Back to Journals » Clinical Epidemiology » Volume 12
The Population Prevalence, Associations of Congenital Heart Defect and Mortality Risk for Down’s Syndrome in South Korea Based on National Health Insurance Service (NHIS) Data
Authors Cho WK , Lee NY , Han K , Suh BK , Park YG
Received 27 February 2020
Accepted for publication 16 May 2020
Published 27 May 2020 Volume 2020:12 Pages 519—525
DOI https://doi.org/10.2147/CLEP.S251637
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Vera Ehrenstein
Won Kyoung Cho,1 Na Young Lee,1 Kyungdo Han,2 Byung-Kyu Suh,3 Yong-Gyu Park2
1Department of Pediatrics, College of Medicine, St. Vincent’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea; 2Department of Biostatistics, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 3Department of Pediatrics, College of Medicine, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea
Correspondence: Byung-Kyu Suh
Department of Pediatrics, College of Medicine, Seoul St. Mary’s Hospital, The Catholic University of Korea, 222, Banpo-daero, Seocho-gu, Seoul 06591, Republic of Korea
Tel +82-2-2258-6185
Fax +82-2-537-4544
Email [email protected]
Yong-Gyu Park
Department of Biostatistics, College of Medicine, The Catholic University of Korea, 222, Banpo-daero, Seocho-gu, Seoul 06591, Republic of Korea
Tel +82-2-2258-6930
Fax +82-2-537-4548
Email [email protected]
Background: In the present study, we estimated the population prevalence, associations of congenital heart defect (CHD) and mortality risk for DS using data from National Health Insurance Service (NHIS) and Rare Diseases Registry (RDR).
Methods: We collected data on subjects with DS who were registered in the RDR between 2010 and 2015. To estimate associations of CHD and mortality risk of DS, the data of DS subjects were compared with 1:5 age- and sex-matched controls.
Results: In 2015, 2077 individuals with DS were identified out of the total population of 51,574,044 South Koreans and the prevalence was 4.03 per 100,000 persons. The trend of DS population prevalence across 10-year-old intervals showed a peak in the group under the age of 10 years (26.0 per 100,000 persons) and then declined sharply after the age of 20 years (0.98 per 100,000 persons at 30– 39 years of age). In subjects with DS, the frequencies of atrial septal defect [odds ratios (OR) =65.9; 95% CI, 84.1– 99.1], ventricular septal defect (OR = 88.1, 95% CI, 57.9– 134.1), patent ductus arteriosus (OR = 56.9, 95% CI, 40.1– 80.8), tetralogy of fallot (OR = 42.1, 95% CI, 19.3– 92.3), or atrioventricular septal defect (OR = 510.0, 95% CI, 126.7– 999.0) were higher than those of age- and sex-matched controls. The risk of death in patients with DS was significantly higher than that of age- and sex-matched controls [hazard ratio (HR) =41.7, 95% CI 20.0– 87.0].
Conclusion: In South Korea, the DS population prevalence was 4.03 per 100,000 persons in 2015. The subjects with DS were more likely to accompany CHD and have higher mortality risk than healthy controls.
Keywords: prevalence, Down’s syndrome, congenital heart defect, mortality
Introduction
Down’s syndrome (DS), originally described by John Langdon Down in 1866, is the most common birth defect.1 DS is caused by the presence of three copies of part or all of chromosome 21. Ninety-five percent of DS cases are due to the trisomy 21 and 1% of persons with trisomy 21 are mosaics. DS can also be caused by translocation or duplications involving chromosome 21.2 Individuals with DS are more prone to congenital heart defects (CHDs), gastrointestinal anomalies, and endocrinopathies including hypothyroidism and diabetes. Delayed development and behavioral problems are often reported in children with DS.3
In children with DS, CHD is the most important clinical phenomenon due to its significant impact on mortality.4 DS is associated with a fixed pattern of CHD with overrepresentation of septal defects. Transposition of the great vessels and aortic coarctation are underrepresented in DS. This specific pattern of CHD might be associated with triplication of the genes on chromosome 21.4 Furthermore, this epigenetic pathway might play an important role in severe DS phenotypes.5 To establish medical surveillance of DS patients, the population prevalence, associations of CHD and mortality risk need to be investigated. However, reliable data for DS in South Korea are not available.
