Back to Journals » Infection and Drug Resistance » Volume 9
The pharmacokinetics of vancomycin during the initial loading dose in patients with septic shock
Authors Katip W , Jaruratanasirikul S, Pattharachayakul S, Wongpoowarak W, Jitsurong A, Lucksiri A
Received 4 September 2016
Accepted for publication 26 October 2016
Published 22 November 2016 Volume 2016:9 Pages 253—260
DOI https://doi.org/10.2147/IDR.S121513
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Wasan Katip,1 Sutep Jaruratanasirikul,2 Sutthiporn Pattharachayakul,3 Wibul Wongpoowarak,4 Arnurai Jitsurong,5 Aroonrut Lucksiri1
1Department of Pharmaceutical Care, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, 2Department of Medicine, Faculty of Medicine, 3Department of Clinical Pharmacy, 4Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, 5Department of Toxicology, Faculty of Medicine, Prince of Songkla University, Songkla, Thailand
Objective: To characterize the pharmacokinetics (PK) of vancomycin in patients in the initial phase of septic shock.
Methods: Twelve patients with septic shock received an intravenous infusion of vancomycin 30 mg/kg over 2 h. The vancomycin PK study was conducted during the first 12 h of the regimen. Serum vancomycin concentration–time data were analyzed using the standard model-independent analysis and the compartment model.
Results: For the noncompartment analysis the mean values ± standard deviation (SD) of the estimated clearance and volume of distribution of vancomycin at steady state were 6.05±1.06 L/h and 78.73±21.78 L, respectively. For the compartmental analysis, the majority of vancomycin concentration–time profiles were best described by a two-compartment PK model. Thus, the two-compartmental first-order elimination model was used for the analysis. The mean ± SD of the total clearance (3.70±1.25 L/h) of vancomycin was higher than that obtained from patients without septic shock. In contrast, the volume of the central compartment (8.34±4.36 L) and volume of peripheral compartment (30.99±7.84 L) did not increase when compared with patients without septic shock.
Conclusion: The total clearance of vancomycin was increased in septic shock patients. However, the volume of the central compartment and peripheral compartment did not increase. Consequently, a loading dose of vancomycin should be considered in all patients with septic shock.
Keywords: pharmacokinetics, vancomycin, MRSA, septic shock patients
Introduction
Septic shock is one of the most lethal illnesses encountered in an intensive care unit (ICU). Especially, in the case of the Sequential (Sepsis-related) Organ Failure Assessment (SOFA) score of ≥2 points, which is associated with an increase in the mortality risk in septic patients in an ICU. Moreover, the primary response in critically ill patients caused by the release of intrinsic mediators by the host as a reaction to bacterial toxins leads to the increase in capillary permeability, edema formation, vasodilatation, and hypotension. These alterations in pathophysiological conditions may result in pharmacokinetic (PK) changes in several antibiotics.1
Vancomycin is a relatively hydrophilic antibiotic. In critically ill patients, the leakage of the fluid from the vessels may result in the larger volume of vancomycin distribution, as well as decrease its plasma drug concentration. However, in general, patients with septic shock would aggressively receive fluid resuscitation during the initial phase of septic shock. The overall impacts of these changes were still unknown. Furthermore, in the absence of significant organ dysfunction, renal perfusion of septic shock patients is often increased and, consequently, may result in the increased creatinine clearance and elimination of hydrophilic antibiotics. Theoretically, because optimal trough concentration for vancomycin is 15–20 mg/L and 24-h area under the vancomycin concentration–time curve/minimum inhibitory concentration (AUC24/MIC) ≥400 μg·h/mL, these may result in subtherapeutic vancomycin serum concentrations and a corresponding potential for developing antibiotic resistance and/or therapeutic failure.2 Currently, despite various vancomycin PK studies in critically ill patients, the data on the PK profiles and dosage requirements of vancomycin in patients with initial phase of septic shock are however limited.3 The objective of this study was to evaluate the PK data of vancomycin in patients with an initial phase of septic shock.
Materials and methods
Subjects
This study was conducted at a university-affiliated hospital located in Southern Thailand between January and December 2012.
