Back to Journals » OncoTargets and Therapy » Volume 13
The Potential Action of Thomsen–Friedenreich Monoclonal Antibody (A78-G/A7) in Thyroid Cancer
Authors Peng Y, Zhan XX, Cao Y, Zhang HW , Cao WH, Su YJ, Diao C, Sun QM, Cheng RC
Received 13 May 2020
Accepted for publication 7 August 2020
Published 25 August 2020 Volume 2020:13 Pages 8677—8689
DOI https://doi.org/10.2147/OTT.S261685
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yao Dai
Ying Peng, 1 Xiang-Xiang Zhan, 2 Yi Cao, 3 Han-Wen Zhang, 1 Wei-Han Cao, 1, 2 Yan-Jun Su, 1, 2 Chang Diao, 2 Qiang-Ming Sun, 4, 5 Ruo-Chuan Cheng 2
1Kunming Medical University of Yunnan Province, Kunming, Yunnan 650500, People’s Republic of China; 2Thyroid Disease Diagnosis and Treatment Center, First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan 650032, People’s Republic of China; 3Longyan Jianhai Medical and Pharmaceutical Technology Co., Ltd., Longyan, Fujian 364000, People’s Republic of China; 4Institute of Medical Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Kunming 650118, People’s Republic of China; 5Yunnan Key Laboratory of Vaccine Research & Development on Severe Infectious Diseases, Kunming 650118, People’s Republic of China
Correspondence: Ruo-Chuan Cheng; Qiang-Ming Sun Email [email protected]; [email protected]
Background: Thomsen–Friedenreich antibody (TF-Ab) is a specific antibody against the Thomsen–Friedenreich antigen (TF-Ag). At present, studies on a number of other tumors have shown that TF-Ab can effectively inhibit metastasis and induce apoptosis in tumor cells. However, the role of TF-Ab in thyroid cancer (TC) remains unclear.
Materials and Methods: Normal subjects and patients with primary papillary TC with or without lymph node metastasis were tested for TF-Ab expression by enzyme-linked immunosorbent assays (ELISAs). Immunofluorescence was used to assess the expression of TF-Ag in thyroid papillary carcinoma with or without lymph node metastasis and undifferentiated cancer tissues. To evaluate the role of TF-Ab in TC, the effects of TF monoclonal antibody (mAb A78-G/A7) on cell biological function were investigated by MTT assays, flow cytometry, adhesion assays and transwell experiments.
Results: Compared with normal individuals, TF-Ab levels in patients with TC were decreased, but no changes were observed with respect to lymph node metastasis. The expression of TF-Ag in TC tissues was relatively higher than that detected in adjacent tissues, but it was not affected by the presence or absence of lymph node metastasis. Upon treatment mAb A78-G/A7 treating, TC cell cycles were affected, meanwhile the abilities to adhere, invade and migrate were also significantly reduced.
Conclusion: The results of the present study showed that mAb A78-G/A7 could affect the invasion and migration of all assayed TC cell lines. The effects of mAb A78-G/A7 on the cell cycle, adhesion, invasion and migration of TC cells were more significant than those observed for proliferation and apoptosis.
Keywords: Thomsen–Friedenreich antibody, TF-Ab, Thomsen–Friedenreich antigen, TF-Ag, mAb A78-G/A7, thyroid cancer, TC
Introduction
Thomsen–Friedenreich antigen (TF-Ag) is a precursor of the MN blood type (MNS,ISBT0002) determinant cluster discovered in 1927 by Thomsen and Friedenreich, respectively, and is widely present in cell membrane glycoproteins.1 In normal cells, TF-Ag is masked by sialic acid and other sugar chains,2 becoming exposed when tumorigenesis occurs and is expressed in most tumor types.3–7 TF-Ag is thought to be involved in immune evasion, tumor growth, apoptosis and metastasis.8,9 The overexpression of TF-Ag is associated with clinical features, such as liver metastasis, remote metastasis, and an undesirable outcome in colorectal cancer (CRC) patients, which may be caused by TF-Ag expressed by tumor cells being able to specifically bind to the glycoprotein receptor of the liver membrane, leading to liver metastases.10 In addition, TF-Ag expressed on the surface of tumor cells can also adhere to vascular endothelial cells, tumor cell attachment in blood vessels.11,12 Thus, TF-Ag is a particularly important tumor target. Studies have demonstrated that the humoral immune response of a vaccine to TF-Ag can kill tumor cells through antibody-dependent cell-mediated cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) and block the ability of tumor cells to spread.13 This function also indicates that this target has strong clinical application value.