In South Korea, a universal compulsory National Health Insurance Service (NHIS) that provides coverage for nearly 100% of the total population was launched by the government in 1989.6 The NHIS initiated the rare and intractable diseases registration (Rare Diseases Registry, RDR) program in 2004.7 In this study, we estimate the population prevalence, associations of CHD and mortality risk for DS in South Korean using NHIS and RDR data.
Methods
The Korean Rare Disease Registry and the National Health Insurance Service Databases
In 2013, the NHIS covered 97.2% (n=49,989,620) of the population, and the Medical Aid system covered the remaining 2.8% (n=1,458,871).8 The nationwide registry for RDR was established by the Korean NHIS in cooperation with the Ministry of Health and Welfare.9 Patients with DS have been registered in the RDR (specific code designation V159) since 2004. The RDR database gathers identifying information on each subject with a physician-certified diagnosis, enabling accurate calculation of prevalence regardless of NHIS usage during a given period. Moreover, the annual incidence of diagnosis could be estimated by the number of newly registered patients with a given disease.
We used information from the NHIS claims database (NHIS-2017-1-202) to collect data on DS patients who registered in RDR between 2010 and 2015. During this period, the total number of registered DS cases and the number of new registrations each year were identified. To estimate the prevalence of DS, the size of the Korean population was ascertained from resident registration data obtained by the Korean Ministry of Security and Public Administration. For mortality risk, we used population and death data from the national health information database (NHID) of the NHIS individually linked to mortality registration data of Statistics Korea.6 This study was approved by the Institutional Review Board (IRB) of The Catholic University of Korea (IRB Number: KC17EISI0016).
Study Subjects
The diagnostic criteria for DS used by the RDR program are as follows: specific clinical findings (up-slanting palpebral fissures, epicanthic folds, and brachycephaly) and chromosomal and radiologic study results including those from echocardiography. International classification of disease, 10th revision (ICD-10) codes Q90, Q90.0, Q90.1, Q90.2, and Q90.9 are included in V159, the specific RDR designation for DS. To estimate associations of CHD and mortality risk of DS, the data of DS subjects were compared with 1:5 age- and sex-matched controls. We compared the frequencies of atrial septal defect (ASD, 10th revision (ICD-10) codes Q21.1), ventricular septal defect (VSD, Q21.0), patent ductus arteriosus (PDA, Q25.0), PV atresia/stenosis (Q22.0/Q22.1), tetralogy of Fallot (TOF, Q21.3), atrioventricular septal defect (AVSD, Q 21.2), coarctation of the aorta (CoA, Q25.1), double outlet right ventricle (DORV, Q20.1), Transposition of the great arteries (TGA, Q20.3) and single ventricle (Q20.4) between DS subjects and age- and sex-matched controls in 2015. We calculated the death rate dividing the incidence of death by the total follow-up period between DS patients and age- and sex-matched controls from 2010 to 2014.
Statistical Analysis
We analyzed prevalence and incidence of DS in South Korea from 2010 to 2015 across 10-year old age group. The annual incidence of DS from 2010 to 2015 was calculated based on the number of newly registered DS patients and the number of registered residents of the corresponding year. Prevalence and incidence in 2015 were reported by accounting for the age- and sex-specific distribution of the Korean population. Conditional logistic regression models were used to compare the odds ratio (OR) and 95% CI of CHD between DS patients and the control group. Multivariate Cox regression models were used to assess the hazard ratios of death for DS patients and the control group. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Population Prevalence and Incidence of DS from 2010 to 2015
The annual prevalence and incidence of DS are summarized in Table 1. The prevalence of DS was 2.75 per 100,000 persons in 2010, 2.90 per 100,000 persons in 2011, 3.04 per 100,000 persons in 2012, 3.25 per 100,000 persons in 2013, 3.88 per 100,000 persons in 2014, and 4.03 per 100,000 persons in 2015. In 2015, 2077 individuals with DS were identified from the total population of 51,574,044 South Koreans. The incidence of DS was 0.43 per 100,000 persons in 2010, 0.36 per 100,000 persons in 2011, 0.40 per 100,000 persons in 2012, 0.29 per 100,000 persons in 2013, 0.30 per 100,000 persons in 2014, and 0.34 per 100,000 persons in 2015.
