastatic melanoma and the average median overall survival (OS) for a patient with metastatic melanoma ranged from 6 to 12 months. Recent treatment methods of advanced melanoma, including immunotherapy, have been a factor in improving survival outcomes. Immunotherapy helps block the negative regulation of T-cell activation, which promotes the immune system’s ability to attack cancer cells. Immunotherapy has proven to be effective in clinical trials both as monotherapy and combination therapy for metastatic melanoma. The 5-year overall survival rate for Pembrolizumab alone in previously treated patients approached 34%, and the 5-year overall survival rate in previously untreated patients was 41%.2 The 4-year overall survival rate for the combination of Nivolumab and Ipilimumab in previously untreated patients exceeded 50%.3 In clinical trials of Nivolumab in previously treated patients, the 5-year OS rate was estimated at 35%.4
Despite great progress in immunotherapy over the past decade, the clinical outcome of some melanoma patients remains poor. To further reduce mortality rates, identifying an effective predictive biomarker for melanoma patients receiving immunotherapy could help clinicians in adopting better preventive and therapeutic treatments. Growing evidence indicates that cancer-related inflammation contributes to the development and progression of various types of cancer.5–7 Cancer-related inflammation could promote the formation of new blood vessels and lymphatic ducts at the early stage of tumorigenesis and could destroy the function of immune cells at later stage.8,9 Further evidence suggests that the major barrier to more successful immunotherapy is the tumor microenvironment, where chronic inflammation plays a predominant role.10 Therefore, several inflammatory markers, such as the Glasgow prognostic score (GPS), the neutrophil-to-lymphocyte ratio (NLR), and the C-reactive protein-to-albumin ratio (CRP/ALB ratio, CAR), are expected to be valuable prognostic and predictive biomarkers in immunotherapy.11–13 Cachexia in cancer patients is the result of the chronic systemic inflammatory response and often indicates a poor outcome for cancer patients.14 Sarcopenia, which has been reported to correlate with BMI, is an important nutritional component of cancer cachexia syndrome.15
The advanced lung cancer inflammation index (ALI) is based on the body mass index (BMI) and several inflammatory markers, such as serum albumin and NLR. Therefore, the ALI has the potential to reflect systemic inflammation better than other biomarkers because it correlates with inflammatory and nutritional indicators. Globally, there have been several studies describing the role of ALI as a prognostic and predictive marker in various types of cancer.16–19 The pretreatment ALI was a significant independent predictor of early progression in patients with advanced non-small-cell lung cancer (NSCLC) receiving Nivolumab and helped to identify patients likely to benefit from continued Nivolumab treatment.20 However, there is little information regarding the association between the ALI and the response to immunotherapy in metastatic melanoma.
The aim of our study was to evaluate the predictive significance of the ALI in patients with metastatic melanoma receiving immunotherapy as second-line therapy.
Patients and Methods
This study is a single-center, observational, retrospective study. Eligible patients were from the Department of Medical Oncology, Sir Run Run Shaw Hospital, Zhejiang University. Our requirements to screen patients for our study were as follows: (1) patients aged 18 years and above with adequate organ functions (neutrophil count ≥1.5*109/L; platelet count ≥100*109/l, hemoglobin ≥80 g/L, serum bilirubin ≤1.5*upper normal limit, transaminase ≤3* upper normal limit, calculated creatinine clearance ≥60 mL/min, etc.), (2) histologically confirmed stage IV melanoma per the American Joint Committee on Cancer’s Staging Manual, 7th edn, (3) Eastern Cooperative Oncology Group Performance Status of 0 or 1, (4) used immunotherapy as second-line therapy, (5) had not received previous immunotherapy as first-line therapy or adjuvant therapy, (6) measurable disease by Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST version 1.1) and (7) no synchronous or metachronous cancer.21 Patients with actively progressing brain metastases, or a history of serious autoimmune disease were excluded from our study.
Baseline and outcome data were collected retrospectively from electronic patient review charts. Weight, height, neutrophil, lymphocyte and serum albumin (g/dL) were collected at baseline prior to receiving immunotherapy. We determined the differential leukocyte count by a hematology analyzer according to the manufacturer’s protocol. We assayed the serum albumin using a chemiluminescent immunoassay according to the manufacturer’s protocol. The BMI was calculated by dividing the weight (kg) by height (m) squared. The neutrophil-to-lymphocyte ratio (NLR) was calculated as absolute neutrophil count divided by absolute lymphocyte count. We calculated the advanced lung cancer inflammation index (ALI), which is defined as follows: ALI=BMI × serum albumin/NLR.