Thomsen–Friedenreich antibody (TF-Ab) is specifically produced by human immune B cells in response to TF-Ag.14 Studies have confirmed that the natural TF-Ab level in tumor patients is significantly correlated with their prognosis, indicating that passive TF-Ab immunotherapy does not cause pathological reactions.15–18 As a specific antibody produced against TF-Ag, studies have shown that the prognosis of patients with high TF-Ab levels was significantly better than that of patients with low TF-Ab levels.14–16 Other studies also showed that the level of TF antibody expression significantly changes in tumor patients,19 providing some evidence that TF-Ab may could be used to treat TF-Ag. In recent years, some scholars have proved that TF-Ab passive immunity can block lung metastasis and improve the survival rate in a passive immunotherapy experiment using the 4T1 mouse model of breast cancer metastasis.20 Furthermore, other scholars have performed in vitro and in vivo immunotherapy experiments with leukemia and further confirmed that TF-Ab passive immunity can induce cell apoptosis.21 Therefore, we believe that the apoptosis of TF-Ag-harboring tumor cells induced by antibodies toward TF-Ag in the human body may be an antitumor immune monitoring mechanism, indicating that TF-Ab could have clinical benefits.
Thyroid cancer (TC) is a common malignant tumor of the endocrine system with an increasing incidence, making there an urgent need to discover new biological targets and treatments for this type of cancer.22 In our previous study,23 TF-Ag, as a pan-oncoantigen, was shown to be significantly overexpressed in TC. However, the potential effect of TF-Ab on TF-Ag has not been demonstrated in TC. Although the results of some studies have provided convincing evidence supporting the anticancer effect of TF-Ab on TF-Ag, this activity in TC has not been confirmed. Therefore, in the present study, the role of mAb A78-G/A7 in the proliferation and metastasis of TC cells was investigated, and the results demonstrated that TF-Ag can be an effective therapeutic target for TC and that TF-Ab has potential use for targeting TF-Ag to treat TC.
Materials and Methods
Human Tissue and Serum Samples
Human tissue and serum samples (N=40) were collected from patients with thyroid cancer from the First Affiliated Hospital Of Kunming Medical University. Control serum samples (N=40) were collected from healthy people in the Physical Examination Center Of The First Affiliated Hospital of Kunming Medical University. Based on the findings from hematoxylin and eosin staining of sections for pathological diagnosis and histological types,24 three groups were included, papillary thyroid carcinoma (PTC) with (N=20) or without (N=20) lymph node metastasis and healthy controls (histologically identified as normal thyroid tissue at a distance of more than 2 cm from the edge of the cancer)25 (N=20). All tissue specimens were immediately frozen and transferred to the Kunming Institute of Biology, after which they were used to generate 10% buffer formalin-fixed and paraffin-embedded sections. The paraffin sections were sliced into 5-µm-thick sections. All serum samples were obtained by centrifugation at 3000 rpm for 15 minutes after the collection of fresh blood and stored at −20°C. The inclusion and exclusion criteria of this study are presented in Table 1.
 |
Table 1 Inclusion and Exclusion Criteria of This Study |
Cell Culture
All TC cell lines were invasive tumor cell lines isolated from human thyroid carcinoma tissues that were provided to us as a gift by the Kunming Biology Institute, Chinese Academy (Kunming, China). TC cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum FBS (Gibco, 10,099–141C, USA) in an incubator and under an atmosphere with 5% CO2 at 37°C.
Enzyme-Linked Immunosorbent Assay (ELISA)
The standard TF-Ag was replaced by asialoglycophorin from human blood group MN (aGP, a9791-1mg, Sigma, Germany), which was coated on ELISA plates and incubated overnight in a refrigerator at 4°C. Human serum samples were diluted 1:2 (50-μL samples diluted with diluent to 100 μL), with a 2-fold gradient dilution of mAb A78-G/A7 used as the standard control. Then, the samples were added to the corresponding perforated plates and placed in a 37°C box for 30 minutes after being washed thrice with 1× washing liquid. Since the standard control uses an IgM-reactive mAb A78-G/A7, 100-μL of enzyme binder (rabbit anti-human IgM, 74293S, CST, USA) was added to each well and incubated at 37°C for 30 minutes after being washed with the liquid three times. Next, color and stop solutions were added in turn, and the absorbance value (OD value) was read at 450 nm on a microplate reader within 15 minutes.
