Back to Journals » International Journal of General Medicine » Volume 15
The Potential Role of Small Nucleolar RNAs in Cancers – An Evidence Map
Authors Wu F , Zhang L , Wu P, Wu Y, Zhang T, Zhang D, Tian J
Received 3 December 2021
Accepted for publication 29 March 2022
Published 8 April 2022 Volume 2022:15 Pages 3851—3864
DOI https://doi.org/10.2147/IJGM.S352333
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fanqi Wu,1,* Longguo Zhang,2,* Pingfan Wu,3 Yi Wu,2 Tao Zhang,4 Dekui Zhang,5 Jinhui Tian6
1Department of Respiratory, Lanzhou University Second Hospital, Lanzhou, Gansu Province, People’s Republic of China; 2The Second Clinical Medical School, Lanzhou University, Lanzhou, Gansu Province, People’s Republic of China; 3Department of Pathology, The 940th Hospital of the Joint Logistic Support of the People’s Liberation Army, Lanzhou, Gansu Province, People’s Republic of China; 4Department of Endocrinology and Metabolism, Lanzhou University Second Hospital, Lanzhou, Gansu Province, People’s Republic of China; 5Department of Gastroenterology, Lanzhou University Second Hospital, Lanzhou, Gansu Province, People’s Republic of China; 6Evidence-Based Medicine Center, Lanzhou University, Lanzhou, Gansu Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dekui Zhang; Jinhui Tian, Tel +86 139 1978 8616 ; +86 136 1934 2312, Email [email protected]; [email protected]
Purpose: Cancer seriously endangers human health in every country of the world. New evidence shows that small nucleolar RNAs play important roles in tumorigenesis. Herein, we created this evidence map to systematically assess the impact of dysregulated snoRNAs on cancers.
Methods: We searched four databases to February 2022 using the keywords, “carcinoma”, “neoplasms”, “tumor”, “cancer”, “snoRNA”, and “small nucleolar rna”. The research data were independently screened by two reviewers. Bubble plot, mind map, heatmap were used to depict the relationship between snoRNAs and cancers.
Results: In total, 102 studies met the inclusion criteria and were analyzed in this evidence map. In this study, we found that dysregulated snoRNAs were statistically associated with the clinicopathological characteristics of cancer patients, and affected tumor cell phenotypes. Abnormally expressed snoRNAs were associated with poor survival in cancer patients. Current research confirmed that snoRNAs have good diagnostic efficiency for cancers. snoRNAs could modulate biological processes and signaling pathways of different cancer cells by altering rRNA, regulating mRNA, and recruiting protein factors.
Conclusion: Taken all together, ectopic snoRNAs may serve as new biomarkers for clinical assessment, diagnostic, prognostic prediction of cancer patients, and provide a potential therapeutic strategy for cancer treatment. This article provided a visual analysis of existing evidence on snoRNAs and cancers, which can offer useful information for different researchers interested in snoRNAs.
Keywords: malignant neoplasms, snoRNAs, biomarkers, prognosis, diagnosis
Introduction
Cancer seriously endangers human health in every country of the world.1 In 2020, estimates showed that 19.3 million new cases emerged and cancer death toll had surpassed 10.0 million globally.2 Many genetic changes and non-genetic alterations are required for cancer genesis and progression, which is a dynamic and complex process. In addition to protein-coding genes, next-generation sequencing techniques give indications that noncoding RNAs (ncRNA) disorders play a crucial role in cancer-related signaling pathways. Therefore, ncRNAs have triggered broader interest in molecular mechanism research of cancers.
Small nucleolar RNAs (snoRNAs), a class of highly conserved ncRNAs of 60–300 nucleotides, have been ranked as one of the most ancient RNAs.3 snoRNAs are further divided into two major classes on account of their defined sequence motifs, consisting of C/D box snoRNAs (SNORDs) and H/ACA box snoRNAs (SNORAs). SNORDs, carrying highly conserved C box (RUGAUGA, R = A or G) and D box and less conserved C’ and D’ box, are bound by four core proteins termed 2ˊ-O-methyltransferase fibrillarin (FBL), nucleolar protein 56 (NOP56), nucleolar protein 58(NOP58), and small nuclear ribonucleoprotein 13 (SNU13) and packaged into C/D box small nucleolar ribonucleoproteins (snoRNPs) which direct 2ˊ-O-methylation of target RNAs. Just like the C/D box snoRNAs, another four core proteins including nucleolar protein 10 (NOP10), NHP2 ribonucleoprotein (NHP2), GAR1 ribonucleoprotein (GAR1), and a dyskerin pseudouridine synthase 1 (DKC1) bind to the conserved H (ANANNA, N = any NT) and ACA triplet boxes of SNORAs to form H/ACA box small nucleolar ribonucleoproteins that guide pseudouridylation of ribosomal RNAs (rRNAs) and small nuclear RNAs. Besides, there are some unusual snoRNAs, namely small Cajal body-specific RNAs with both box C/D, and/or H/ACA,4 extremely short single-domain box C/D RNAs,5 snoRNA-related long non-coding RNAs (lncRNAs),6 microRNAs (miRNAs)-like ncRNAs derived from small nucleolar RNAs,7 and snoRNA-derived and C (C′)/D′ (D)-box conserved PIWI interacting RNAs (piRNAs).8 sno-lncRNAs, sno-miRNAs, and snoRNA-derived piRNAs are functionally distinct from canonical snoRNAs.
