Back to Journals » Integrated Pharmacy Research and Practice » Volume 11
The Practice of the Community Pharmacists in Managing Potential Drug-Drug Interactions: A Simulated Patient Visits
Authors Hamadouk RM , Albashair ED , Mohammed FM , Yousef BA
Received 1 January 2022
Accepted for publication 9 March 2022
Published 15 March 2022 Volume 2022:11 Pages 71—84
DOI https://doi.org/10.2147/IPRP.S355675
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Jonathan Ling
Riham M Hamadouk,1 Esra D Albashair,1 Fatimah M Mohammed,1 Bashir A Yousef2
1Department of Clinical Pharmacy, Faculty of Pharmacy, University of Khartoum, Khartoum, Sudan; 2Department of Pharmacology, Faculty of Pharmacy, University of Khartoum, Khartoum, Sudan
Correspondence: Bashir A Yousef, Department of Pharmacology, Faculty of Pharmacy, University of Khartoum, Al-Qasr Ave, Khartoum, 11111, Sudan, Tel +249155662037, Fax +249183780696, Email [email protected]
Background: Drug-drug interactions (DDIs) can cause treatment failure and serious adverse drug reactions, leading to morbidity and mortality. Due to their significant effects on the patient’s health, community pharmacists (CPs) competence in detecting and preventing these interactions is essential to provide optimal health services. Thus, this study aimed to explore the performance of the CPs in situations involving the presence of potential DDIs.
Methods: A cross-sectional, simulated patient study was conducted in 235 community pharmacies in the Khartoum locality. Two scenarios were used to evaluate the performance of the CPs. Ten final year B. Pharm. students were selected to act as simulated patients (SPs); they were trained for two weeks to familiarize their roles. All encounters were documented immediately after leaving the pharmacy by the SPs in the data collection form.
Results: All planned SPs visits were completed, resulting in 470 visits. None of the CPs asked about the patients’ medication history in both scenarios. After the SPs provided information about the drug used currently by the patient, 13.6% and 23.4% of the CPs had identified the potential DDIs in scenario 1 and scenario 2, respectively. In scenario 1, 59.4% distinguished the interaction of simvastatin with both drugs, while, in scenario 2, 74.5% recognized the interaction of warfarin with both drugs. In identifying DDIs, around half of the CPs were dependent on their knowledge or using drug interaction checker programs. The most common intervention made by the CPs was referring the patient to the prescriber (56.3% CPs in scenario 1 and 60% CPs in scenario 2).
Conclusion: CPs practice in identifying and managing potential DDIs was poor. The current CPs practices need substantial improvement. Therefore, professional education and the use of software programs in community pharmacies should be encouraged.
Keywords: drug-drug interactions, simulated patient, community pharmacist, medication history
Introduction
Drug-drug interactions (DDIs) are a phenomenon in which one drug changes the efficacy or toxicity of another drug when used concomitantly.1 DDIs were classified as pharmacokinetic drug-drug interactions, which occur when one drug alters another drug’s systemic concentration; thus, its amount and presence in the site of action changes. Mechanisms of pharmacokinetic interactions include absorption, distribution, metabolism, and excretion. The other type is pharmacodynamic drug-drug interactions, which occur when the presence of one drug synergizes, diminishes, or even cancels the effect of the other drug.2,3
Drug-drug interactions can be beneficial, like when they use for their additive effects in treating certain conditions or can be harmful, causing treatment failure and serious adverse drug reactions (ADRs) leading to morbidity and mortality.3,4 Many factors are associated with DDIs, including the simultaneous use of drugs with herbal medications, or with over-the-counter medications, multidrug regimens, visiting different prescribers, polypharmacy, and the aging of the population.5 The clinically important DDIs usually associated with certain drug-related factors, like narrow therapeutic index drugs, and drugs that can induce or inhibit the metabolic enzyme activity.6
The high hazard of unwanted DDIs occurs in developed countries, like in the United States alone, 20% of the adverse drug events responsible for almost 770,000 deaths are due to DDIs.7 In developing countries like Sudan, primary health services are not affordable to the majority of the patients, so the impact of DDIs will be higher.8 Community pharmacists play as guardians to protect patients from risk and consequences of DDIs, because Sudanese physicians show inadequate communication with patients, particularly for medications’ history and potential DDIs.9 According to a self-assessment Sudanese study conducted in 2017, the majority of the community pharmacists faced several drug-drug interactions while dispensing, and they routinely asked their patients about their current prescription and over-the-counter medications and herbal medications.9
Many studies have investigated DDIs; some have shown the risk of harm with particular drug interactions, like the study by Antoniou et al, which showed the administration of cotrimoxazole in patients taking spironolactone associated with an increased risk of sudden death.10 Other studies assessed the prevalence of potential drug-drug interactions in prescriptions dispensed in hospital and community pharmacies.11,12 A study from Greece that evaluated the prevalence and type of potential DDIs in prescriptions in community pharmacies found that patients are at risk of adverse drug reactions due to potential DDIs, and pharmacists can help in detecting and avoiding these potential DDIs.13