Immunofluorescence
Tumor tissues were treated as previously reported for the immunofluorescence analysis. The appropriate fixative was selected to fix the tissues, and then the permeability treatment was performed to ensure that the antibody could reach the antigen site. Subsequently, the slides were blocked with 5% BSA and then incubated with mAb A78-G/A7. The slides were washed with PBS thrice and then incubated with a secondary antibody (goat anti-mouse IgM, AP128F, Millipore, Germany). The same methods described above were also performed using an antibody against CDH2 (N-cadherin, 13116S, CST, USA). A Nikon Eclipse TE2000-U microscope was used to mount and observe the slides, and the results were analyzed using ImageJ.
Cell Proliferation Assay
The number of live cells was assessed using a CCK8 kit (341–07761, DOJINDO, Japan). BCPAP and 8305C cells were cultured in DMEM supplemented with 10% FBS and plated in 96-well plates at a density of 2000 cells/well. After treating the cells with mAb A78-G/A7, the CCK8 working solution was added to the wells and incubated for 4 hours at 37°C. The cells were stained after 1 day to quantitate cell viability, and an EPOCH™ Spectrophotometer System (BioTek, Winooski, VT, USA) was used to measure the absorbance of the samples at 450 nm.
Cell Apoptosis Assay
After 72 hours17 of treatment with mAb A78-G/A7, the cells were washed with PBS, fixed in 2% paraformaldehyde 30 minutes, and permeated for 30 minutes with PBS containing 0.1% Triton X-100. Then, the cells were centrifuged for 5 minutes at 1000 rpm, resuspended in 1×binding buffer at a density of 1×106 cells/mL and stained with fluorescein isothiocyanate (FITC)-conjugated annexin V using an Annexin V FITC Apoptosis kit (556,547, BD, USA) for 15 minutes before flow cytometry analysis. The blank control group received the same treatment. A flow cytometer (Becton Dickinson Immunocytometry Systems) and CellQuest Pro (version 3.3) (BD Biosciences) were using to determine the apoptotic rate.
Cell Cycle Analysis
mAb A78-G/A7-treated cells were grown to 70–80% confluence in 6-cm dishes. After culturing for 72 hours, the supernatant was collected, and the adherent cells were trypsinized, washed with ice-cold PBS, and then fixed in PBS containing 70% ethanol at −20°C overnight. Subsequently, the cells were resuspended in 1× binding buffer (10× buffer diluted with distilled water), stained at room temperature in the dark for 30 minutes with PI/RNase Staining Buffer Solution (550825.0, BD Pharmingen, USA), and then immediately analyzed using a flow cytometer. The accompanying software (CellQuest Pro) was used to analyze the data, and the data are presented as the means and standard deviation. The experiment was performed three times.
Cell Adhesion Assay
BCPAP and 8305C cells in DMEM supplemented with 10% FBS were added to 96-well plates at a density of 2000 cells/well. After incubating with mAb A78-G/A7 for 72 hours, the adherent cells were collected through trypsinization. Then, the cells were cultured again in 96-well plates in DMEM supplemented with 10% FBS on a shaking table at 37°C and with shaking 80 rpm/minute for one hour. The 96-well plates were placed under a microscope (Nikon Eclipse TE2000-U) to observe the cells that adhered to the matrix. Then, the culture medium was refreshed before adding CCK8 working solution (341–07761, DOJINDO, Japan) to the wells and incubating for 2 hours at 37°C, after which the OD value was read at 450 nm on a microplate reader.
Cell Migration Assay
Boyden chambers (EMD Millipore) were used to assess cell migration ability. DMEM supplemented with 10% FBS was added to the bottom of each chamber, and then aliquots of mAb A78-G/A7-treated cells (1.5×105) suspended in DMEM were placed into the top of each chamber. The cells were cultured for 24 hours, after which the unmigrated cells were removed from the film. Then, the cells that moved to the bottom of the film were stained with crystal violet and observed using a microscope (200× magnification). ImageJ was used to scan and analyze four random fields. A bar graph of the sample pixel intensities was generated to show the results, which were reported as the means and standard deviations.