Ninety percent of snoRNAs distribute in intronic regions of mRNA without their own promoters and the rest of snoRNAs are independently transcribed genes possessing their own promoters.9 The expression of snoRNAs can be regulated by genetic changes,10,11 such as genomic amplification,12 chromosome deletion,13 translocation,14 and mutation.15 In addition, Guerrieri et al found that SNORA67 expression is upregulated by DKC1(a H/ACA box core protein) overexpression.16 Kim et al reported that Prx1, a major scavenger of reactive oxygen species in cells, post-transcriptionally regulates a set of snoRNAs expression by stabilizing snoRNAs.17 These observations demonstrated that some genes processing snoRNAs, such as DKC1 and post-transcriptional modification also can lead to ectopic expression of several snoRNAs. The primary role of snoRNAs is to direct the posttranscriptional modification of target RNAs, including ribosomal RNAs, small nuclear RNAs, transfer RNAs, and probably other cellular RNAs, which has been well described.18 In recent years, in addition to the above-mentioned classical role of snoRNAs, emerging studies have exposed their potential novel functions, such as regulation of mRNA splicing,19,20 suppressants of oxidative stress,21 regulators of systemic glucose metabolism chromatin structure,22 and precursors of miRNAs7 and piRNAs.8
For many years, as the most well-established ncRNA, snoRNAs were thought to be housekeeping genes or transcriptional noise. However, with the flying progress of molecular biology, our understanding of snoRNAs grows. Up to date, there are five snoRNAs database: snoDB,23 SNORic,24 snoRNAbase,25 snoRNA Atlas,26 and snOPY,27 and more than 2000 snoRNAs have been annotated in humans according to the snoDB database. Furthermore, it has been reported that increasingly snoRNAs are involved in pathophysiological processes of various tumors.28–30 Currently, there is an increasingly large literature focusing on the association between snoRNAs dysregulation and cancer occurrence and development.
However, no systematic research of a very broad area covering many topics on snoRNAs in cancers has been carried out so far. Therefore, we conducted this evidence map to provide an expansive overview of extant research in regard to snoRNAs affecting oncogenesis.
Materials and Methods
Literature Search
A comprehensive search was performed in PubMed, Embase, the Web of Science, and Cochrane from database inception to February 2022 for studies published in English. In addition, in order to further determine other eligible papers, we manually searched the reference list of articles that may meet the conditions. The applied search terms include (“carcinoma” OR “neoplasms” OR “tumor” OR “cancer”) AND (“snoRNA” OR “small nucleolar rna”).
Inclusion and Exclusion Criteria
We included researches on the relationship between snoRNAs and malignant neoplasms, including but not limited to early diagnosis of malignant neoplasms, prognostic markers, drug resistance, and tumorigenesis mechanisms. Meta-analysis and systematic reviews that assess the association of snoRNAs with neoplasms are also included, if any. Studies that met the following criteria were excluded: 1. Non-malignant tumor research; 2. Bioinformatics studies without subsequent validation; 3. non-English studies; 4. non-snoRNA research; 5. Review.
Data Extraction
Publications identified in the comprehensive search were sent into Endnote X9 and screened on title and abstract for qualification. The research data were independently screened by two reviewers (W-FQ and Z-LG) according to the inclusion and exclusion criteria. Disagreements between the two investigators were settled by group discussion with a third researcher (T-JH). Microsoft Excel 2019 was utilized to set up a “literature information extraction table” for the extracted research information.
The extracted items mainly included the following: 1. basic characteristics: research title, author, country, publication period; 2. research characteristic: cancer types, snoRNA types, expression levels, prognostic indicators (eg, OS, overall survival, DFS/RFS, disease-free survival/recurrence-free survival), diagnostic indicators (eg, area under a curve, sensitivity, specificity), cell gene function research (eg, proliferation, migration, invasion, cell cycle, apoptosis, self-renewal, colony formation, epithelial-mesenchymal transition), clinicopathological characteristics, signal pathway and so on.
Data Analysis and Charting
We analyzed the basic characteristics of the literatures and presented the results as medians, percent, or other descriptive statistics as applicable. We used three bubble plots to present (a) the number of included studies by continent (X-axis: Continents, Y-axis: cancer types, bubble size: number of studies), (b) the number of included studies over a 15-year period (X-axis: year of publications, Y-axis: cancer types, bubble size: number of studies), and (c) the effect of deregulated snoRNAs on the prognosis. A stacked plot was used to analyze and compare the longitudinal trends in the proportion of articles from each continent. A heatmap was utilized to create a visual representation of the relationship between snoRNA types and gene function research. We used two mind maps to describe the relationship between aberrant expression of snoRNAs and (a) cancer cell phenotypes; (b) clinicopathological features.
Results
Literature Research
A total of 5011 studies were obtained from the four databases. Endnote X9 software was used to remove duplicates, and there were 2235 records excluded after removing duplicates. And 2433 articles were excluded after screening titles and abstracts; 2 reports were not retrieved. After further reading the literature, 56 non-malignant tumor studies were excluded, 13 articles were bioinformatics studies without subsequent validation, 7 articles were non-English studies, 20 articles were non-snoRNA research, 84 articles were reviews, and 59 articles were excluded for other reasons. Finally, a total of 102 studies (Table S1) were included, and the literature screening process is shown in Figure 1.
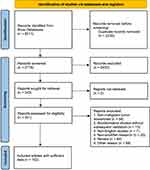 |
Figure 1 Flow diagram of study selection. |
Characteristics of Included Studies
One hundred and two included studies were published in English from 2008 to 2022, the number of new articles per year increased steadily (Figure S1). The map included a total of 20 tumor types, the top 3 tumor types were as follows: (1) breast cancer, (2) hepatocellular carcinoma (HCC), (3) non-small cell lung cancer (NSCLC), as shown in Figure 2. The types of tumors were clustered into four systems: genitourinary system, digestive system, respiratory system, and other systems (Figure S2).
From 2008 to 2016, the research on snoRNAs and malignant tumors was mainly concentrated in Europe and North America. In the past six years, the research center has tilted towards Asia (Figure S3). In North American countries, studies were more inclined towards NSCLC and breast cancer, and scientists in Europe were more interested in leukemias. While in Asian countries, research mainly focused on the digestive system, such as HCC, colorectal cancer, and gastric cancer (Figure S4).