Moreover, several studies have discussed community pharmacists’ knowledge, attitude, and practice towards DDIs. From these studies; a study conducted in central Saudi Arabia showed that community pharmacists’ knowledge about DDIs was insufficient.14 Another study from Saudi Arabia concluded that community pharmacists’ knowledge about DDIs was inadequate.15 Drug interactions were also discussed in studies that evaluated the dispensing practice. In a study that assessed the dispensing practice in the community pharmacies, almost 95% of the dispensers provided a drug that interacted with the patient prescribed medicine.16 However, the physician and pharmacist’s ability to identify and effectively manage potential DDIs requires further studies.4
According to FIP/WHO the pharmacist’s first concern must be the welfare of patients, this can be achieved through providing effective medication. Thus, the pharmacist must assure the appropriateness of the prescribed medicines, they have to provide clear instructions about the use of the drugs, consider the most cost-effective medicines while dispensing, minimize the unnecessary treatments, and detect and prevent any drug-drug and drug-food interactions.17
Despite what was reported about developing countries regarding the insufficient number of pharmacists and provision of Pharmacy services by unqualified personnel,18 in Sudan, there are strict penalties for community pharmacies that run without registered pharmacists, for that pharmacies owners are keen to have a pharmacist in their pharmacies. In this context, and because community pharmacists are considered the last line that ensures providing safe and effective medication; this research was conducted to explore community pharmacists’ performance in situations involving the presence of potential DDIs, and if they request patient medication history during their counseling. The study was focused on two drugs, warfarin as a drug with a narrow therapeutic index where a slight change in its concentration leads to serious consequences and maybe death,19 and simvastatin as one of the statins that used widely in controlling dyslipidemia and its toxicity associated with myopathy and risk of rhabdomyolysis.20
Methodology
Study Design
A cross-sectional design was used in this study conducted in the Khartoum locality. The study design was according to the STROBE Statement – Checklist of items that must be included in reports of cross-sectional studies,21 using the simulated patient approach to explore the performance of community pharmacists in events involving the presence of DDIs. The simulated patient methodology is world widely known method to assess the quality of pharmacy services. This approach allows the investigation with the minimum rate of the Hawthorne effect (under observation, the subjects tend to change their response), and avoids the desirable correct answers observed in the self-assessment method.22,23
Scenarios
To evaluate the community pharmacists’ performance regarding DDIs, the author designed two test scenarios in consultation with a pharmacist and a physician. The scenarios were pretested to evaluate their content validity by showing them to four faculty members in the Faculty of the Pharmacy, University of Khartoum. Two prescriptions were made in the proper format of a standard prescription, including the necessary patient information, to be used by the simulated patients (SPs). Each prescription contains two enzyme inhibitor drugs that interact with the patient’s specific drug.
Scenario 1
The (SP) enters the pharmacy to dispense a prescription for 72 Years old woman who was on simvastatin for hyperlipidemia, without informing the pharmacist about the patient’s medications. The prescription contains oral itraconazole and clarithromycin, both drugs severely interact with simvastatin. If the pharmacist dispensed the prescribed medications without asking any questions, then the SP will mention the current drug taken by the patient, and ask the pharmacist if there are any problems with using these drugs concomitantly.
Scenario 2
The (SP) enters the pharmacy to dispense a prescription for 76 Years old man who was on warfarin for deep vein thrombosis (DVT), without informing the pharmacist about the patient’s medications. The prescription contains oral metronidazole and cotrimoxazole, metronidazole has moderate interaction with simvastatin while cotrimoxazole severely interacts with simvastatin. If the pharmacist dispensed the prescribed medications without asking any questions, then the SP will mention the current drug taken by the patient, and ask the pharmacist if there are any problems with using these drugs concomitantly. Pairs of the interacting drugs and the scenarios presented in Table 1.
The simulation was constructed to determine if pharmacists will ask about the patient’s medication history; because it significantly decreases the risk of drug-related problems such as DDIs.24 Also, aging is associated with chronic diseases that require the use of multidrug regimens to manage them, and with aging, many physiological changes occur leading to a decrease in the functions of many organs like kidneys and liver; hence the metabolism of drugs reduces, making this population more prone to potential DDIs.25,26
Sample Size and Sampling
A list was obtained from the Sudanese General Directorate of Pharmacy to determine the sample size, containing all registered community pharmacies and their location. The registered community pharmacies in the Khartoum locality N=568, using the equation: n= N/1 + N(e)2, where (e) an error margin of 0.05,27 the necessary sample size was determined, which was n=235. The sampling technique applied in this study was systematic sampling. Microsoft Excel 2019 was used in selecting the sample, the equation k=N/n was used, where N= the population (list of registered community pharmacies in the Khartoum locality), n= the sample size, and k= systematic sample interval.28 Starting from a random point in the list the sample size was achieved by taking every (k) pharmacy on the list.