Cell Invasion Assay
A DMEM suspension of 1.5×105 mAb A78-G/A7-treated cells was placed onto the top of a Boyden chamber (EMD Millipore), which contained 50 μL of Matrigel (1 mg/mL final concentration), with medium DMEM supplemented with 10% FBS added to the lower chamber. Crystal violet was used to stain the cells that invaded to the bottom of the membrane and were observed under a microscope (200× magnification). ImageJ was used to scan and analyze four random fields. A bar graph of the sample pixel intensities was generated to show the results, which were reported as the means and standard deviations.
Statistical Analysis
Independent Student’s t-test was used to assess differences between different groups. The data are reported as the means±standard deviation, where P<0 demonstrated a significant difference between different two groups.
Results
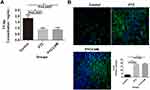
Serum TF-Ab IgM Levels are Dramatically Lower in Patients with Primary TC Than in Healthy Individuals
The level of TF-Ab IgM expression was investigated in primary TC patients and healthy individuals using ELISAs (N=20). The results showed that healthy individuals expressed TF-Ab IgM at higher levels than primary TC patients (Figure 1A). Additionally, our results showed that the expression of TF-Ab IgM in the serum of TC patients with lymph node metastasis was not different from that in those without lymph node metastasis (Figure 1A).
TF-Ag is Highly Expressed in TC Tissue
Immunofluorescence analysis was used to assess the expression of TF-Ag in TC and normal tissues, with the results showing that TF-Ag expression was higher in TC tissues than that observed in normal tissues (Figure 1B). However, we failed to detect an obvious difference between TC tissues with lymph node metastasis and those without lymph node metastasis (Figure 1B).
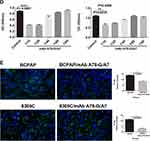
TF-Ag is Obviously Expressed in TC Cell Lines
The level of TF-Ag expression in TC cell lines was evaluated by immunofluorescence analysis. Based on our results, TF-Ag was prominently overexpressed in the different TC cell lines when compared to that observed in normal human thyroid cells (both P<0.05) (Figure 2A). In addition, we observed that TF-Ag expression was dramatically high in BCPAP cells (papillary cancer cells) and in 8305C cells (undifferentiated cancer cells) (Figure 2A).
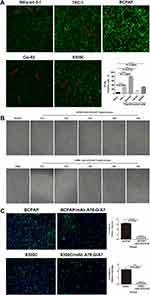 |
Figure 2 Continued. |
mAb A78-G/A7 Effectively Inhibits the Adhesion of TC Cells
Since TF-Ag-mediated adhesion plays an important role in the process of metastasis, as a specific antibody to TF-Ag, we investigated whether mAb A78-G/A7 could effectively inhibit the expression of the adhesion factor N-cadherin (Figure 2C), thereby weakening cell adhesion (Figure 2B). We observed that the percentage of adherent cells in the mAb A78-G/A7-treated group was significantly decreased and that the expression of the adhesion factor N-cadherin was markedly decreased compared to that observed in the control group. These results suggest that mAb A78-G/A7 has a negative effect on the overall metastatic mechanism of TC and other tumors. Furthermore, we determined the optimal concentration of mAb A78-G/A7 to use against TC cells by assessing the cell adhesion rate using a dilution series of the antibody (Figure 2D).
TF-Ag Expression in TC Cells Can Be Effectively Inhibited by mAb A78-G/A7 Treatment
To further verify the blocking effect of mAb A78-G/A7 toward TF-Ag, we used immunofluorescence staining to assess the expression of TF-Ag in TC cells with or without mAb A78-G/A7 treatment (Figure 2E). Our data indicated that a 1:20 medium/antibody ratio could significantly decrease TF-Ag expression in tumor cells. These results provide evidence that TF-Ab expression is negatively correlated with TF-Ag expression in TC and that TF-Ab is a potential regulator of TF-Ag.