Clinicopathologic Features
Twenty-three studies evaluated the relationship between dysregulation snoRNAs and clinicopathological characteristics. Eight studies included nine snoRNAs found that dysregulated snoRNAs were associated with metastasis and invasion of gastrointestinal tumors, for example: distant metastasis (5 snoRNAs), lymph node metastasis (5 snoRNAs), TNM stage (4 snoRNAs), venous invasion (1 snoRNAs), lymphatic invasion (1 snoRNA). In HCC, abnormal expression of snoRNAs was mainly related to TNM stage (6 snoRNAs), tumor diameter (4 snoRNAs), capsular invasion (3 snoRNAs). In addition, Wu et al found that HBV infection also affected the expression of SNORD76 in HCC.31 In pancreatic ductal adenocarcinoma, Cui et al reported that the abnormal expression of SNORA23 was mainly associated with venous invasion, intraductal papillary mucinous neoplasm and mucinous cystic neoplasm.32 Besides, one study found that downregulated snoRNA was associated with higher TNM staging in glioblastoma,33 as shown in Figure 3.
Cell Gene Function Research
The results in this category showed the relationship between dysregulated snoRNAs and tumor cell phenotypes, as shown in Figure 4. The most commonly reported gene function research was proliferation (38 snoRNAs), followed by migration (26 snoRNAs), invasion (25 snoRNAs), cell cycle (22 snoRNAs), apoptosis (20 snoRNAs), colony formation (19 snoRNAs), epithelial-mesenchymal transition (7 snoRNAs), and self-renewal (4 snoRNAs). From this heatmap, it can be seen that deregulated snoRNA promotes tumorigenesis mainly by affecting the proliferation, migration and invasion of tumor cells.
 |
Figure 4 Heatmap of the cell gene function research. Abbreviation: EMT, epithelial–mesenchymal transition. |
We used a mind map to reflect the relationship between snoRNAs and cellular phenotypes in different cancers (Figure S5). The current research primarily focused on the relationship between upregulated snoRNAs and tumors. However, the connection between downregulated snoRNAs and tumors is rare, particularly in the respiratory system.
snoRNAs Expression and Prognosis
The association between snoRNAs dysregulation and tumor prognosis is presented in Figure 5. X-axis: prognostic indicators-deregulated snoRNAs. The Y-axis: tumor systems. Bubble’s size: the number of patients included in those studies. Twenty-seven studies examined the relationship between prognosis and ectopic snoRNA in eleven tumor types, and the number of patients in these studies ranged from 40 to 318. In terms of tumor systems, the hotspots of research were mainly in digestive tumors, particularly in HCC. Among the included studies, snoRNAs were mostly upregulated and associated with poor prognosis, the opposite was true for downregulated snoRNAs. OS is the main indicator to evaluate tumor prognosis. Twenty-five studies used overall survival to evaluate the prognosis of tumors and 15 studies used DFS/RFS.
 |
Figure 5 Bubble plot of included studies by prognosis and by tumor system. Abbreviations: OS, overall survival; DFS/RFS, disease-free survival/recurrence-free survival. |
Risk formulas were developed to predict the survival of cancer patients based on the expression of snoRNAs derived from sequencing data and public databases. These risk formulas are useful tools for the prognostic assessment of tumors. However, we observed that most of them had not been verified in clinical cases (Table S2).
snoRNAs Expression and Diagnosis
The diagnostic potential of snoRNAs was assessed among eleven articles involving six types of neoplasm. In these studies, the number of snoRNAs ranged from 1 to 16. Seven studies reported the relationship between dysregulation snoRNAs and NSCLC diagnosis. These researches found that detection of snoRNAs in plasma, sputum and tumor tissue displayed high accuracy in the diagnosis of lung cancer. Three studies have found that the expression of snoRNAs in tissues and serum can be used to identify gastrointestinal tumors. Interestingly, Kitagawa group reported that SNORA74A and SNORA25 may be useful noninvasive tools for the early detection of pancreatic cancer.34 In genitourinary tumors, two studies assessed the diagnostic ability of eight snoRNAs in plasma, urinary sediment, serum, and tissues for clear cell renal cell carcinoma30,35 (Table S3).
Signaling Pathways
snoRNAs can modulate biological processes and signaling pathways of different cancer cells by altering rRNA, regulating mRNA, and recruiting protein factors, as detailed in Figure 6.
 |
Figure 6 snoRNAs related signaling pathway. |
Discussion
Since 2008,11 snoRNAs in cancers have gradually garnered research interest. Due to the accumulating volume of literature, we performed this evidence map to provide an expansive overview of extant research concerning snoRNAs in cancers. This evidence map consisted of 102 studies and no systematic review and meta-analysis for snoRNAs are available until now.
Emerging studies suggest that snoRNAs deregulation could play a crucial part in promoting tumorigenesis by modulating the malignant phenotype of tumors.36 SNORD52 is significantly upregulated in HCC tissues and cells than paracancerous samples and normal human hepatic cells, respectively.37 SNORD52 knockdown represses the proliferation, invasive, migration and colony formation capability of HCCLM9 and HCCLM3 cells, and induces G2/M arrest and apoptosis. Whereas SNORD52 overexpression promotes the above-mentioned malignant phenotypes of HCC cells. Ectopic expression of snoRNAs also influences cancer initiation and progression correlating closely with stemness maintenance of cancer cells.29,38–41 For instance, SNORA72 has an impact on stemness maintenance of ovarian cancer cells and is upregulated in ovarian cancer stem cells.29 Besides, SNORA72 knockdown decreases the self-renewal and migration abilities of ovarian cancer cells. These data suggested abnormal snoRNAs might be a potential biomarker for diagnosis, prognosis, and molecularly targeted therapy.
Both bioinformatics analyses and experimental studies have shown that snoRNAs deregulation was an independent prognostic factor of various cancers, comprising solid tumors and leukemias.42–45 In experimental studies, high expression of SNORA21 and SNORA42 acts as an independent risk factor for prognosis in colorectal cancer and may serve as potential prognostic biomarkers. Furthermore, several studies developed risk assessment models based on snoRNAs expression to predict overall survival of cancers by next-generation deep sequencing or silico analysis.46–48 Gao et al developed a risk formula identifying SNORA47, SNORA68 and SNORA78 in the training set of 77 NSCLC patients through multivariate Cox regression analysis, and it was validated in the testing set of 49 cases.46 A study by Zhao et al established a risk assessment model with a six-snoRNA signature from the TCGA database in clear cell renal cell carcinoma and also confirmed it in 64 clinical tissue cases and 50 serum samples achieving higher sensitivity and specificity.35 However, the majority of risk assessment formulae in silico analysis are not validated in clinical cases. Therefore, more investigations should be undertaken in a large population to identify the prognostic value of snoRNAs.