Assessment Form
To assess the practice, the author developed a data collection form specifically for this study to document the information from the visits (Table 2). Four academic members from the University of Khartoum who had excellent experiences in pharmacy practice validated the assessment form, and their feedback was used to modify the assessment form. Moreover, the assessment form has been piloted in thirty community pharmacies, and the data was excluded from this study. The collection form started firstly with closed questions which include information such as medication history, presence of any potential DDI identified by the community pharmacist, and type of drug-drug interactions identified in each scenario. Secondly, it contained open-ended questions, including how pharmacists identify DDIs and how they intervene to resolve these problems.
 |
Table 2 Data Collection and Assessment Form for Scenario 1 and Scenario 2 |
Data Collection
Ten final year B. Pharm. students from the Faculty of the Pharmacy University of Khartoum were selected to play the role of the patients in this simulation, four males and six females. We used ten students because, according to a systematic review by Watson et al the minimum recommended number of SPs is two.29 Written informed consents to volunteer as a simulated patient were collected from the students. The students were trained for two weeks to familiarize their roles. They were trained to act as patients, introduce and develop the scenarios, use lay language, and avoid using jargon during scenarios presentation. Also, repeated rehearsals were done to ensure the scenarios were performed reliably.
After that, a pilot study was done to check the SPs’ ability to perform the scenarios. Thirty community pharmacies were visited during the pilot study (three pharmacies for each SP), and these community pharmacies were not included in the study. Randomly from the ten SPs, five were assigned for each scenario. Also, community pharmacies were randomized across the SPs in each scenario. As a result, each SP made 47 visits to complete 235 pharmacy visits. Additionally, different lists containing the names and locations of community pharmacies were made and handled to the responsible SP to ensure that there were no repeated visits to any of the pharmacies. The test visits were carried out over two months, between August 2021 and October 2021, on different days and times. A total of 235 pharmacies were visited twice by one different scenario each time. At the beginning of each visit, the SP requested a pharmacist to ensure that the study examined only pharmacists. The information gathered during the visits was recorded immediately after leaving the pharmacy by the SPs in the data collection form.
Data Management and Analysis
For analysis of data, Statistical Package for Social Sciences software, version 23.0 (IBM SPSS Inc., Chicago, IL) was used.30 Initially, all information was gathered via a data master sheet then coded into variables. Descriptive statistics involving frequency tables were used to present the results. The relationship between variables was tested using an appropriate statistical method (One Way ANOVA analysis of variances) with a p-value of less than 0.05 was considered statistically significant.
Ethical Approval
This study was approved by the Ethical Committee of the Faculty of Pharmacy, University of Khartoum (FPEC-11-2020). Additional approval was obtained from the scientific research authority ministry of health, and due to the covert nature of the study, written informed consent was collected from the community pharmacists involved in this study two months before the study. The pharmacists were not aware of the nature and the time of the study, but they were informed that the study well assessed their practice, and they all agreed to participate. Throughout the research procedure, strict anonymity was maintained, and the information was kept confidential.
Results
Demographic Characteristics of the Community Pharmacists
As planned, all SPs visits were completed, 235 community pharmacies were visited twice, resulting in 470 visits. A total of 235 community pharmacists were tested in this study, of these 235 pharmacists, the highest category was those aged 26–30 years old (37%) (Table 3). More than half of the participants were females (57.4%). The majority were bachelor’s degree holders (70.6%), and those with working experience of 2–5 years represented the highest category (48.5%). The majority of the pharmacists were working daily between 6–10 hours (74.5%) (Table 3).
 |
Table 3 Socio-Demographic Characteristics of the Respondents (n=235) |
Medication History Taking Practice
None of the 235 community pharmacists asked about the patient’s medication history in both scenarios (Table 4). Because community pharmacists did not assess medication history, identifying the potential DDIs was done after the SPs provided information about the drug used currently by the patient and asked if there are any problems with using them concomitantly.
Scenario 1
Two hundred and three pharmacists failed to identify any potential DDIs in the prescription presented by the SP in this scenario. Among the 32 (13.6%) pharmacists who identified the presence of potential DDIs, 59.4% of them were able to identify the interaction of simvastatin with both drugs (Table 4). In identifying potential DDIs; 53.1% of the pharmacists were able to identify the DDIs depending on their knowledge, while 46.9% used drug interaction checker programs through their mobile phones (Figure 1). In the DDIs management (Figure 2), the most common intervention made by the community pharmacists to resolve the problem was referring the patient to the prescriber (56.3%), followed by suggesting a time interval between the interacting drugs (40.6%).
 |
Figure 1 Methods of DDIs identification by the community pharmacists in scenario 1 (n= 32) and scenario 2 (n= 55), (y-axis represents percentage). |
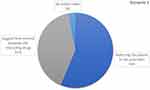 |
Figure 2 Type of interventions made by the community pharmacists to resolve DDIs in scenario 1 (n= 32). |
Scenario 2
The pharmacists who succeeded in identifying potential DDIs in the prescription provided by the SP in this scenario were 55 (23.4%). Of these 55 pharmacists, 74.5% were able to distinguish the interaction of warfarin with both drugs (Table 4). Regarding the method of identification, the majority of the community pharmacists, 56.4% depended on their knowledge, this was followed by using drug interaction checker programs through mobile phones 40%. (Figure 1). With regards to the intervention made by community pharmacists to resolve the problem (Figure 3), the majority of them (60%) referred the patient to the prescriber. This was followed by suggesting a time interval between the interacting drugs (21.8%).