The patients were eligible for the study if they:
- were older than 18 years;
- developed septic shock:
- persistent hypotension (systolic blood pressure [SBP] <90 mmHg, mean arterial pressure <60 mmHg or decreased SBP 40 mmHg from baseline) despite adequate volume resuscitation, in the absence of other causes for hypotension, and
- presented with more than two of the following clinical findings:
- heart rate of >90 beats/min;
- respiratory rate of >20 breaths/min or arterial partial pressure of carbon dioxide (PCO2) of <32 mmHg;
- core temperature of <36°C or >38°C; and
- white blood cell count of <4×109 or >12×109 cells/L or >10% immature (band) forms; and
- received vancomycin in the setting of suspected methicillin-resistant staphylococcus aureus (MRSA) infection.
Patients were excluded from the study if they were: 1) exposed to intravenous (IV) vancomycin within the last 7 days; 2) on hemodialysis; 3) on renal replacement therapy (continuous venovenous hemofiltration, continuous venovenous hemodialysis, continuous venovenous hemodiafiltration, slow continuous ultrafiltration, continuous arteriovenous hemodialysis); 4) pregnant; 5) treated for burns; 6) diagnosed with a hematologic malignancy; or 7) allergic to vancomycin. The study protocol was approved by the Ethics Committee of Songklanagarind Hospital, and written informed consent was obtained from each patient. The data recorded on the day when the patients developed septic shock were age, gender, main diagnosis, and SOFA scores. Body weight, mechanical ventilation status, nutritional support, fluid balance, serum albumin, and estimated creatinine clearance (CLCr), according to the Cockroft-Gault method,4 as well as concurrent administration of vasoactive drugs were also recorded during the time of septic shock.
Study design
This is a prospective, non-comparative PK study.
Drug administration
Vancomycin was reconstituted according to the manufacturer’s guidelines. It was diluted into preparations: 1 g in 100 mL of normal saline solution. Each subject received a vancomycin loading dose of 30 mg/kg (based on actual body weight) 2 h infusion via central line.
Blood sampling
Blood samples of ~2.5 mL were obtained each time by direct venipuncture before and at 30, 60, 120, 130, 140, 160, 180, 210, 240, 360, 540, and 720 min after the initiation of vancomycin infusion. All blood samples were allowed to clot and then centrifuged at 2,000 rpm. The serum obtained was stored at −80°C until analyzed.
Vancomycin assays
Concentrations of vancomycin in serum were determined by fluorescence polarization immunoassay (AxSYM; Abbott Laboratories, Abbott Park, IL, USA). The assay limit of detection of vancomycin was 2 µg/mL, and the intraday and interday assay coefficients of variation were <7% over the entire calibration range (7–75 µg/mL).
PK analyses
Vancomycin PK analyses were conducted using two approaches: the noncompartmental modeling (model-independent methods) and the compartmental modeling using Phoenix® WinNonlin® Version 6.3 (CertaraTM, St. Louis, MO, USA) to determine the PK parameters of interest in each individual patient.
The elimination rate constant (Ke) of each patient was estimated with linear regression of the last three points which is at least 4 h after the completion of infusion on the semilogarithmic vancomycin concentration–time plots. The area under the serum concentration–time curve from time zero to 12 h (AUC0→12) was calculated for each subject by the linear-log trapezoidal rule. The AUC0→24 at steady state (AUC0→24,ss) was estimated assuming the patients received the same dose of vancomycin every 24 h and vancomycin PK remained the same. Then, the effects of patients’ demographic and clinical data on the PK of vancomycin were explored.
For the model dependent analysis, serum vancomycin concentration–time curves were fit to one, two, and three compartmental first-order elimination models. The Akaiki information criterion (AKI) and the Schwarz Bayesian criterion were used to select the best fit model.
Pharmacodynamics analyses
The probability of achieving the PD target of AUC24/MIC ≥400 in patients with septic shock treated with vancomycin was assessed using AUC24/MIC model and parameters estimated from the PK analysis. Simulations of 10,000 patients were conducted with different vancomycin dosages (30 mg/kg loading and 20 mg/kg subsequent dose every 8, 12, and 24 h) and MICs different for MRSA to vancomycin.
Statistical analysis
The correlations between patients’ demographic data, clinical data, and PK parameters were assessed via simple linear regression analysis.
Results
Twelve patients (nine males and three females) were enrolled in the study with a mean age of 57±19 years (range 26–86 years) and mean actual body weight of 62±9 kg (range 50–80 kg). The characteristics of all patients and the regimens of vancomycin are shown in Table 1.