Numerous studies have shown that many snoRNAs might be novel diagnostic biomarkers for malignant tumors.49 Aberrant expression of SNORD16,36 SNORD33,50 and SNORD7631 could be detected in tissues of colon cancer, colorectal cancer and HCC, respectively, and discriminate between normal and neoplastic tissues. As part of them are stable and measurable in body fluids, some snoRNAs could help in the early diagnosis and the classification of subtypes of several tumors.34 SNORD63 and SNORD96A are stable in plasma and urinary sediment, and also potentially play as noninvasive early biomarkers for clear cell renal cell carcinoma.30 Compared with normal people, downregulated tumor-educated platelets SNORD55 is significantly related to TNM stage I/II patients with lung adenocarcinoma and lung squamous cell carcinoma,51 and increased plasma SNORD83A was related to tumor size.52 Tumor-educated platelets SNORD55 and plasma SNORD83A can distinguish NSCLC patients, even early NSCLC from controls with sufficient specificity and sensitivity. Teittinen et al observed that significant differences in snoRNAs expression can be used to classify various subgroups of leukemia, containing acute myeloid leukemia, acute lymphoblastic leukemias, and Burkitt’s lymphoma/leukemia.53 Besides, combining multimodal biomarkers, such as multiple snoRNAs or combination snoRNAs and other biomarkers, can improve the diagnostic efficiency of cancers. The combination of SNORD66, SNORD78, and miRNAs in sputum is cooperative for early detection of NSCLC with high sensitivity and specificity.54 The combination of CEA with plasma SNORD83A and serum SNORD1C offers better predictive value for the diagnosis of early-stage NSCLC52 and CRC49 respectively compared with snoRNA or CEA alone. Additionally, coexpression of SNORD66 and SNORD77 can improve the entire sensitivity and specificity for identifying lung cancer.55 Although current studies confirmed the diagnostic ability of snoRNAs for cancer, the majority of authors do not mention the true negatives, false negatives, true positives and false positives used in their articles. Therefore, it is not possible to assess the diagnostic value of snoRNAs through meta-analysis and obtain a higher level of evidence to support them.
Recently, a study in China with 712 HCC patients and 801 cancer-free controls reported a significant association of SNORD105 rs2305789 AA genotypes with a high risk of HCC.56 To the best of our knowledge, this is the first study that confirms SNP within snoRNAs affecting its expression level and function in cancer. Considerable evidence has shown that non-coding RNA single nucleotide polymorphisms (SNPs) are related to the risk of tumors.57–59 However, Gail60 reported that a model with several SNPs is less accurate in projecting individualized breast cancer risk than the National Cancer Institute’s Breast Cancer Risk Assessment Tool (BCRAT), a traditional method. In addition, how snoRNA SNPs result in tumorigenesis and whether snoRNA SNPs are a cause or consequence of cancer are not properly defined. Therefore, larger and further studies of snoRNA SNPs from different ethnic populations are needed to achieve high discriminatory accuracy and gain as much information as possible about the role of snoRNA SNPs in tumors.
snoRNAs can modulate biological processes and signaling pathways of various cancer cells by modifying rRNA, regulating mRNA, and recruiting protein factors.61 For example, as oncogenes, SNORD12C,62 SNORD78,62 SNORD42A,63 and SNORA6716 direct rRNA methylation and pseudouridylation to promote tumorigenesis in several tumors. In addition, SNORA23 suppresses tumorigenesis by impairing 2’-O-ribose methylation of 28S rRNA.64 Mechanistic studies revealed that snoRNAs affect the development of tumors by regulating various pathways. For instance, SNORD50A/B deletion is frequent and correlated with poor prognosis in multiple human malignancies by computational prediction.65 Furthermore, they revealed that SNORD50A/B directly binds K-Ras protein and suppresses the Ras-ERK1/2-MAPK pathway. Dong et al demonstrated that the deletion of U50 (SNORD50A) is common in breast cancer and prostate cancer.11–13 SNORD50A/B is found to be a tumor suppressor gene in these investigations. SNORD105b advances gastric cancer through activating the ALDOA/C-myc pathway.66 snoU2_19 plays as an oncogene in HCC and affects the development of HCC via the regulation of the Wnt/β-catenin signaling pathway by inhibiting β-catenin translocation.67 ACA11 deteriorates the prognosis of HCC by activation of the PI3K/AKT pathway to induce epithelial-mesenchymal transition.68 SNORD8941 and SNORA7229 promote cell stemness and ovarian carcinogenesis by modulating the Notch1-c-Myc pathway. In HCC, SNORD113-1 significantly inhibits HCC cell growth by decreasing the phosphorylation of SMAD2/3 and ERK1/2 in TGF-β pathways and MAPK/ERK.69 SNORA71A promotes the progression of NSCLC by upregulating the phosphorylation of MEK and ERK1/2 in the MAPK/ERK signal pathway. Furthermore, SNORA71A acts as a promoter of metastasis chiefly by regulating EMT and downregulated SNORA71A reduces cell proliferation in NSCLC cell lines.45 SNORD44 overexpression is associated with activating the caspase-dependent apoptosis pathway which facilitates the apoptosis of glioma cells.70 SNORA42 facilitates esophageal squamous carcinoma cell growth and metastasis by protecting DHX9 from being ubiquitinated and enhancing phosphorylation of p65 through the NF-κB pathway.71 SNORA42 functions as an oncogene in NSCLC and knockdown of SNORA42 is associated with inhibited tumorigenicity by initiating p53-dependent apoptosis.12 In esophageal squamous cell carcinoma, SNORD12B results in accelerated growth by activating the AKT-mTOR-4EBP1 signaling through nucleus partitioning of protein phosphatase 1 catalytic subunit alpha.72 Whether as oncogenes or tumor suppressor genes, these studies point to snoRNAs playing vital role in modulating tumor cellular processes and molecular mechanisms.