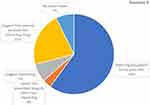 |
Figure 3 Type of interventions made by the community pharmacists to resolve DDIs in scenario 2 (n= 55). |
Comparison Between Pharmacists’ Demographics and Their Practice
In scenario 1, (Table 5) concerning age group, gender, professional qualification, years of experience, and daily working hours in the pharmacy; comparisons showed no significant differences between these independent variables and pharmacist’s practice in identifying and resolving potential DDIs. For scenario 2, Table 6 shows variability in the practice of pharmacists in identifying and resolving potential DDIs by demographic categories. Females’ rating of their practice toward DDIs identification and management was significantly higher than males (ANOVA p-value=0.021). Comparing other demographic groups with the practice there were no significant differences.
 |
Table 5 Variability in the Practice of Pharmacists in Identifying and Resolving Potential DDIs by Demographic Categories in Scenario 1 |
 |
Table 6 Variability in the Practice of Pharmacists in Identifying and Resolving Potential DDIs by Demographic Categories in Scenario 2 |
Discussion
Drug-drug interactions (DDIs) were the center of many studies, and due to the significant effects of these interactions on the patient’s health, and their economic burden, more attention should be paid by the health care members in all sectors.7 Regarding pharmacists and as an attempt to prevent DDIs, the United States Pharmacopeia (USP) put instructions to guide the pharmacist through patient counseling.31 Moreover, one of the core counseling items is asking the patient about medication history, including OTC medications, prescribed medications, and herbal medications, as patients are usually unaware of the impact DDIs on their health.32
Although the findings of the self-assessment study conducted in Sudan revealed that 75.6% of the participating pharmacists claimed they usually ask about medication history, asking about medication history was not done by any community pharmacists in this study. The same result was observed in a simulated patient study assessing the practice of counseling by the community pharmacist in Saudi Arabia, where none of the pharmacists asked if the patients were taking any other medications.33 In another study from Ethiopia, higher results were found as 11.7% of the pharmacists asked about current medications and 21.7% asked about medication history.34
In scenario 1 the patient was on simvastatin. Simvastatin is metabolized by the CYP3A4 iso-enzyme. Drugs that inhibit this enzyme increase the plasma level of simvastatin, which is associated with muscle-related adverse events, including the serious event rhabdomyolysis.20 Furthermore, elderly patients are at high risk of developing rhabdomyolysis, which seems to occur with simvastatin more than with the other statins, and appears in cases where statins are co administrated with potent enzyme inhibitors. Both clarithromycin and itraconazole are strong CYP3A4 inhibitors and their use with simvastatin is contraindicated.35 The results showed that out of 235 pharmacists, the number of pharmacists who identified the interaction between simvastatin and both drugs was only 19 pharmacists. Six pharmacists noticed the interaction of simvastatin with clarithromycin alone, while seven pharmacists identified the interaction of simvastatin and itraconazole alone.
Regarding detecting potential DDIs, slightly more than half (53.1%) of pharmacists depended on their knowledge, while the rest (46.9%) used drug interaction checker programs. The interventions made by pharmacists in this scenario revealed that 56.3% of the pharmacists referred the patient to the prescriber, 40.6% of the pharmacists suggested time intervals between the interacting drugs, and 3.1% made no action. Moreover, the community pharmacists’ practice in identifying and managing potential DDIs in this scenario 1 was poor. There was no significant difference between the demographic characteristics of pharmacists and their current practice. In a study by Toklu et al, the dispensing practice was significant with community pharmacists who had working experience of 5 years or less.36
In scenario 2, the patient was on warfarin, which has widespread drug interaction. Cotrimoxazole is considered one of the antibiotics associated with increasing the risk of bleeding.37,38 Moreover, a study in 2010 concluded that the risk of upper gastrointestinal tract hemorrhage in older patients taking warfarin is significantly higher with cotrimoxazole than other antibiotics.37 Even though the concomitant co-administration of warfarin and cotrimoxazole is considered a severe drug-drug interaction that must be avoided and alternative antibiotics should be selected,39 most pharmacists in this study did not identify this serious interaction. Metronidazole is also considered one of the drugs that increase warfarin toxicity. It was reported that the concomitant use of metronidazole with warfarin caused intracranial hemorrhage.40 If possible, this combination should be avoided, or the international normalized ratio (INR) should be closely monitored.
Concerning community pharmacists’ ability to detect potential DDIs, 41 pharmacists noticed warfarin’s interaction with both medications, 12 pharmacists identified only the interaction of warfarin with metronidazole alone, and two pharmacists identified only the interaction of warfarin with cotrimoxazole. As in scenario 1, most of the pharmacists in this scenario (56.4%) depended on their knowledge in identifying DDIs, 40% of the pharmacists used drug interaction checker programs, and 3.6% asked another pharmacist. The interventions made by pharmacists in this scenario vary; the most frequent intervention observed was referring to the prescriber, as 33 pharmacists referred the patient to their doctors. Twelve pharmacists suggested time intervals, four pharmacists suggested monitoring, and two pharmacists took their own decision to change the prescribed drugs to non-interacting ones. Unfortunately, taking no action regarding these interactions was the response of four community pharmacists.