All patients signed informed consent to receive immunotherapy. Immunotherapy agent was administered to the patients, which was continued until disease progression, treatment-related serious adverse events, or death. The choice of immunotherapy was at the discretion of the treating oncologist; typically, Nivolumab was given every 2 weeks, Pembrolizumab was given every 3 weeks, and Toripalimab was given every 3 weeks. All patients were followed up with a physical examination, serum tumor marker evaluation, chest CT and abdominal CT every two cycles of immunotherapy. When required, whole-body bone scan, positron emission tomography/computed tomography (PET/CT) scan, cranial and abdominal magnetic resonance imaging (MRI) and were additionally performed. After completion of all cycles of immunotherapy, each patient was monitored every 3 months until the confirmation of disease progression.
This study was conducted in accordance with the Declaration of Helsinki. This study was performed with the approval of the Ethics Committee of Sir Run Run Shaw Hospital, Zhejiang University.
Receiver operating characteristic (ROC) curve analysis was used to find the optimal cut-off value of ALI in predicting disease control. The response status and the date of progression were determined according to the RECIST criteria version 1.1. The disease control included complete response (CR), partial response (PR) and stable disease (SD) among all of the patients. The sensitivity, specificity, positive predictive value, negative predictive value and Youden index were calculated for the optimal cut-off values. We selected the cut-off value and patients were dichotomized to either the high ALI group (>the cut-off value) or the low ALI group (≤ the cut-off value) group accordingly. Categorical baseline variables were compared using Fisher’s exact test or Chi2 test and continuous baseline variables were compared using t test. In this study, we regarded the progression-free survival (PFS) as the primary endpoint for predicting the effects of immunotherapy. The PFS was defined as the time from the start of immunotherapy to progression or death from any cause, whichever occurred first. PFS was estimated using the Kaplan–Meier method. Differences between groups were compared using the Log rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Analyses were performed with SPSS software (version 23.0). A P < 0.05 was considered statistically significant.
Results
Patient Characteristics
Between 2016 and 2019, a total of 43 patients with metastatic melanoma receiving immunotherapy as second-line therapy were included in this retrospective cohort study. The median patient age at diagnosis was 57 years (range 26–84 years). Nineteen patients (44.2%) were male and 24 (55.8%) were female. All patients had an ECOG PS of 0 or 1. In the 43 patients, 25 patients had pulmonary metastasis, 35 patients had lymphatic metastasis, 13 patients had pulmonary metastasis, 1 patient had lymphatic metastasis and 4 patients had lymphatic metastasis, 15 patients (34.9%) had single-organ metastasis, and 28 patients (65.1%) had multi-organ metastases. The characteristics of the 43 patients are summarized in Table 1.
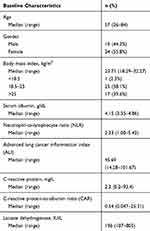 |
Table 1 Patient Characteristics at Baseline (n = 43) |
Preoperative lymphocyte count and neutrophil count, serum albumin, weight and height were available in all 43 patients. The median pretreatment values to calculate the ALI of our study were as following: lymphocyte count was 1.38 G/L (range 0.62–3.53 G/L), neutrophil count was 3.30 G/L (range 1.60–7.30 G/L), albumin was 4.15 g/dL (range 3.35–4.86 g/dL) and BMI was 23.71 (range 18.29–32.27). The median neutrophil-to-lymphocyte ratio was calculated as 2.33 (range 1.00–5.43). The median ALI was calculated as 45.69 (range 14.28–101.67).
Classification According to the ALI, CRP/ALB Ratio, NLR
We set 50.98 (sensitivity: 72.7%; specificity: 85.7%) as the optimal cut-off value for the ALI by calculating the ROC curve (Figure 1A). The patients were classified into high-ALI (n = 19) and low-ALI (n = 24) groups. We compared the baseline characteristics between the two groups. The background of the patients in each group is summarized in Table 2. No differences were observed for sex, age, serum albumin and lactate dehydrogenase. But the continuous variables BMI, NLR, CRP and CAR all had significantly worse scores in patients of the low ALI group.
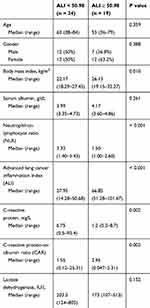 |
Table 2 Patient Characteristics at Baseline Classified by the Advanced Lung Cancer Inflammation Index (n = 43) |
We set 0.426 (sensitivity: 81.0%; specificity: 63.6%) as the cut-off value for the CRP/ALB ratio by ROC curve (Figure 1B). The patients were classified into high CRP/ALB ratio (n = 25) and low CRP/ALB ratio (n = 18) groups.