Due to being targeted both by siRNA and antisense oligonucleotide (ASO), dysregulated snoRNAs could be a potential target for cancer therapy. The siRNA-mediated SNORD16 knockdown resulted in reduced cell growth, proliferation, migration, and invasion of colon cancer cells by inducing cell apoptosis.36 In HCC xenograft-transplanted nude mouse tumor models, targeting SNORD52 with ASO resulted in a marked reduction in overall tumor growth and mass.37 However, snoRNAs are also involved in many normal physiological functions like ribosome formation, alternative splicing, and regulators of systemic glucose metabolism chromatin structure.22 In addition, aberrant expression of snoRNAs not only promote tumorigenesis but also connect to numerous normal physiological function like ribosome formation, and non-tumor benign diseases73 such as Prader Willi syndrome,74,75 tetralogy of Fallot,76 and LPS-mediated liver injury,21 etc. Therefore, it will be necessary to find tumor-specific snoRNAs for molecularly targeted therapy.
Deregulated snoRNAs have been implicated to induce treatment resistance in tumors. SNORD3A, SNORA13, SNORA28 involve in doxorubicin resistance in human doxorubicin resistant osteosarcoma cells through modulating multiple genes promoting proliferation, ribosome biogenesis, DNA damaging sensing, and DNA repair.77 Abnormal expression of snoRNAs contributing to tamoxifen resistance is identified by deep sequencing.78 Cancer stem cells are the main cause of chemotherapeutic resistance. Evidence showed that snoRNAs deregulation has an impact on stemness maintenance of various cancers.29,38–41 Thus, combined chemotherapy and snoRNAs targeted by siRNA and ASO regimens might be promising strategies for chemotherapy resistance. We observed the paucity of other therapy resistance like radio-resistance, targeted therapy resistance, and immunotherapy resistance. Therefore, there should be more investigations to explore how ectopic expression of snoRNAs contributes to recurrence, radio-resistance, targeted therapy resistance, and immunotherapy resistance, and the correlation between snoRNAs deregulation and tumor-infiltrating immune cells.
Besides, retrospective analyses of high-quality sequencing data, which were derived from common databases, were carried out to delineate the tumorigenesis or tumor suppressor property and molecular mechanisms of snoRNAs in cancers.79–81 These existing silico analyses require further verification in vivo or in vitro experiments. Notably, snoRNAs perform similar functions with other ncRNAs, such as miRNAs and lncRNAs. However, very little research is concerning their crosstalk and regulatory patterns in tumors. Simultaneously, we look forward that novel snoRNA mechanisms will be identified in future investigations.
Conclusion
Since 2008, the number of articles on snoRNAs and oncogenesis has gradually increased. snoRNAs can function as oncogenes or antioncogenes, and can be upregulated or downregulated in tumors. However, most snoRNAs are upregulated because of their low expression under normal conditions. snoRNAs are closely associated with clinicopathological features of cancer patients and the malignant phenotype of cancer cells. snoRNAs can affect tumor development by modifying the cancer-related signaling pathways directly or indirectly. They also can play as potential diagnosis or prognosis biomarkers and may serve as potential new therapeutic targets in cancers. This article provided a visual analysis of existing evidence on snoRNAs and cancers, which can offer useful information for different researchers interested in snoRNAs.
Abbreviations
snoRNAs, small nucleolar RNAs; ncRNAs, noncoding RNAs; SNORDs, C/D box snoRNAs; SNORAs, H/ACA box snoRNAs; snoRNPs, small nucleolar ribonucleoproteins; rRNAs, ribosomal RNAs; lncRNAs, long non-coding RNAs; miRNAs, microRNAs; piRNAs, PIWI interacting RNAs; BC, Breast cancer; HCC, hepatocellular carcinoma; NSCLC, non-small cell lung cancer; HBV, hepatitis B virus; ASO, antisense oligonucleotide.
Data Sharing Statement
All data supporting the conclusion of this article are included within the article.
Acknowledgments
We are grateful to all researchers of enrolled studies.
Funding
This work was supported by the Industry support and guidance project of colleges and universities in Gansu Province (2019C-21), Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital – Key Cultivation Projects (CY2018-ZD01), and Cuiying Scientific Training Program for Undergraduates of Lanzhou University Second Hospital (CYXZ2021-54).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi:10.1002/cncr.33587
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Kiss T. Small nucleolar RNAs: an abundant group of noncoding RNAs with diverse cellular functions. Cell. 2002;109(2):145–148. doi:10.1016/S0092-8674(02)00718-3
4. Darzacq X, Jády BE, Verheggen C, et al. Cajal body-specific small nuclear RNAs: a novel class of 2’-O-methylation and pseudouridylation guide RNAs. EMBO J. 2002;21(11):2746–2756. doi:10.1093/emboj/21.11.2746
5. Deryusheva S, Gall JG. Small, smaller, smallest: minimal structural requirements for a fully functional box C/D modification guide RNA. Biomolecules. 2019;9(9):457. doi:10.3390/biom9090457