Pharmacists’ practice in this scenario was also poor. Gender was significantly associated with the practice, as females performed better than males in this scenario. However, by age, professional qualification, years of experience, and daily working hours in the pharmacy there was no significant difference in pharmacist’s practice. Our results were similar to what was found in a Turkish study that assessed the practice in community pharmacies, as gender was significant in influencing the dispensing practice of pharmacists and females had higher scores than males. While age and years of working experience did not influence the dispensing practice of pharmacists.16
As drug experts, pharmacists’ knowledge of DDIs is crucial in optimizing patient care, their ability to accurately identify these interactions will improve the therapeutic outcomes and reduce hospitalization.41 In this study, and after the SPs informed the pharmacists about patient medication history, many pharmacists failed to identify the potential DDIs in both scenarios. For instance, community pharmacists ignored this essential counseling item and the consequences of neglecting it.
In a comparable study by Teressa et al, which consist of two parts; a self-reported part and actual practice through a simulated approach,42 similar results were observed regarding the information provided to the patient about DDIs as in the simulated part, 80.95% of the community pharmacists did not give information about DDIs. Also, in a simulated patient study from Germany, the identification of DDIs was low. Although community pharmacies are equipped with interaction checkers software, only 30% of pharmacists informed the simulated patient about the interaction.43 Also, a similar study assessed the practice of community pharmacists found that 92.4% of the pharmacists did not inform the patient about the DDIs presented in the prescription.16
Moreover, our findings were in accordance with the results from the Saudi SP study, where almost 83% of the participating pharmacists did not provide information about drug interaction.33 Another study revealed that the knowledge of community pharmacists regarding DDIs was inadequate. Major and minor DDIs were not identified by most of the participated pharmacists.14 Further, a study from Libya, that evaluated over-the-counter counseling practices by community pharmacists showed that 23% of the pharmacists provided information about drug-drug or drug-food interactions only upon request of patients.44 In contrast, a study from Lebanon showed a better community pharmacist practice toward drug interactions, as 64.8% of the pharmacists reported that they check for drug interactions.45
The identification of DDIs by practitioner or pharmacist can be made using reference books like British National Formulary and Stockley’s Drug Interactions. Drug interaction checker programs are also helpful and accessible through the internet. However, healthcare professionals should alert potent enzyme inhibitors and potent enzyme inducers. Also, attention must be paid when handling drugs with a narrow therapeutic window because these drugs are frequently linked to more serious concerns.46 In Sudan, the lack of electronic systems that include interaction checker software in the community pharmacy setting made pharmacists’ knowledge crucial in medication safety. In the current study, there was a low frequency of identification in both scenarios; most of the participated pharmacists had inadequate knowledge about the DDIs presented in the two scenarios. These results were similar to what found in a study from Saudi Arabia,15 which concluded that pharmacists had inadequate knowledge about DDIs.
It was also noticed that 15 pharmacists in scenario 1 and 22 pharmacists in scenario 2 used drug interaction checker programs; these results are high compared to the Irani study,47 where only nine pharmacists used these programs to check the DDIs. However, 90.2% of the community pharmacists in Switzerland were using these programs to identify potential DDIs.48 Such programs are vital as the pharmacist cannot remember all DDIs. A study from Turkey found that the use of drug interaction checker programs provides enormous help to community pharmacists in preventing potential DDIs, as these programs, when combined with the health care member’s medical and practical skills, could ensure the provision of safe healthcare services.49
In addition to identifying potential DDIs, pharmacists’ intervention to avoid such preventable DDIs is essential. According to Tannenbaum et al, when the pharmacist identifies a potential DDI, he or she must tell the prescriber and recommend a management plan, including monitoring the adverse drug reaction or modification in the drug therapy.50 Community pharmacists are usually faced with challenging situations in managing prescription errors or drug-related problems, including DDIs, as they lack some information that allows safety evaluation of prescriptions, like patient’s medical history or information such as blood test.51 Therefore, community pharmacist needs to collaborate with physicians.
In both scenarios, most pharmacists who identified the potential DDIs referred the patient to the prescriber. These findings are consistent with a Lebanese study, where 69% of the pharmacists inform the patient/or call the prescriber.45 A higher result was observed in another study,15 where 98.1% of pharmacists used to contact the prescribers upon detecting DDIs before dispensing the prescription. Moreover, in a study conducted in Swiss community pharmacies, direct contact with the prescriber was involved in more than one-third of the pharmacists’ interventions to solve drug-related problems, including DDI.52 While in a study from the United Arab Emirates, 38% of pharmacists referred simulated patients to their physicians.53
What was noticeable was that 13 (40.6%) pharmacists in scenario 1 and 12 (21.8%) pharmacists in scenario 2 suggested a time interval between the interacting drugs. The time interval between drugs is used when the interaction between two drugs occurs in the gastrointestinal tract (absorption), so giving the object drug two hours before or four hours after the perpetrator drug will allow the object drug to be absorbed into the blood circulation and prevent the interaction.39 In this study, the mechanism of drug-drug interactions in the two scenarios is inhibition of the metabolizing enzymes. Thus, spacing dosing times between the interacting drugs will not eliminate the interactions.