 |
Table 1 Prevalence and Incidence of Down’s Syndrome in South Korea |
Distributions of DS Prevalence Across 10-Year-Old Age Groups and by Sex in 2015
DS distribution across 10-year-old age groups and by sex are shown in Table 2. The prevalence trend across 10-year-old interval showed a peak in group under the age of 10 then declined sharply after the group after the age of 20 years. In children under the age of 10, the prevalence of DS was 28.4 per 100,000 persons in males and 23.4 per 100,000 persons in females. The prevalence of DS then decreased rapidly to 8.67 per 100,000 persons in teenagers, 3.09 per 100,000 persons in twentieth, 0.98 per 100,000 persons in thirtieth, 0.55 per 100,000 persons in fortieth, and 0.26 per 100,000 persons in fiftieth.
 |
Table 2 Down’s Syndrome Prevalence Distributions Across 10-Years-Old Age Groups and by Sex in 2015 |
Congenital Heart Defects in DS
In patients with DS, the frequencies of ASD [odds ratios (OR) = 65.9, 95% confidence interval (CI), 84.1–99.1], VSD (OR = 88.1, 95% CI, 57.9–134.1), PDA (OR = 56.9, 95% CI, 40.1–80.8), PV_AS (OR = 10.0, 95% CI, 3.8–26.6), TOF (OR = 42.1, 95% CI, 19.3–92.3), AVSD (OR = 510.0, 95% CI, 126.7–999.0), CoA (OR = 80.0, 95% CI, 10.6–603.2) or TGA (OR = 2.5, 95% CI, 0.2–27.6) were significantly higher than those of age- and sex-matched control subjects. In cases of DORV and single ventricle, odds ratios of DS were not calculated by zero counting (Table 3). Patients with more than 1 CHD could be counted more than once. Among the total 2077 DS patients, the numbers of DS patient without any CHD were 1039 (50.0%).
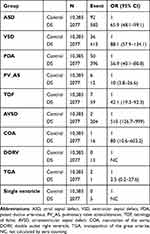 |
Table 3 Odds Ratios of Congenital Heart Defects in Down’s Syndrome Compared with Age- and Sex-Matched Controls in 2015 |
Hazard Ratios for Death in Patients with DS Compared with Age- and Sex-Matched Controls from 2010 to 2014
During 4.01 years, the risk of death in patients with DS was significantly higher than that of age- and sex-matched control subjects (HR=41.7, 95% CI 20.0–87.0). In patients with DS, the risk of death in twentieth (HR= 2.6, 95% CI 1.1–6.2), thirtieth (HR= 4.7, 95% CI 2.1–10.9), fortieth (HR= 16.8, 95% CI 7.9–35.4) and fiftieth (HR= 33.6, 95% CI 14.6–77.3) were significantly higher than that in group under the age of 10 (Table 4).
 |
Table 4 Hazard Ratios of Death in Down’s Syndrome Compared with Age and Sex Matched Controls from 2010 to 2014 |
Discussion
In this study, we report a DS population prevalence of 4.03 per 100,000 persons in 2015 (Table 1). DS population prevalence under the age of 10 years (26.0 per 100,000 persons) was the highest among all age groups. As age group increased, the DS prevalence continued to decline sharply at 0.98 per 100,000 persons at 30–39 years of age (Table 2). In England and Wales, the DS population prevalence was 66 per 100,000 persons in 2011.10 In the United Kingdom, the DS population prevalence was 59 per 100,000 persons in female and 68 per 100,000 persons in male in 2011.11 In the United States, the DS population prevalence were 82.7 per 100,000 persons in 2008, and the DS birth prevalence was 150 per 100,000 births in 2000.12 In England and Wales, the DS birth prevalence was 99 per 100,000 births in 2010.13 In Southern Thailand, the DS birth prevalence was 121 per 100,000 births from 2009 to 2013. The DS population prevalence under the age of 10 years in this study was relatively lower than other countries, but might be valid compared to the previous South Korea DS birth prevalence data during 2005–2006 (37 per 100,000 persons).14,15 However, the South Korea DS population prevalence across all ages were strikingly lower than that in Europe, the United States, and Asia.