6. Yin QF, Yang L, Zhang Y, et al. Long noncoding RNAs with snoRNA ends. Mol Cell. 2012;48(2):219–230. doi:10.1016/j.molcel.2012.07.033
7. Yu F, Bracken CP, Pillman KA, et al. p53 represses the oncogenic Sno-MiR-28 derived from a SnoRNA. PLoS One. 2015;10(6):e0129190. doi:10.1371/journal.pone.0129190
8. Zhong F, Zhou N, Wu K, et al. A SnoRNA-derived piRNA interacts with human interleukin-4 pre-mRNA and induces its decay in nuclear exosomes. Nucleic Acids Res. 2015;43(21):10474–10491. doi:10.1093/nar/gkv954
9. Dieci G, Preti M, Montanini B. Eukaryotic snoRNAs: a paradigm for gene expression flexibility. Genomics. 2009;94(2):83–88. doi:10.1016/j.ygeno.2009.05.002
10. Su X, Feng C, Wang S, et al. The noncoding RNAs SNORD50A and SNORD50B-mediated TRIM21-GMPS interaction promotes the growth of p53 wild-type breast cancers by degrading p53. Cell Death Differ. 2021;28(8):2450–2464. doi:10.1038/s41418-021-00762-7
11. Dong XY, Rodriguez C, Guo P, et al. SnoRNA U50 is a candidate tumor-suppressor gene at 6q14.3 with a mutation associated with clinically significant prostate cancer. Hum Mol Genet. 2008;17(7):1031–1042. doi:10.1093/hmg/ddm375
12. Mei YP, Liao JP, Shen J, et al. Small nucleolar RNA 42 acts as an oncogene in lung tumorigenesis. Oncogene. 2012;31(22):2794–2804. doi:10.1038/onc.2011.449
13. Dong XY, Guo P, Boyd J, et al. Implication of snoRNA U50 in human breast cancer. J Genet Genomics. 2009;36(8):447–454. doi:10.1016/S1673-8527(08)60134-4
14. Chu L, Su MY, Maggi LB Jr, et al. Multiple myeloma-associated chromosomal translocation activates orphan snoRNA ACA11 to suppress oxidative stress. J Clin Invest. 2012;122(8):2793–2806. doi:10.1172/JCI63051
15. Jenkinson EM, Rodero MP, Kasher PR, et al. Mutations in SNORD118 cause the cerebral microangiopathy leukoencephalopathy with calcifications and cysts. Nat Genet. 2016;48(10):1185–1192. doi:10.1038/ng.3661
16. Guerrieri AN, Zacchini F, Onofrillo C, et al. DKC1 overexpression induces a more aggressive Cellular behavior and increases intrinsic ribosomal activity in immortalized mammary gland cells. Cancers. 2020;12(12):3512. doi:10.3390/cancers12123512
17. Kim EK, Lee SY, Kim Y, Ahn SM, Jang HH. Peroxiredoxin 1 post-transcriptionally regulates snoRNA expression. Free Radic Biol Med. 2019;141:1–9. doi:10.1016/j.freeradbiomed.2019.05.030
18. Reichow SL, Hamma T, Ferré-D’Amaré AR, Varani G. The structure and function of small nucleolar ribonucleoproteins. Nucleic Acids Res. 2007;35(5):1452–1464. doi:10.1093/nar/gkl1172
19. Cavaillé J, Buiting K, Kiefmann M, et al. Identification of brain-specific and imprinted small nucleolar RNA genes exhibiting an unusual genomic organization. Proc Natl Acad Sci USA. 2000;97(26):14311–14316. doi:10.1073/pnas.250426397
20. Falaleeva M, Pages A, Matuszek Z, et al. Dual function of C/D box small nucleolar RNAs in rRNA modification and alternative pre-mRNA splicing. Proc Natl Acad Sci USA. 2016;113(12):E1625–E1634. doi:10.1073/pnas.1519292113
21. Michel CI, Holley CL, Scruggs BS, et al. Small nucleolar RNAs U32a, U33, and U35a are critical mediators of metabolic stress. Cell Metab. 2011;14(1):33–44. doi:10.1016/j.cmet.2011.04.009
22. Lee J, Harris AN, Holley CL, et al. Rpl13a small nucleolar RNAs regulate systemic glucose metabolism. J Clin Invest. 2016;126(12):4616–4625. doi:10.1172/JCI88069
23. Bouchard-Bourelle P, Desjardins-Henri C, Mathurin-St-Pierre D, et al. snoDB: an interactive database of human snoRNA sequences, abundance and interactions. Nucleic Acids Res. 2020;48(D1):D220–d225. doi:10.1093/nar/gkz884
24. Gong J, Li Y, Liu CJ, et al. A Pan-cancer analysis of the expression and clinical relevance of small nucleolar RNAs in human cancer. Cell Rep. 2017;21(7):1968–1981. doi:10.1016/j.celrep.2017.10.070
25. Lestrade L, Weber MJ. snoRNA-LBME-db, a comprehensive database of human H/ACA and C/D box snoRNAs. Nucleic Acids Res. 2006;34(90001):D158–62. doi:10.1093/nar/gkj002
26. Jorjani H, Kehr S, Jedlinski DJ, et al. An updated human snoRNAome. Nucleic Acids Res. 2016;44(11):5068–5082. doi:10.1093/nar/gkw386
27. Yoshihama M, Nakao A, Kenmochi N. snOPY: a small nucleolar RNA orthological gene database. BMC Res Notes. 2013;6(1):426. doi:10.1186/1756-0500-6-426
28. Zhang Z, Tao Y, Hua Q, et al. SNORA71A promotes colorectal cancer cell proliferation, migration, and invasion. Biomed Res Int. 2020;2020:8284576. doi:10.1155/2020/8284576
29. Zhang L, Ma R, Gao M, et al. SNORA72 activates the notch1/c-Myc pathway to promote stemness transformation of ovarian cancer cells. Front Cell Dev Biol. 2020;8:583087. doi:10.3389/fcell.2020.583087
30. Shang X, Song X, Wang K, et al. SNORD63 and SNORD96A as the non-invasive diagnostic biomarkers for clear cell renal cell carcinoma. Cancer Cell Int. 2021;21(1):56. doi:10.1186/s12935-020-01744-4
31. Wu L, Chang L, Wang H, et al. Clinical significance of C/D box small nucleolar RNA U76 as an oncogene and a prognostic biomarker in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2018;42(1):82–91. doi:10.1016/j.clinre.2017.04.018
32. Cui L, Nakano K, Obchoei S, et al. Small nucleolar noncoding RNA SNORA23, Up-regulated in human pancreatic ductal adenocarcinoma, regulates expression of spectrin Repeat-containing nuclear envelope 2 to promote growth and metastasis of xenograft tumors in mice. Gastroenterology. 2017;153(1):292–306.e2. doi:10.1053/j.gastro.2017.03.050