In this study, other interventions were also observed. Monitoring is considered one of the management options of DDIs,39 it was suggested by 7.3% of pharmacists in scenario 2. Changing the prescribed drugs to another drug was the intervention done by 3.6% of the pharmacists in scenario 2. In contrast, community pharmacists did not make this decision in scenario 1, even though selecting a drug that does not interact with statins is considered the adequate choice when possible.54 One pharmacist in scenario 1 and four pharmacists in scenario 2 identified the potential DDIs. However, they did not take any action and dispensed the prescription. Taking no action regarding drug-drug interaction puts the patient at risk of harm. A low level of pharmacist intervention to prevent DDIs has also been observed in a study exploring pharmacists’ involvement in identifying and managing potential DDIs among patients in the outpatient psychiatric clinics.41
This study has some limitations that should be considered when drawing any conclusions. The study is a cross-sectional study conducted only in the Khartoum locality therefore, the generalizability of the results to all community pharmacists in Sudan cannot be done. Additionally, although the simulated patient methodology effectively studies actual practices and reduces observation bias, audio recording recommended in the literature to provide quality assurance,55 by minimizing recall bias, was not used for data privacy reasons, thus, recall bias cannot be completely ruled out. Another limitation; the current study only assessed the practice of pharmacists regarding drug-drug interactions for two drugs (warfarin and simvastatin); many other drugs with important DDIs were not investigated. Moreover, this study did not assess the basis of the interventions made by the community pharmacists understudy to resolve the DDIs situation and if they possessed the clinical skills to take the right decision. Also, this study did not examine the relationship between pharmacists’ practice in managing DDIs and the operational features in the pharmacy. Finally, the performance feedback, which informs the pharmacists how close or far their practice is from the ideal and helps optimize their practice, was not delivered due to limited resources and many community pharmacies.
The study’s findings suggest that a minimal standard of practice should be established. Continuing education is fundamental for pharmacists to have the necessary knowledge and skills to improve their practice. Therefore, intensive educational and training programs must be endorsed by stakeholders. Enhancing software programs in community pharmacies should be encouraged to support and achieve optimal pharmacists’ practice. Also, further SP studies should be conducted emphasizing the dispensing and counseling practice of the community pharmacists followed by performance feedback to promote future changes in pharmacists’ performance.
Conclusion
This study explored the community pharmacists’ practice in dealing with potential drug-drug interactions. The results revealed that community pharmacists’ practice in identifying potential drug-drug interactions was poor. All community pharmacists did not perform the critical counseling item asking about medication history. The study also showed a low rate of using drug interaction checker programs. The majority of the pharmacists who identified the potential DDIs recommended referral to the prescriber. However, inappropriate intervention like suggesting time intervals was also highly observed.
Acknowledgments
The authors acknowledge all the students who participated as simulated patients.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Shetty V, Chowta MN, Chowta KN, Shenoy A, Kamath A, Kamath P. Evaluation of potential drug-drug interactions with medications prescribed to geriatric patients in a tertiary care hospital. J Aging Res. 2018;2018. DOI:10.1155/2018/5728957
2. Shah H, Shah NJ. Drug Interactions. In: Raj GM, Raveendran R, editors. Introduction to Basics of Pharmacology and Toxicology: Volume 1: General and Molecular Pharmacology: Principles of Drug Action. Singapore: Springer Singapore; 2019:197–204.
3. Snyder BD, Polasek TM, Doogue MP. Drug interactions: principles and practice. Aust Prescr. 2012;35(3):85–88. doi:10.18773/austprescr.2012.037
4. Hasan SS, Lim KN, Anwar M, et al. Impact of pharmacists’ intervention on identification and management of drug-drug interactions in an intensive care setting. Singapore Med J. 2012;53(8):526–531.
5. Malone DC, Abarca J, Hansten PD, et al. Drug – drug Interactions. J Am Pharm Assoc. 2004;44(2):142–151. doi:10.1331/154434504773062591
6. Palleria C, Di Paolo A, Giofrè C, et al. Pharmacokinetic drug-drug interaction and their implication in clinical management. J Res Med Sci. 2013;18(7):600–609.