8305C Cell Proliferation is Significantly Inhibited by mAb A78-G/A7 Interference
To directly estimate the influence of mAb A78-G/A7 on TC cell proliferation, we assessed cell division using a CCK8 kit to stain cells. CCK8 is a fluorescent dyestuff that can be used to monitor cell proliferation via decreased fluorescence intensity that occurs after each cellular division. The OD values of the mAb A78-G/A7-treated 8305C cells after 24 hours were significantly lower than those observed for the untreated cells (P<0.05), demonstrating that cell proliferation was notably suppressed. In contrast, the OD values of the mAb A78-G/A7-treated BCPAP cells after 24 hours were not obviously different from those of the untreated cells (P>0.05), proving that cell growth was not suppressed. These results demonstrate that interference of TF-Ag by mAb A78-G/A7 treatment has different potential effects on cell proliferation in different TC cell lines (Figure 3A).
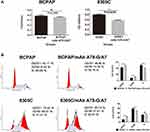
mAb A78-G/A7 Affects BCPAP and 8305C Cell Cycle Distribution
To verify the observed effects on cell growth, the cell cycle distribution of BCPAP and 8305C cells 72 hours after the addition of mAb A78-G/A7 was assessed by flow cytometry. Our results showed that the mAb A78-G/A7-treated 8305C cells had more cells in G1 phase and fewer cells in G2 phase than that observed in the untreated 8305C cells (Figure 3B). Similarly, the mAb A78-G/A7-treated BCPAP cells had more cells in G1 phase than was observed for the untreated BCPAP cells. Although the number of BCPAP cells in G2 phase was increased, the number of cells in S phase was significantly decreased. Therefore, overall, it appears that the doubling time was suppressed. The number of cells in G0/G1 stage notably increased (P<0.05), showing that mAb A78-G/A7 could significantly affect the cell cycle of both 8305C and BCPAP cells (Figure 3B). With respect to the BCPAP cells, we believe that there were other factors that resulted in inconsistencies in proliferation.
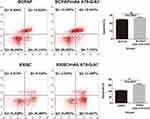
mAb A78-G/A7 Promotes the Apoptosis of 8305C Cells
Fluorescence-activated cell sorting (FACS) analyses based on annexin V/7-AAD staining revealed a survival disadvantage of the mAb A78-G/A7-treated 8305C cells but not the mAb A78-G/A7-treated BCPAP cells. The number of apoptotic cells in the mAb A78-G/A7-treated BCPAP group (27.0%) was not significantly different from that observed in the untreated cell group (25.0%) (P>0.05). However, the number of apoptotic cells in the mAb A78-G/A7-treated 8305C cell group (63.0%) was significantly higher than that observed in the untreated cell group (45.8%) (P<0.05). These results indicate that interference of TF-Ag by mAb A78-G/A7 promotes the apoptosis of 8305C cells but not BCPAP cells (Figure 4). We hypothesize that mAb A78-G/A7 may also affect some specific surface molecules of different cell lines in different manners, resulting in different therapeutic effects, which is worth further investigation.
BCPAP and 8305C Cell Migration is Dramatically Reduced by mAb A78-G/A7 Treatment
To determine the effect of TF-Ab treatment on BCPAP and 8305C cell migration, cells treated with or without mAb A78-G/A7 were tested using transwell assays. Our data indicated that the migration capacities of BCPAP cells were clearly reduced (P<0.05) by treatment with mAb A78-G/A7 (Figure 5), with similar results were observed for 8305C cells (P<0.05) (Figure 5).
mAb A78-G/A7 Notably Decreases the Invasive Ability of BCPAP and 8305C Cells
To assess the effects of mAb A78-G/A7 on BCPAP and 8305C cell invasion, cell invasion assays were performed. The ability of cells that were treated with or without mAb A78-G/A7 to go through the Matrigel was analyzed through invasion assays. The results showed that the invasive abilities of BCPAP and 8305C cells were dramatically inhibited (P<0.05) after the addition of mAb A78-G/A7 (Figure 6).
Discussion
Immunotherapy is thought to be of great benefit to cancer patients,26 and recent studies have shown that anti-cancer vaccines targeting tumor-related carbohydrates (such as TF-Ag) have beneficial effects in the prevention and treatment of various tumors. The results of several previous studies have shown that the TF-Ag vaccine is effective in improving the survival of breast cancer patients.27,28 In addition to TF-Ag-specific antibody response activation, TF-Ag-specific T cell activation has also been observed in TF-Ag passive immunized patients.29 The results of an experimental animal study also showed that mice immunized with a synthetic TF-Ag glycopeptide vaccine produced cytotoxic T cell responses toward TF-Ag-positive tumor cells.30 These results suggest that tumor vaccines targeting TF-Ag can activate specific humoral and cellular antitumor responses. Thus, we believe that TF-Ag can be viewed as an important tumor target.