33. Xu B, Ye MH, Lv SG, et al. SNORD47, a box C/D snoRNA, suppresses tumorigenesis in glioblastoma. Oncotarget. 2017;8(27):43953–43966. doi:10.18632/oncotarget.16693
34. Kitagawa T, Taniuchi K, Tsuboi M, et al. Circulating pancreatic cancer exosomal RNAs for detection of pancreatic cancer. Mol Oncol. 2019;13(2):212–227. doi:10.1002/1878-0261.12398
35. Zhao Y, Yan Y, Ma R, et al. Expression signature of six-snoRNA serves as novel non-invasive biomarker for diagnosis and prognosis prediction of renal clear cell carcinoma. J Cell Mol Med. 2020;24(3):2215–2228. doi:10.1111/jcmm.14886
36. He JY, Liu X, Qi ZH, et al. Small nucleolar RNA, C/D Box 16 (SNORD16) acts as a potential prognostic biomarker in colon cancer. Dose Response. 2020;18(2):1559325820917829. doi:10.1177/1559325820917829
37. Li C, Wu L, Liu P, et al. The C/D box small nucleolar RNA SNORD52 regulated by Upf1 facilitates Hepatocarcinogenesis by stabilizing CDK1. Theranostics. 2020;10(20):9348–9363. doi:10.7150/thno.47677
38. Zhou F, Liu Y, Rohde C, et al. AML1-ETO requires enhanced C/D box snoRNA/RNP formation to induce self-renewal and leukaemia. Nat Cell Biol. 2017;19(7):844–855. doi:10.1038/ncb3563
39. Mannoor K, Shen J, Liao J, Liu Z, Jiang F. Small nucleolar RNA signatures of lung tumor-initiating cells. Mol Cancer. 2014;13(1):104. doi:10.1186/1476-4598-13-104
40. Zheng D, Zhang J, Ni J, et al. Small nucleolar RNA 78 promotes the tumorigenesis in non-small cell lung cancer. J Exp Clin Cancer Res. 2015;34(1):49. doi:10.1186/s13046-015-0170-5
41. Zhu W, Niu J, He M, et al. SNORD89 promotes stemness phenotype of ovarian cancer cells by regulating Notch1-c-Myc pathway. J Transl Med. 2019;17(1):259. doi:10.1186/s12967-019-2005-1
42. Liu CX, Qiao XJ, Xing ZW, Hou MX. The SNORA21 expression is upregulated and acts as a novel independent indicator in human gastric cancer prognosis. Eur Rev Med Pharmacol Sci. 2018;22(17):5519–5524. doi:10.26355/eurrev_201809_15812
43. Okugawa Y, Toiyama Y, Toden S, et al. Clinical significance of SNORA42 as an oncogene and a prognostic biomarker in colorectal cancer. Gut. 2017;66(1):107–117. doi:10.1136/gutjnl-2015-309359
44. Ronchetti D, Mosca L, Cutrona G, et al. Small nucleolar RNAs as new biomarkers in chronic lymphocytic leukemia. BMC Med Genomics. 2013;6(1):27. doi:10.1186/1755-8794-6-27
45. Tang G, Zeng Z, Sun W, et al. Small nucleolar RNA 71A promotes lung cancer cell proliferation, migration and invasion via MAPK/ERK pathway. J Cancer. 2019;10(10):2261–2275. doi:10.7150/jca.31077
46. Gao L, Ma J, Mannoor K, et al. Genome-wide small nucleolar RNA expression analysis of lung cancer by next-generation deep sequencing. Int J Cancer. 2015;136(6):E623–9. doi:10.1002/ijc.29169
47. Xing L, Zhang X, Zhang X, Tong D. Expression scoring of a small-nucleolar-RNA signature identified by machine learning serves as a prognostic predictor for head and neck cancer. J Cell Physiol. 2020;235(11):8071–8084. doi:10.1002/jcp.29462
48. Wang X, Xu M, Yan Y, et al. Identification of eight small nucleolar RNAs as survival biomarkers and their clinical significance in gastric cancer. Front Oncol. 2019;9:788. doi:10.3389/fonc.2019.00788
49. Liu Y, Zhao C, Sun J, et al. Overexpression of small nucleolar RNA SNORD1C is associated with unfavorable outcome in colorectal cancer. Bioengineered. 2021;12(1):8943–8952. doi:10.1080/21655979.2021.1990194
50. Yang X, Li Y, Li L, et al. SnoRNAs are involved in the progression of ulcerative colitis and colorectal cancer. Dig Liver Dis. 2017;49(5):545–551. doi:10.1016/j.dld.2016.12.029
51. Dong X, Song X, Ding S, et al. Tumor-educated platelet SNORD55 as a potential biomarker for the early diagnosis of non-small cell lung cancer. Thorac Cancer. 2021;12:659–666. doi:10.1111/1759-7714.13823
52. Wang K, Song X, Li X, et al. Plasma SNORD83A as a potential biomarker for early diagnosis of non-small-cell lung cancer. Future Oncol. 2021;18(7):821–832. doi:10.2217/fon-2021-1278
53. Teittinen KJ, Laiho A, Uusimäki A, et al. Expression of small nucleolar RNAs in leukemic cells. Cell Oncol. 2013;36(1):55–63. doi:10.1007/s13402-012-0113-5
54. Su Y, Guarnera MA, Fang H, Jiang F. Small non-coding RNA biomarkers in sputum for lung cancer diagnosis. Mol Cancer. 2016;15(1):36. doi:10.1186/s12943-016-0520-8
55. Su J, Liao J, Gao L, et al. Analysis of small nucleolar RNAs in sputum for lung cancer diagnosis. Oncotarget. 2016;7(5):5131–5142. doi:10.18632/oncotarget.4219
56. Chen X, Zhang Q, Yang Z, et al. An SNP reducing SNORD105 and PPAN expression decreases the risk of hepatocellular carcinoma in a Chinese population. J Clin Lab Anal. 2021;35(12):e24095. doi:10.1002/jcla.24095
57. Chen X, Zhou Y, Wan Y, et al. The expression of NLK is functionally associated with colorectal cancers (CRC). J Cancer. 2021;12(23):7088–7100. doi:10.7150/jca.62526
58. Shaker O, Ayeldeen G, Abdelhamid A. The impact of single nucleotide polymorphism in the long non-coding MEG3 gene on microRNA-182 and microRNA-29 expression levels in the development of breast cancer in Egyptian women. Front Genet. 2021;12:683809. doi:10.3389/fgene.2021.683809.