7. Peabody J, Czarina M, Robert T, Hild C. Drug-drug interaction assessment and identification in the primary care setting. J Clin Med Res. 2018;10(11):806–814. doi:10.14740/jocmr3557w
8. Awad AI, Eltayeb IB, Capps PA. Self-medication practices in Khartoum State, Sudan. Eur J Clin Pharmacol. 2006;62(4):317–324. doi:10.1007/s00228-006-0107-1
9. Tokka ASA. Assessment of the awareness, knowledge, attitude, and practice of Sudanese Community Pharmacists, in Khartoum State, about drug interactions. World J Pharm Res. 2017;6(4):409–426. doi:10.20959/wjpr20174-8257
10. Antoniou T, Hollands S, Macdonald EM, Gomes T, Mamdani MM, Juurlink DN. Trimethoprim-sulfamethoxazole and risk of sudden death among patients taking spironolactone. CMAJ. 2015;187(4):E138–E143. doi:10.1503/cmaj.140816
11. Kafeel H, Rukh R, Qamar H, et al. Possibility of drug-drug interaction in prescription dispensed by community and hospital pharmacy. Pharmacol Pharm. 2014;05(04):401–407. doi:10.4236/pp.2014.54048
12. Mousavi S, Tabrizian K, Afshari A, Ashrafi M, Dirin M. Potential drug-drug interactions in prescriptions dispensed in community and hospital pharmacies in East of Iran. J Res Pharm Pract. 2014;3(3):104. doi:10.4103/2279-042x.141118
13. Chatsisvili A, Sapounidis I, Pavlidou G, et al. Potential drug-drug interactions in prescriptions dispensed in community pharmacies in Greece. Pharm World Sci. 2010;32(2):187–193. doi:10.1007/s11096-010-9365-1
14. Alrabiah Z, Alhossan A, Alghadeer SM, Wajid S, Babelghaith SD, Al-arifi MN. Evaluation of community pharmacists ’ knowledge about drug – drug interaction in Central Saudi Arabia. Saudi Pharm J. 2019;27(4):463–466. doi:10.1016/j.jsps.2019.01.008
15. Imam M, Abdel-Sattar RM, AlOmeir O, et al. Assessment of knowledge, attitude, and practice of pharmacists towards drug interactions in Saudi Arabia. J Pharm Res Int. 2021;33:538–545. doi:10.9734/jpri/2021/v33i46b32973
16. Gokcekus L, Toklu HZ, Demirdamar R, Gumusel B. Dispensing practice in the community pharmacies in the Turkish Republic of Northern Cyprus. Int J Clin Pharm. 2012;34(2):312–324. doi:10.1007/s11096-011-9605-z
17. World Health Organization. Joint Fip/Who guidelines on good pharmacy practice. In: WHO Technical Report Series. World Health Organization; 2010:1–19.
18. Abdullah M, Wahab A, Khan N, Khan A, Muhammad Imran Khan VN. Current scenario and future perspective of community pharmacy in developed, developing and sub-developing countries: a review. Int J Basic Med Sci Pharm. 2018;8(1):e205.
19. Johnson JA, Cavallari LH. Warfarin pharmacogenetics. Trends Cardiovasc Med. 2015;25(1):33–41. doi:10.1016/j.tcm.2014.09.001
20. Bellosta S, Corsini A. Statin drug interactions and related adverse reactions: an update. Expert Opin Drug Saf. 2018;17(1):25–37. doi:10.1080/14740338.2018.1394455
21. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):1623–1627. doi:10.1371/journal.pmed.0040296
22. IBjörnsdottr I, Granas AG, Bradley A, Norris P. A systematic review of the use of simulated patient methodology in pharmacy practice research from 2006 to 2016. Int J Pharm Pract. 2020;28(1):13–25. doi:10.1111/ijpp.12570
23. Hamadouk RM, Arbab AH, Yousef BA. Assessment of community pharmacist’s practice and patient counselling toward acute diarrhea treatment in Khartoum locality: a simulated patient study. Integr Pharm Res Pract. 2021;10:145–152. doi:10.2147/iprp.s340528
24. Fitzgerald RJ. Medication errors: the importance of an accurate drug history. Br J Clin Pharmacol. 2009;67:671–675. doi:10.1111/j.1365-2125.2009.03424.x
25. Cross A, Elliott R, Petrie K, Kuruvilla L, George J. Adherence in older adults prescribed multiple medications. Cochrane Database Syst Rev. 2020;5(5). doi:10.1002/14651858.CD012419.pub2
26. Fujie K, Kamei R, Araki R, Hashimoto K. Prescription of potentially inappropriate medications in elderly outpatients: a survey using 2015 Japanese guidelines. Int J Clin Pharm. 2020;42(2):579–587. doi:10.1007/s11096-020-00967-9
27. Kasiulevičius V, Šapoka V, Filipavičiūtė R. Sample size calculation in epidemiological studies. Gerontologija. 2006;7(4):225–231.
28. Kalton G. Systematic sampling. In: Wiley StatsRef: Statistics Reference Online. Wiley Online Library; 2017:1–6. doi:10.1002/9781118445112.stat03380.pub2
29. Watson MC, Norris P, Granas AG. A systematic review of the use of simulated patients and pharmacy practice research. Int J Pharm Pract. 2010;14(2):83–93. doi:10.1211/ijpp.14.2.0002
30. IBM Corp. IBM SPSS Statistics for Windows, Version 23.0. US: IBM Corp; 2021.
31. American Society of Health-System Pharmacists. ASHP guidelines on pharmacist-conducted patient education and counseling. Am J Heal Pharm. 1997;54(4):431–434. doi:10.1093/ajhp/54.4.431
32. International Pharmaceutical Federation. Counselling, concordance and communication - innovative education for pharmacists. 2012:145.