In clinical medicine, antibody immunotherapy targeting carbohydrate-associated antigens has been shown to have distinct benefits for cancer patients. Natural carbohydrate-reactive IgM Abs that can induce the apoptosis of tumor cells are viewed as a key components of innate immune surveillance against tumors.31 Currently, a number of antibody drugs are used in the clinical treatment of cancer patients. For example, trastuzumab, which triggers her2-positive breast cancer cells apoptosis,32 and rituximab, which targets the B-cell malignant tumor surface antigen CD20, have been well used clinical treatment.31 TF-Ab is specifically produced by human immune B cells in response to TF-Ag. Naturally produced TF-Ab (the specific antibody against TF-Ag) can be detected in all adult serum,33 which may be part of the humoral immunosurveillance mechanism against TF-Ag positive tumor cells.34 However, studies have shown that naturally produced TF-Ab is potentially degraded and is present in lower titers in cancer patients. The naturally occurring TF-Ab in healthy humans indicates that passive TF-Ab immunity will not cause pathological reactions. A previous study35 showed that TF-Ab can promote the apoptosis of TF-Ag positive cancer cells, demonstrating its potential use for cancer biotherapy. Thus, we proposed a hypothesis that the TF-Ag antibody vaccine can increase and further maintain a high anti-TF-Ag immune response and high TF-Ab titers that could prevent the recurrence and metastasis of tumors.
TC is a common malignant tumor of the endocrine system, the occurrence of which has rapidly increased in recent years.21 Although TC treatments and their effects have improved recent years, the trauma caused by surgery and the recurrence of tumors are still intractable problems, decreasing the quality of life and increasing the economic burden on patients.36,37 Therefore, it is of great importance to further elucidate the pathogenesis of TC and identify molecular targets with potential for use in early diagnosis, prevention, and treatment. In the present study, we observed that TF-Ag was highly expressed among the assayed TC tissues and four human TC cell lines with different metastatic potential, and significantly high TF-Ag expression was confirmed in BCPAP and 8305C cells, demonstrating that TF-Ag can also be an effective target in TC. Thus, exploring the potential effects of the TF-Ag-targeting TF-Ab is important for the development of therapeutic approaches for TC.
In the present study, we observed a significant decrease in serum TF-Ab expression in primary TC patients. We further investigated the effect of mAb A78-G/A7 treatment on TC cell proliferation, apoptosis and invasion. Our results demonstrated that interference of TF-Ab by mAb A78-G/A7 inhibits cell proliferation and tumor spread, suggesting a tumor suppressor-like role for TF-Ab in TC. Through in vitro experiments, we observed that treatment with mAb A78-G/A7 significantly inhibited the proliferation of 8305C cells (P<0.05), and the number of cells in G0/G1 stage and that underwent apoptosis were obviously increased (P<0.05). However, a similar effect was not observed for BCPAP cells, with cell proliferation and apoptosis not being significantly inhibited. Nevertheless, the adhesion, invasion and migration of all mAb A78-G/A7-treated TC cells were dramatically decreased (P<0.05), proving that TF-Ag blockade may reduce the invasive potential and proliferation of TC cells. To avoid the influence of cell viability, cell invasion was examined 24 hours after mAb A78-G/A7 treatment. To assess cell viability, CCK8, a fluorescent dyestuff that that can be used to monitor cell proliferation via decreased fluorescence intensity that occurs after each cellular division, was used. The effect of mAb A78-G/A7 treatment on cell adhesion, invasion, and migration were also tested in the high TF-Ag expression cell lines (BCPAP and 8305C). Our results showed that mAb A78-G/A7 could decrease the invasive potential and migration of BCPAP and 8305C cells by blocking TF-Ag (Figures 4 and 5). Our findings provide new insights into possible treatments for patients with TC.
In summary, the results of our study confirmed important biological roles for TF-Ag in TC progression and spread. Abnormal TF-Ab levels are expected to be a new noninvasive predictor of primary TC. Importantly, we observed a potential effect of mAb A78-G/A7 against TF-Ag in TC, providing the first evidence for a new therapeutic target for TC. The effectiveness of mAb A78-G/A7 on TC cells also provides evidence for the possible anticancer effect of TF-Ab in TC. Therefore, TF-Ab passive immunotherapy may be beneficial to TC patients. Further investigation of the mechanism of TF-Ab passive immunity in TC patients will be conducive to the development of targeted anti-TC treatment.