Since this is registration-system-based study, underestimation of the prevalence of DS due to unregistered cannot be ruled out. However, there are health care benefits entitled for reduced coinsurance rate, 10% of medical expenses regardless of hospitalized or outpatient after RDR registrations and DS registrations began in 2004 and settled steadily in 2010. Therefore, these results in this study might reflect the overall prevalence of DS in South Korea. In addition, considering no difference in embryonic aneuploidy according to ethnicity,16 ethnic diversity as a reason for low DS prevalence in South Korea might be premature. In South Korea, maternal serum screening test for fetal aneuploidy in the first trimester has been covered by the NHIS and performed in more than 95% of pregnancies.17 Whereas, termination of pregnancy including DS pregnancy was designated as a crime and was subject to punishment by criminal law and a physician only to perform a termination in exceptional cases under the Maternal and Child Health law.18 It is difficult to provide legal data on artificial abortion of DS pregnancy. However, the total artificial abortion rate in South Korea is estimated to be 4.8 ‰ and the number of artificial abortions is estimated to be 50,000 in 2017.19 In these contradictory medical environments, the prenatal diagnosis and selective pregnancy termination could be the explanations for the low DS prevalence in South Korea.
Unlike the Western cases, the DS population prevalence in South Korea is highest in group under the age of 10 and sharply declined, reaching less than 1 per 100,000 people after age 30 (Table 2). The proportions of DS patients aged 35–60 years in the United States, 45–55 years in the United Kingdom, and 40–50 in England and Wales are approximately equal to that of South Korea DS prevalence under the age of 10.11,12 We also found that the DS mortality risk in South Korea is 41.7 fold higher than age- and sex-matched control from 2010 to 2014. In 2013, patients with DS in Denmark had 5–11 fold higher mortality risk than that of the general population.20 These results might suggest lower life expectancy of DS in South Korea than those in other countries, which was recently determined to be 60 years4 and reflect the vulnerability of health care of DS patients. The impact of first decades is seemed to be important to life expectancy of DS in South Korea. However, there was no data on cause of death for the DS population in this register-based study. In Sweden, the median ages at death were 3.6 years in 1969–1973 and 56.8 years in 1999–2003, respectively.21 The dramatic increase in survival seen in DS is over the recent decades and is mainly due to improved cardiac surgery.21 There were racial disparities in improvement of median age at death in DS.22 In South Korea, there was no specific medical care program for DS patients.23 Development of more strengthened medical management of DS are necessary. Many factors might be responsible for low DS population prevalence and further study is needed to investigate the health care environment that affects the survival of DS patients.
In the present study, the number of DS Patients without any CHD were 1039 (50.0%) in 2015. Previous cross-sectional study on CHD and DS, there were 47% (572/1222) DS without CHD in Japan and 43.1% (170/394) in South Korea.14,24 European countries and the US, endocardial cushion defect (43%), which results in AVSD/AV canal defect, was the main cardiac abnormality, followed by VSD (32%), secundum ASD (10%), TOF (6%), and isolated PDA (4%).4,25 However, ASD (28.0%) was the most common CHD in DS, followed by VSD (19.9%), PDA (19.1%), and AVSD (9.8%) in this study. These frequency orders are similar with the previous study conducted using data from all Korean medical institutes between 2005 and 2006.14 We also found the odds ratios of ASD, VSD, PDA, PV_AS, TOF, AVSD, CoA or TGA in patients with DS were statistically higher than those of age- and sex-matched control subjects. The endogenous gene expressions of DS critical region-1 have been reported to be associated with heart valve formations.26 The genes outside chromosome 21, CRELD1 has been found to participate in AVSD development in DS patients.27,28 Recent studies attempted to investigate the basic biological association of cardiac anomalies with DS through overexpression of chromosome 21 microRNAs (miRNAs).29 However, the mechanism of CHD development in DS is not clearly understood.