59. Sun Q, Chong F, Jiang X, et al. Association study of SNPs in LncRNA CDKN2B-AS1 with breast cancer susceptibility in Chinese Han population. Int J Biochem Cell Biol. 2022;143:106139. doi:10.1016/j.biocel.2021.106139
60. Gail MH. Discriminatory accuracy from single-nucleotide polymorphisms in models to predict breast cancer risk. J Natl Cancer Inst. 2008;100(14):1037–1041. doi:10.1093/jnci/djn180
61. Bergeron D, Fafard-Couture É, Scott MS. Small nucleolar RNAs: continuing identification of novel members and increasing diversity of their molecular mechanisms of action. Biochem Soc Trans. 2020;48(2):645–656. doi:10.1042/BST20191046
62. Wu H, Qin W, Lu S, et al. Long noncoding RNA ZFAS1 promoting small nucleolar RNA-mediated 2’-O-methylation via NOP58 recruitment in colorectal cancer. Mol Cancer. 2020;19(1):95. doi:10.1186/s12943-020-01201-w
63. Pauli C, Liu Y, Rohde C, et al. Site-specific methylation of 18S ribosomal RNA by SNORD42A is required for acute myeloid leukemia cell proliferation. Blood. 2020;135(23):2059–2070. doi:10.1182/blood.2019004121
64. Liu Z, Pang Y, Jia Y, et al. SNORA23 inhibits HCC tumorigenesis by impairing the 2’-O-ribose methylation level of 28S rRNA. Cancer Biol Med. 2021;19:104. doi:10.20892/j.issn.2095-3941.2020.0343
65. Siprashvili Z, Webster DE, Johnston D, et al. The noncoding RNAs SNORD50A and SNORD50B bind K-Ras and are recurrently deleted in human cancer. Nat Genet. 2016;48(1):53–58. doi:10.1038/ng.3452
66. Zhang C, Zhao LM, Wu H, et al. C/D-box snord105b promotes tumorigenesis in gastric cancer via ALDOA/C-Myc pathway. Cell Physiol Biochem. 2018;45(6):2471–2482. doi:10.1159/000488265
67. Wu L, Zheng J, Chen P, Liu Q, Yuan Y. Small nucleolar RNA ACA11 promotes proliferation, migration and invasion in hepatocellular carcinoma by targeting the PI3K/AKT signaling pathway. Biomed Pharmacother. 2017;90:705–712. doi:10.1016/j.biopha.2017.04.014
68. Wang H, Ma P, Liu P, Chen B, Liu Z. Small nucleolar RNA U2_19 promotes hepatocellular carcinoma progression by regulating Wnt/β-catenin signaling. Biochem Biophys Res Commun. 2018;500(2):351–356. doi:10.1016/j.bbrc.2018.04.074
69. Xu G, Yang F, Ding CL, et al. Small nucleolar RNA 113-1 suppresses tumorigenesis in hepatocellular carcinoma. Mol Cancer. 2014;13(1):216. doi:10.1186/1476-4598-13-216
70. Xia XR, Li WC, Yu ZT, et al. Effects of small nucleolar RNA SNORD44 on the proliferation, apoptosis and invasion of glioma cells. Histochem Cell Biol. 2020;153(4):257–269. doi:10.1007/s00418-020-01848-y
71. Shan Y, Wei S, Xiang X, et al. SNORA42 promotes oesophageal squamous cell carcinoma development through triggering the DHX9/p65 axis. Genomics. 2021;113(5):3015–3029. doi:10.1016/j.ygeno.2021.06.036
72. Tian B, Liu J, Zhang N, et al. Oncogenic SNORD12B activates the AKT-mTOR-4EBP1 signaling in esophageal squamous cell carcinoma via nucleus partitioning of PP-1α. Oncogene. 2021;40(21):3734–3747. doi:10.1038/s41388-021-01809-2
73. Deogharia M, Majumder M. Guide snoRNAs: drivers or passengers in human disease? Biology. 2018;8(1). doi:10.3390/biology8010001
74. Cavaillé J. Box C/D small nucleolar RNA genes and the Prader-Willi syndrome: a complex interplay. Wiley Interdiscip Rev RNA. 2017;8(4). doi:10.1002/wrna.1417
75. Butler MG, Wang K, Marshall JD, et al. Coding and noncoding expression patterns associated with rare obesity-related disorders: Prader-Willi and Alström syndromes. Adv Genomics Genet. 2015;2015:53–75. doi:10.2147/AGG.S74598
76. Nagasawa C, Ogren A, Kibiryeva N, et al. The role of scaRNAs in adjusting alternative mRNA splicing in heart development. J Cardiovasc Dev Dis. 2018;5(2). doi:10.3390/jcdd5020026.
77. Godel M, Morena D, Ananthanarayanan P, et al. Small nucleolar RNAs determine resistance to doxorubicin in human osteosarcoma. Int J Mol Sci. 2020;21(12):4500. doi:10.3390/ijms21124500
78. Huber-Keener KJ, Liu X, Wang Z, et al. Differential gene expression in tamoxifen-resistant breast cancer cells revealed by a new analytical model of RNA-Seq data. PLoS One. 2012;7(7):e41333. doi:10.1371/journal.pone.0041333
79. Huang L, Liang X-Z, Deng Y, et al. Prognostic value of small nucleolar RNAs (snoRNAs) for colon adenocarcinoma based on RNA sequencing data. Pathol Res Pract. 2020;216(6):152937. doi:10.1016/j.prp.2020.152937
80. Yang H, Lin P, Wu HY, et al. Genomic analysis of small nucleolar RNAs identifies distinct molecular and prognostic signature in hepatocellular carcinoma. Oncol Rep. 2018;40(6):3346–3358. doi:10.3892/or.2018.6715
81. Zhang S, Ding L, Li X, Fan H. Identification of biomarkers associated with the recurrence of osteosarcoma using ceRNA regulatory network analysis. Int J Mol Med. 2019;43(4):1723–1733. doi:10.3892/ijmm.2019.4108
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.