33. Qarni A, Alrahbeni T, AlQarni A, Alqarni A. The practice of counseling by community pharmacists in bisha health directorate, Saudi Arabia – simulated patient visit. 2019:1–15. DOI:10.21203/rs.2.16862/v1
34. Netere AK, Erku DA, Sendekie AK, Gebreyohannes EA, Muluneh NY, Belachew SA. Assessment of community pharmacy professional’s knowledge and counseling skills achievement towards headache management: a cross-sectional and simulated-client based mixed study. J Headache Pain. 2018;19. doi:10.1186/s10194-018-0930-7
35. Florentin M, Elisaf MS. Simvastatin interactions with other drugs. Expert Opin Drug Saf. 2012;11:439–444. doi:10.1517/14740338.2012.670633
36. Toklu HZ, Akici A, Cali S, Sezen SF, Keyer-uysal M, Sezen S. The pharmacy practice of community pharmacists in Turkey. Marmara Pharma J. 2010;(16):53–60. doi:10.12991/201014464
37. Fischer HD, Juurlink DN, Mamdani MM, Kopp A, Laupacis A. Hemorrhage during warfarin therapy associated with cotrimoxazole and other urinary tract anti-infective agents: a population-based study. Arch Intern Med. 2010;170(7):617–621. doi:10.1001/archinternmed.2010.37
38. Schelleman H, Bilker WB, Brensinger CM, Han X, Kimmel SE, Hennessy S. Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: interactions and the risk of hospitalization for gastrointestinal bleeding. Clin Pharmacol Ther. 2008;84(5):581–588. doi:10.1038/clpt.2008.150
39. Ansari J. Drug interaction and pharmacist. J Young Pharm. 2010;2(3):326–331. doi:10.4103/0975-1483.66807
40. Majid S, Elnaz S, Moradi V, Mohammad S, Kakhki BR, Rahmani S. Warfarin interactions: a letter to editor. J Patient Saf Qual Improve. 2019;3–5. DOI:10.22038/psj.2019.44292.1250
41. Alruthia Y, Alkofide H, Dakheel F, et al. Drug-drug interactions and pharmacists’ interventions among psychiatric patients in outpatient clinics of a teaching hospital in Saudi Arabia. Saudi Pharm J. 2019;27(6):798–802. doi:10.1016/j.jsps.2019.05.001
42. Surur AS, Getachew E, Teressa E, Hailemeskel B, Getaw NS, Erku DA. Self-reported and actual involvement of community pharmacists in patient counseling: a cross-sectional and simulated patient study in Gondar. Ethiopia. 2017;15(1):1–7.
43. Alte D, Weitschies W, Ritter CA. Mystery shoppers background. Objective. 2007;41:1023–1030. doi:10.1345/aph.1H565
44. Alseid S, Elmahjoubi E, Rghebi N. Evaluation of over-the-counter counselling practices by Libyan community pharmacists. AlQalam J Med Appl Sci. 2021;4(2):126–136.
45. Makkaoui N, Halaoui A, Atoui Z, et al. Knowledge, attitudes, and practices regarding drug interactions among community pharmacists. J Public Health. 2020;29(6):1357–1363.
46. Tsui VWL, Thomas D, Tian S, Vaida AJ. Chapter 16 - adverse drug events, medication errors, and drug interactions. In: Thomas D, editor. Clinical Pharmacy Education, Practice and Research. Elsevier; 2019:227–245.
47. Larki-harchegani A, Mehrpooya M, Qadermazi A, Shabib S, Ataei S. Evaluation the professional Practice of community pharmacists in dealing with important drug interactions in prescription. J Appl Pharma Sci. 2018;8(05):129–133. doi:10.7324/JAPS.2018.8517
48. Indermitte J, Erba L, Beutler M, Hersberger KE, Haefeli WE, Hersberger KE. Management of potential drug interactions in community pharmacies: a questionnaire-based survey in Switzerland. Eur J Clin Pharmacol. 2007;63:297–305. doi:10.1007/s00228-006-0237-5
49. Ilaç P, Önlenmesinde E, Eczanelerde S, et al. The importance of computerized drug interaction checker programs used in community pharmacies to avoid potential drug interactions: a preliminary study with clarithromycin. Istanbul Med J. 2019;20(1):67–71. DOI:10.4274/imj.galenos.2018.45712
50. Tannenbaum C, Sheehan NL, Sheehan NL. Drug – gene interactions understanding and preventing drug – drug and drug – gene interactions. Expert Rev Clin Pharma. 2014;2433. doi:10.1586/17512433.2014.910111
51. Chen Y, Neil KE, Avery AJ, Dewey ME, Johnson C. Prescribing errors and other problems reported by community pharmacists. Therap Clin Risk Manag. 2005;1(4):333–342.
52. Krähenbühl JM, Kremer B, Guignard B, Bugnon O. Practical evaluation of the drug-related problem management process in Swiss community pharmacies. Pharm World Sci. 2008;30(6):777–786. doi:10.1007/s11096-008-9217-4
53. Policy H, Buabeid M, Ashames A. Patient safety culture in handling prescriptions and interprofessional collaboration practices amongst community pharmacists: an investigative simulated patient study from the United Arab Emirates. Risk Manag Healthc Policy. 2020;13:3201–3209. doi:10.2147/RMHP.S282571
54. Samardzic I, Benkovic I, Vrca VB. Incidence of statin-drug interactions in Croatian community pharmacy. Pharmazie. 2017;72(3):187–191. doi:10.1691/ph.2017.6855
55. Werner JB, Hons BP, Benrimoj SI. Audio taping simulated patient encounters in community pharmacy to enhance the reliability of assessments. Am J Pharm Educ. 2008;72(6). doi:10.5688/aj7206136
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