There are some limitations in this registration-system-based study. Since data were pre-collected by NHID and RDR system, necessary information is lacking and underestimation cannot be ruled out.
To the best of our knowledge, this is the first study to determine population prevalence, associations with CHD and mortality risk in DS patients based on NHIS and RDR with a maximum follow-up of 4 years. In 2015, the DS population prevalence was 4.03 per 100,000 persons. The trend of DS population prevalence across 10-year-old intervals showed a peak in the group under the age of 10 years (26.0 per 100,000 persons) and then declined sharply after the age of 20 years (0.98 per 100,000 persons at 30–39 years of age). In South Korea, DS population prevalence after the age of 20 years was strikingly lower than that in Europe, the United States, and Asia. The subjects with DS are more likely to accompany CHD and have higher mortality risk than in healthy controls. Further research is needed to investigate the low DS population prevalence. The present study could be used as baseline data for improving the survival of DS patients in South Korea.
Data Sharing Statement
All files for the analysis of the present study are available at the national health insurance sharing service webpage (https://nhiss.nhis.or.kr). Raw data requests can be made through the homepage.
Acknowledgments
The authors wish to acknowledge the financial support of the Catholic Medical Center Research Foundation in the program year 2018. The abstract of this paper was presented at the 58th Annual ESPE as a poster presentation. The poster’s abstract was published in “Poster Abstracts” in Hormone Research in Paediatrics: [https://www.eurospe.org/meetings/2019/espe2019/abstracts/].
Disclosure
The authors have no competing interests to declare.
References
1. Down JL. Observations on an ethnic classification of idiots. 1866. Ment Retard. 1995;33(1):54–56.
2. Plaiasu V. Down syndrome - genetics and cardiogenetics. Maedica. 2017;12(3):208–213.
3. Chapman RS, Hesketh LJ. Behavioral phenotype of individuals with down syndrome. Ment Retard Dev Disabil Res Rev. 2000;6(2):84–95. doi:10.1002/1098-2779(2000)6:2<84::Aid-mrdd2>3.0.Co;2-p
4. Vis JC, Duffels MG, Winter MM, et al. Down syndrome: a cardiovascular perspective. J Intellect Disabil Res. 2009;53(5):419–425. doi:10.1111/j.1365-2788.2009.01158.x
5. Do C, Xing Z, Yu YE, Tycko B. Trans-acting epigenetic effects of chromosomal aneuploidies: lessons from down syndrome and mouse models. Epigenomics. 2017;9(2):189–207. doi:10.2217/epi-2016-0138
6. Cheol Seong S, Kim YY, Khang YH, et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol. 2017;46(3):799–800. doi:10.1093/ije/dyw253
7. Kim HS, Lyoo CH, Lee PH, et al. Current status of Huntington’s disease in Korea: a nationwide survey and national registry analysis. J Mov Disord. 2015;8(1):14–20. doi:10.14802/jmd.14038
8. Song SO, Jung CH, Song YD, et al. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab J. 2014;38(5):395–403. doi:10.4093/dmj.2014.38.5.395
9. Service NHI. Research Report on Health Care Utilization of Patients with Rare and Intractable Disorder and Improvement of Individual Copayment Beneficiaries Program for Rare and Intractable Disorder. Wonju: National Health Insurance Service; 2010. doi:10.1097/mib.0000000000000313
10. Wu J, Morris JK. The population prevalence of down’s syndrome in England and Wales in 2011. Eur J Hum Genet. 2013;21(9):1016–1019. doi:10.1038/ejhg.2012.294
11. Alexander M, Ding Y, Foskett N, Petri H, Wandel C, Khwaja O. Population prevalence of down’s syndrome in the United Kingdom. J Intellect Disabil Res. 2016;60(9):874–878. doi:10.1111/jir.12277
12. Presson AP, Partyka G, Jensen KM, et al. Current estimate of down syndrome population prevalence in the United States. J Pediatr. 2013;163(4):1163–1168. doi:10.1016/j.jpeds.2013.06.013