We set 2.22 (sensitivity: 81.0%; specificity: 68.2%) as the cut-off value for the NLR by ROC curve (Figure 1C). The patients were classified into high NLR (n = 24) and low NLR (n = 19) groups.
Survival Analyses According to the ALI, CRP/ALB Ratio, NLR
The disease control rate was 51.2% (22/43). PR was seen in 2 patients (4.7%), and SD was seen in 20 patients (46.5%). Out of 43 patients, 29 (67.4%) suffered from disease progression. The median PFS was 5.067 months (range 1.03–30.77 years, Figure 2A).
Patients in high-ALI group had significantly improved PFS compared to patients in low-ALI group (11.17months vs 2.60 months, P = 0.023, Hazard Ratio: 0.446, 95% Confidence Interval: 0.196–0.857, Figure 2B). Patients with a low CRP/ALB ratio had significantly improved PFS compared to patients with a high CRP/ALB ratio (11.30 months vs 2.77 months, P = 0.047, Hazard Ratio: 0.480, 95% Confidence Interval: 0.225–0.966, Figure 2C). The low NLR group had a longer median PFS compared with high NLR group (11.17 months vs 2.73 months, P = 0.026, Hazard Ratio: 0.444, 95% Confidence Interval: 0.203–0.875, Figure 2D).
Univariate and multivariate analyses on factors influencing the response to immunotherapy are presented in Table 3. Only ALI was indicated as an independent predictor for response.
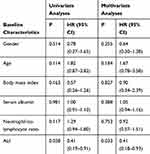 |
Table 3 Univariate Analyses and Multivariate Analyses for the Response to Immunotherapy |
Discussion
Immunotherapy agents that target the programmed death 1 receptor (PD-1) within the tumor microenvironment or its ligands PD-L1 on tumor cells, thus blocking their interaction, have shown that survival times can be extended beyond chemotherapy. Some updated results show that a durable, sustained clinical benefit and improved survival outcomes can be achieved with immunotherapy in patients with metastatic melanoma. Systemic inflammation plays a key role in tumor survival, proliferation, angiogenesis, immunosuppression, and nutritional depletion. Nutritional status is an important factor in immune responses, with malnutrition being the main cause of immunodeficiency. Cancer-associated inflammation and malnutrition are key determinants not only of tumor progression but also of the response to immunotherapy. Therefore, the efficacy of immunotherapy is thought to be heavily dependent on the patients’ inflammatory and nutritional status at baseline. The ALI is composed of several values, namely BMI, the serum albumin, the absolute neutrophil count and absolute lymphocyte count in the peripheral blood, all of which play pivotal roles in patients’ nutritional, immune, and systemic inflammation status. Low BMI and serum albumin mirror a pre-cachexia or cachexia status.22 Likewise, decreased levels of serum albumin and lymphocyte count, and increased levels of neutrophil count indicate depressed host immunity with an accompanying enhanced inflammatory condition.23
To the best of our knowledge, our study is the first to evaluate the relationship between second-line immunotherapy and the advanced lung cancer inflammation index (ALI) in metastatic melanoma patients. The results of our study clearly discovered using pre-immunotherapy ALI as a novel relevant predictive biomarker. ALI can place metastatic melanoma patients into two fundamentally distinct PFS groups following immunotherapy as second-line therapy, with results favoring ALI > 50.98 over its ≤50.98 counterpart. The group with high ALI had significantly longer PFS than that with low ALI. Consequently, the ALI could become a candidate biomarker to select metastatic melanoma patients who could benefit from immunotherapy. These results also suggested that patients who have both inflammation and malnutrition are not likely to benefit from immunotherapy. Although we set 50.98 as the optimal cut-off value of the ALI based on the ROC curve in our study, the range of the optimal cut-off values used in previous reports are relatively broad in other types of cancer.24–26 We suspected that it might be related to the nature of the tumor, TNM stage and other factors. Therefore, the optimal cut-off value of the ALI used in our study may be a provisional value.
The importance of determining whether ALI can predict early progression is to help clinicians screen for patients who are unlikely to benefit from immunotherapy and to assess whether to switch patients to another treatment if tumor progression occurs relatively soon after the initiation of immunotherapy. Therefore, the ALI can be used as a simple biomarker for monitoring the therapeutic effects of immunotherapy as second-line therapy and can also be used for detecting early progression after initiation of immunotherapy in patients with metastatic melanoma. Our data suggest that the discontinuation of immunotherapy treatment in patients with low ALI scores may be justified.