Nonetheless, our study failed to successfully establish animal models of papillary TCs for a further investigation, limiting the full therapeutic effects that could be observed. However, the future elucidation of TF-Ab effects will further enrich the development of anticancer drug therapy for TC.
Ethics Affirmation
This research has met all the guidelines outlined in the Declaration of Helsinki and was approved by Kunming Medical University First Affiliated Hospital’s Ethical Committee [(2020) L no. 2]. All healthy volunteers and patients included in this study provided informed oral consent. The informed verbal consent process and the use of the cell lines was approved by the ethics committee of Kunming Medical University First Affiliated Hospital [(2020) L no. 2].
Disclosure
Yi Cao is an employee of Longyan Jianhai Medical and Pharmaceutical Technology Co., Ltd. The authors report no other potential conflicts of interest for this work.
References
1. Heimburg-Molinaro J, Lum M, Vijay G, et al. Cancer vaccines and carbohydrate epitopes. Vaccine. 2011;29(48):0–8826. doi:10.1016/j.vaccine.2011.09.009
2. Videira PA, Amado IF, Crespo HJ, et al. Surface alpha 2−3- and alpha 2−6-sialylation of human monocytes and derived dendritic cells and its influence on endocytosis. Glycoconjugate. 2008;25(3):259–268. doi:10.1007/s10719-007-9092-6
3. Videira PA, Correia M, Malagolini N, et al. ST3Gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer. 2009;9(1):357. doi:10.1186/1471-2407-9-357
4. Leoyklang P, Malicdan MC, Yardeni T, et al. Sialylation of Thomsen-Friedenreich antigen is a noninvasive blood-based biomarker for GNE myopathy. Biomark Med. 2014;8(5):641–652. doi:10.2217/bmm.14.2
5. Karsten U, Goletz S. What controls the expression of the core-1 (Thomsen—Friedenreich) glycotope on tumor cells? Biochem (Mosc). 2015;80(7):801–807. doi:10.1134/S0006297915070019
6. Wen L, Liu D, Zheng Y, et al. A one-step chemoenzymatic labeling strategy for probing sialylated Thomsen–Friedenreich antigen. ACS Cent Sci. 2018;4(4):451–457. doi:10.1021/acscentsci.7b00573
7. Karsten U, Goletz S. What makes cancer stem cell markers different? Springerplus. 2013;2(1):301. doi:10.1186/2193-1801-2-301
8. Jin KT, Lan HR, Chen XY. Recent advances in carbohydrate-based cancer vaccines. Biotechnol Lett. 2019;41(6–7):641–650. doi:10.1007/s10529-019-02675-5
9. Bezu L, Kepp O, Cerrato G, et al. Trial watch: peptide-based vaccines in anticancer therapy. Oncoimmunology. 2018;7(12):e1511506. doi:10.1080/2162402X.2018.1511506
10. Goletz S, Cao Y, Danielczyk A, et al. Thomsen-Friedenreich antigen: the ́hidden ́tumour antigen. Adv Exp Med Biol. 2003;535:147–162.
11. Glinsky VV, Glinsky GV, Rittenhouse-Olson K, et al. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61(12):4851–4857.
12. Yu LG, Andrews N, Zhao Q, et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated muc1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;282(1):773–781. doi:10.1074/jbc.M606862200
13. Zhou Z, Lin H, Chen L, et al. Recent progress of fully synthetic carbohydrate-based vaccine using TLR agonist as build-in adjuvant. Chin Chem Lett. 2018;29(01):35–42.
14. Schwartzalbiez R. Naturally occurring antibodies directed against carbohydrate tumor antigens. Adv Exp Med Biol. 2012;750(750):27.