13. Wu J, Morris JK. Trends in maternal age distribution and the live birth prevalence of Down’s syndrome in England and Wales: 1938–2010. Eur J Hum Genet. 2013;21(9):943–947. doi:10.1038/ejhg.2012.288
14. Kim MA, Lee YS, Yee NH, Choi JS, Choi JY, Seo K. Prevalence of congenital heart defects associated with down syndrome in Korea. J Korean Med Sci. 2014;29(11):1544–1549. doi:10.3346/jkms.2014.29.11.1544
15. Kim MA, Yee NH, Choi JS, Choi JY, Seo K. Prevalence of birth defects in Korean livebirths, 2005–2006. J Korean Med Sci. 2012;27(10):1233–1240. doi:10.3346/jkms.2012.27.10.1233
16. Franasiak JM, Olcha M, Shastri S, et al. Embryonic aneuploidy does not differ among genetic ancestry according to continental origin as determined by ancestry informative markers. Hum Reprod. 2016;31(10):2391–2395. doi:10.1093/humrep/dew195
17. Kim SH, Kim KW, Han YJ, et al. Korean physicians’ attitudes toward the prenatal screening for fetal aneuploidy and implementation of non-invasive prenatal testing with cell-free fetal DNA. Lung Cancer. 2018;143. doi:10.5734/JGM.2018.15.2.72
18. Wikipedia. Abortion in South Korea. Wikimedia Foundation, Inc. [Updated January 12, 2020]. Available from: https://en.wikipedia.org/wiki/Abortion_in_South_Korea#cite_note-:0-2.
19. Lee SY, Byoun SJ, Kim JH, et al. Artificial Abortion Survey. Korea: Korea Institute for Health And Social Affairs; 2018.
20. Zhu JL, Hasle H, Correa A, et al. Survival among people with down syndrome: a nationwide population-based study in Denmark. Genet Med. 2013;15(1):64–69. doi:10.1038/gim.2012.93
21. Englund A, Jonsson B, Zander CS, Gustafsson J, Anneren G. Changes in mortality and causes of death in the Swedish down syndrome population. Am J Med Genet A. 2013;161a(4):642–649. doi:10.1002/ajmg.a.35706
22. Centers for Disease Control and Prevention (CDC). Racial disparities in median age at death of persons with down syndrome–United States, 1968–1997. MMWR Morb Mortal Wkly Rep. 2001;50(22):463–465. Published 2001/06/21.
23. Pueschel SM, Anneren G, Durlach R, Flores J, Sustrova M, Verma IC. Guidelines for optimal medical care of persons with down syndrome. International League of Societies for Persons with Mental Handicap (ILSMH). Acta Paediatr. 1995;84(7):823–827. doi:10.1111/j.1651-2227.1995.tb13768.x
24. Sawatari H, Chishaki A, Nishizaka M, et al. A nationwide cross-sectional study on congenital heart diseases and symptoms of sleep-disordered breathing among Japanese Down’s syndrome people. Intern Med. 2015;54(9):1003–1008. doi:10.2169/internalmedicine.54.3989
25. Freeman SB, Taft LF, Dooley KJ, et al. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998;80(3):213–217. doi:10.1002/(SICI)1096-8628(19981116)80:3<213::AID-AJMG6>3.0.CO;2-8
26. Wu H, Kao SC, Barrientos T, et al. Down syndrome critical region-1 is a transcriptional target of nuclear factor of activated T cells-c1 within the endocardium during heart development. J Biol Chem. 2007;282(42):30673–30679. doi:10.1074/jbc.M703622200
27. Maslen CL, Babcock D, Robinson SW, et al. CRELD1 mutations contribute to the occurrence of cardiac atrioventricular septal defects in Down syndrome. Am J Med Genet A. 2006;140(22):2501–2505. doi:10.1002/ajmg.a.31494
28. Wang L, Li Z, Song X, Liu L, Su G, Cui Y. Bioinformatic analysis of genes and MicroRNAs associated with atrioventricular septal defect in Down syndrome patients. Int Heart J. 2016;57(4):490–495. doi:10.1536/ihj.15-319
29. Izzo A, Manco R, de Cristofaro T, et al. Overexpression of chromosome 21 miRNAs may affect mitochondrial function in the hearts of Down syndrome fetuses. Int J Genomics. 2017;2017:8737649. doi:10.1155/2017/8737649
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