The novel biomarkers predictive of immunotherapy efficacy could identify patients who would derive durable responses. There has been result reported that tumoral PD-L1 and macrophage PD-L1 expression was higher in the immunotherapy responders than immunotherapy non-responders in patients with unresectable stage III/IV melanoma receiving Pembrolizumab or Nivolumab.27 A study conducted by Balatoni showed an association between FOXP3+ cells and CD8+ T lymphocytes and response to ipilimumab treatment in patients with metastatic melanoma.28 Regarding tumor mutation burden (TMB), a recent study showing high TMB at the first follow-up seems to be associated with response and overall survival under combined immunotherapy in metastatic melanoma.29 However, the biomarkers such as PD-L1, tumor-infiltrating immune cells, and tumor mutation burden are expensive, time-consuming, and unavailable in wide clinical practice.30 Alternatively, an ALI assessment could be performed rapidly and precisely by an inexpensive blood test, performed in routine clinical practice.
In addition to the ALI, other inflammatory biomarkers have been reported to be correlated with the prognosis of patients with melanoma treated with immunotherapy. In our study as well as the previous reports, the NLR was found to be correlated with the prognosis of patients with advanced melanoma treated with Nivolumab or other PD-1 inhibitor monotherapy, and there is no doubt that the NLR is a useful prognostic biomarker.31,32 Although there are only a few studies reporting the association between the CAR and the response to immunotherapy in metastatic melanoma, the usefulness of the C-reactive protein for predicting the outcomes of melanoma patients receiving immune checkpoint inhibition was reported.33 According to the AUC in the ROC curves and Kaplan–Meier survival curves of progression-free survival, we decided that the ALI was superior over CAR and NLR at the present time.
The present study has several drawbacks. Our study is a retrospective analysis from a single-center experience. Due to the small population of eligible patients and the short duration of the follow-up study, we could not fully evaluate the impact of various immunotherapy regimens. To determine the predictive effect of different immunotherapy regimens, a more extensive study should be conducted with a larger sample of patients with long term follow-up period. Moreover, as our study enrolled only Chinese metastatic melanoma patients, results may differ across Eastern countries and Western countries. An international study could lead to more valuable research results. Lastly, considering this study focused on the pre-immunotherapy factors, there may be other factors such as infections or cancer-related complications that could have altered the results.
As the ALI is a dynamic marker that may show remarkable variations during and post immunotherapy periods due to the changes in host immunity, nutritional status and systemic inflammation response status, our subsequent studies would particularly concentrate on the ALI dynamics. While our study only focused on second-line immunotherapy in metastatic melanoma, we would focus on the role of the ALI in the first-line treatment of metastatic melanoma in subsequent studies as immunotherapy has become more and more widely used in first-line therapy.
In conclusion, we revealed the pretreatment ALI to be a strong independent predictive biomarker of progression in patients with metastatic melanoma who received immunotherapy as second-line therapy. As ALI assessment appears to be easily calculated following widely available tests without additional costs, this parameter could help identify patients with poor prognoses and provide useful information when considering treatment strategies for metastatic melanoma.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Xi Cheng and Yong Dong are co-first authors and contributed equally to this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray FI, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–588. doi:10.1093/annonc/mdz011
3. Hodi FS, Chiarionsileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, Phase 3 trial. Lancet Oncology. 2018;19(11):1480–1492. doi:10.1016/S1470-2045(18)30700-9
4. Hodi FS, Kluger HM, Sznol M, et al. Abstract CT001: durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a Phase I trial. Cancer Res. 2016;76(5):999–1008. doi:10.1158/0008-5472.CAN-15-1439