15. Khasbiullina NR, Bovin NV. Hypotheses of the origin of natural antibodies: a glycobiologist’s opinion. Biochem (Mosc). 2015;80(7):820–835. doi:10.1134/S0006297915070032
16. Smorodin EP, Sergeyev BL. The level of IgG antibodies reactive to TF, Tn and alpha-Gal polyacrylamide-glycoconjugates in breast cancer patients: relation to survival. Exp Oncol. 2016;38(2):117–1121. doi:10.31768/2312-8852.2016.38(2):117-121
17. Smorodin E, Sergeyev B, Klaamas K, et al. The relation of the level of serum anti-TF, -Tn and -Alpha Gal IgG to survival in gastrointestinal cancer patients. Int J Med Sci. 2013;10(12):1674–1682. doi:10.7150/ijms.6841
18. Reily C, Stewart TJ, Renfrow MB, et al. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15(6):346–366. doi:10.1038/s41581-019-0129-4
19. Oleg K, Kaire I, Boris S, et al. The Thomsen-Friedenreich antigen-specific antibody signatures in patients with breast cancer. Biomed Res Int. 2018;2018:1–8.
20. Swetha Tati JC, Julia Abdullah F. Humanization of JAA-F11, a highly specific anti-Thomsen-Friedenreich pancarcinoma antibody and in vitro efficacy analysis. Neoplasia. 2017;19(9):716–733. doi:10.1016/j.neo.2017.07.001
21. Yi B, Zhang M, Schwartz-Albiez R, et al. Mechanisms of the apoptosis induced by CD176 antibody in human leukemic cells. Int J Oncol. 2011;38(6):1565–1573. doi:10.3892/ijo.2011.992
22. Bikas A, Burman KD. Epidemiology of thyroid cancer: a comprehensive guide for the clinician. In: The Thyroid and Its Diseases. 2019:541–547.
23. Xiangxiang Z, Bing Z, Chang D, et al. Expression of MUC1 and CD176 (Thomsen-Friedenreich antigen) in papillary thyroid carcinomas. Endocr Pathol. 2015;1(26):21–26.
24. Tallini G, Biase DD, Repaci A, et al. What’s new in thyroid tumor classification, the 2017 World Health Organization classification of tumours of endocrine organs. In: Thyroid FNA Cytology. 2019.
25. Jeffus S. Histology for pathologists, 4th edition. Am J Surg Pathol. 2014;38(4):582. doi:10.1097/PAS.0000000000000175
26. Nisbet I. Cancer immunotherapy comes of age (finally!). Australas Biotechnol. 2016;26(2):38–40.
27. MacLean GD, Bowen-Yacyshyn MB, Samuel J, et al. Active immunization of human ovarian cancer patients against a common carcinoma (Thomsen-Friedenreich) determinant using a synthetic carbohydrate antigen. J Immunother. 1992;11(4):292–305. doi:10.1097/00002371-199205000-00008
28. Slovin SF, Ragupathi G, Musselli C, et al. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother. 2005;54(7):694–702. doi:10.1007/s00262-004-0598-5
29. Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75(8):594–602. doi:10.1007/s001090050144
30. Xu Y, Gendler SJ, Franco A. Designer glycopeptides for cytotoxic T cell-based elimination of carcinomas. J Exp Med. 2004;199(5):707–716. doi:10.1084/jem.20031865
31. Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N Engl J Med. 2012;366(21):2008–2016. doi:10.1056/NEJMct1114348
32. Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27(1):83–117. doi:10.1146/annurev.immunol.021908.132544
33. Butschak G, Karsten U. Isolation and characterization of Thomsen-Friedenreich-specific antibodies from human serum. Tumor Biol. 2002;23(3):113–122. doi:10.1159/000064026
34. Cao Y, Merling A, Karsten U, et al. Expression of CD175 (Tn), CD175s (sialosyl-Tn) and CD176 (Thomsen-Friedenreich antigen) on malignant human hematopoietic cells. Int J Cancer. 2008;123(1):89–99. doi:10.1002/ijc.23493
35. Heimburg J, Yan J, Morey S, et al. Inhibition of spontaneous breast cancer metastasis by anti-Thomsen-Friedenreich antigen monoclonal antibody JAA-F11. Neoplasia. 2006;8(11):939–948. doi:10.1593/neo.06493
36. Calò PG, Medas F, Conzo G, et al. Intraoperative neuromonitoring in thyroid surgery: is the two-staged thyroidectomy justified? Int J Surg. 2017;41:S13–S20. doi:10.1016/j.ijsu.2017.02.001
37. Gambardella C, Polistena A, Sanguinetti A, et al. Unintentional recurrent laryngeal nerve injuries following thyroidectomy: is it the surgeon who pays the bill? Int J Surg. 2017;41(Suppl 1):S55–S59. doi:10.1016/j.ijsu.2017.01.112
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.