5. Grivennikov SI, Greten FR, Karin M, et al. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899.
6. Diakos CI, Charles KA, Mcmillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncology. 2014;15:11. doi:10.1016/S1470-2045(14)70263-3
7. Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi:10.1038/nrc3611
8. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
9. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
10. Nakamura K, Smyth MJ. Targeting cancer-related inflammation in the era of immunotherapy. Immunol Cell Biol. 2017;95(4):325–332. doi:10.1038/icb.2016.126
11. Kasahara N, Sunaga N, Tsukagoshi Y, et al. Post-treatment glasgow prognostic score predicts efficacy in advanced non-small-cell lung cancer treated with Anti-PD1. Anticancer Res. 2019;39(3):1455–1461. doi:10.21873/anticanres.13262
12. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi:10.1016/j.lungcan.2017.07.024
13. Tamiya M, Tamiya A, Hosoya K, et al. Efficacy and safety of pembrolizumab as first-line therapy in advanced non-small cell lung cancer with at least 50% PD-L1 positivity: a multicenter retrospective cohort study (HOPE-001). Invest New Drugs. 2019;37(6):1266–1273. doi:10.1007/s10637-019-00843-y
14. Sadeghi M, Keshavarz-Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N. Cancer cachexia: diagnosis, assessment, and treatment. Crit Rev Oncol Hematol. 2018;127:91–104. doi:10.1016/j.critrevonc.2018.05.006
15. Peterson SJ, Mozer M. Differentiating sarcopenia and cachexia among patients with cancer. Nutr Clin Pract. 2017;32(1):30–39. doi:10.1177/0884533616680354
16. Jafri SH, Shi R, Mills G, et al. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review.. BMC Cancer. 2013;13(1):158. doi:10.1186/1471-2407-13-158
17. Hua X, Chen J, Wu Y, et al. Prognostic role of the advanced lung cancer inflammation index in cancer patients: a meta-analysis. World J Surg Oncol. 2019;17(1):1–9. doi:10.1186/s12957-019-1725-2
18. Shibutani M, Maeda K, Nagahara H, et al. The prognostic significance of the advanced lung cancer inflammation index in patients with unresectable metastatic colorectal cancer: a retrospective study. BMC Cancer. 2019;19(1):241. doi:10.1186/s12885-019-5468-9
19. Topkan E, Mertsoylu H, Ozdemir Y, et al. Prognostic usefulness of advanced lung cancer inflammation index in locally-advanced pancreatic carcinoma patients treated with radical chemoradiotherapy. Cancer Manag Res. 2019;11:8807–8815. doi:10.2147/CMAR.S222297
20. Shiroyama T, Suzuki H, Tamiya M, et al. Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med. 2018;7(1):13–20. doi:10.1002/cam4.1234
21. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
22. Muscaritoli M, Anker SD, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–159. doi:10.1016/j.clnu.2009.12.004
23. Jacobs NL, Holtan SG, Porrata LF, Markovic SN, Tefferi A, Steensma DP. Host immunity affects survival in myelodysplastic syndromes: independent prognostic value of the absolute lymphocyte count. Am J Hematol. 2010;85:160–163. doi:10.1002/ajh.21618
24. Brkic FF, Kadletz L, Jank B, et al. Impact of pretherapeutic neutrophil-to-lymphocyte ratio, serum albumin, body-mass index, and advanced lung cancer inflammation index on clinical outcome in sinonasal squamous cell carcinoma. J Craniomaxillofac Surg. 2020;48(1):33–37. doi:10.1016/j.jcms.2019.11.010
25. Park YH, Yi HG, Lee MH, et al. Prognostic value of the pretreatment advanced lung Cancer Inflammation Index (ALI) in Diffuse Large B cell lymphoma patients treated with R-CHOP chemotherapy. Acta Haematol. 2017;137(2):76–85. doi:10.1159/000452991
26. Barth DA, Brenner C, Riedl JM, et al. External validation of the prognostic relevance of the advanced lung cancer inflammation index (ALI) in pancreatic cancer patients. Cancer Med. 2020;9(15):5473–5479. doi:10.1002/cam4.3233
27. Vilain RE, Menzies AM, Wilmott JS, et al. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clin Cancer Res. 2017;23(17):5024–5033. doi:10.1158/1078-0432.CCR-16-0698
28. Balatoni T, Mohos A, Papp E, et al. Tumor-infiltrating immune cells as potential biomarkers predicting response to treatment and survival in patients with metastatic melanoma receiving ipilimumab therapy. Cancer Immunol Immunother. 2018;67(1):141–151. doi:10.1007/s00262-017-2072-1
29. Forschner A, Battke F, Hadaschik D, et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma - results of a prospective biomarker study. J Immunother Cancer. 2019;7(1):180. doi:10.1186/s40425-019-0659-0
30. Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417–424. doi:10.1158/2326-6066.CIR-16-0325
31. Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. doi:10.1186/s40425-018-0383-1
32. Bartlett EK, Flynn JR, Panageas KS, et al. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer. 2020;126(1):76–85. doi:10.1002/cncr.32506
33. Laino AS, Woods D, Vassallo M, et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer. 2020;8(1):e000842. doi:10.1136/jitc-2020-000842
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