The semilogarithmic plots of the observed serum vancomycin concentration–time curve are shown in Figure 1. Each line refers to a serum vancomycin concentration–time profile of an individual patient. Ten out of the twelve patients had serum vancomycin concentration monitored for 12 h after vancomycin administration. Two patients (subject numbers 6 and 10) died before the study completed, and the last vancomycin serum concentrations of them were collected at 4 and 9 h after infusion was completed. Two out of the ten patients had the observed vancomycin serum concentration at 12 h after the dose (minimum concentration, Cmin) within the therapeutic range (15–20 mg/L), while six patients had the lower Cmin than the therapeutic range (<15 mg/L). Two patients had the higher observed Cmin than the therapeutic range (>20 mg/L). Observed and predicted vancomycin concentration versus time plots using two-compartmental model analysis for individual subjects receiving vancomycin 30 mg/kg infused over 2 h are shown in Figure 2.
  | Figure 1 Semilogarithmic plots of the observed serum vancomycin concentration–time curve (A) and observed vancomycin concentrations versus time curve (B) in twelve patients. |
  | Figure 2 Observed () and predicted (—) vancomycin concentration versus time plots using two-compartmental model analysis for individual subjects receiving vancomycin 30 mg/kg infused over 2 h. |
The Ke of each patient was estimated with linear regression of the last three points (at least 4 h after the completion of infusion) on the semilogarithmic vancomycin concentration–time plots. The noncompartmental analysis was performed in ten patients. The elimination phase cannot be predicted in the other two patients due to insufficient concentration observed during their elimination phases. The area under the serum concentration–time curve (AUC) was calculated by the linear-log trapezoidal rule. The AUCs of non- and two-compartmental model analyses were presented in Tables 2 and 3, respectively. The median terminal half-life was 8.93 h (range 6.85–15.68 h). The median estimate of vancomycin total body clearance at steady state (CLss = dose/AUC0→∞) was 5.87 L/h (range 4.78–8.05), and the median estimate of volume of distribution at steady state (Vss = mean residence time extrapolated to infinity [MRTINF]*total clearance [CL]) was 78.90 L (range 48.27–111.51 L). Table 2 presents the patients’ PK parameter obtained from the noncompartmental analysis.
The relationship of patients’ demographic and their clinical outcomes and the values of PK parameters were explored. Only patients’ weight was observed to be related with the values of vancomycin Vss (Vss = 0.2554 × (weight) + 42.794, r2 =0.330).
As for the model-dependent analysis, serum vancomycin concentration–time curves were fit to one, two, and three compartmental first-order elimination models. Based on the AKI and the Schwarz Bayesian criterion, the three-compartment model was best fit for the vancomycin concentration–time data of patients numbered 7, 8, and 9, while the two-compartment model was best fit for the rest of the patients.
The two-compartment analysis was performed in order to summarize the characteristics of vancomycin PK among this population. The PK parameters were summarized in Table 3. The median systemic and distributive clearance values of vancomycin are 3.87 L/h (range 1.73–5.97 L/h) and 23.25 L/h (range 14.17–30.83 L/h), respectively. The median central and peripheral volume of distribution values of vancomycin are 7.33 L (range 3.60–18.71 L) and 31.60 L (range 19.02–45.61 L), respectively. The scatter plot of observed against predicted concentrations of the 12 patients with septic shock, using the two-compartmental first-order elimination model is shown in Figure 3. The observed vancomycin concentrations fell on and equally all along both sides of the line of unity. Figure 4 presents the scatter plot of residual versus predicted vancomycin concentrations from the same model analysis. The residual values were roughly equally distributed on both sides of the zero line.
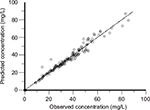  | Figure 3 Scatter plot of observed against predicted concentrations of twelve septic shock patients (two-compartment model). |
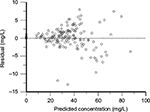  | Figure 4 Scatter plot of predicted concentrations against weighted residuals of twelve septic shock patients (two-compartment model). |
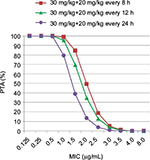
The correlation between patients’ demographic, clinical data and PK parameters were explored. Systemic (r2 = 0.286) and distributive (r2 =0.370) clearance values were found as weakly correlated with the SOFA score. The higher the SOFA score, the lower the systemic and distributive clearance values. However, vancomycin clearance and creatinine clearance (r2 =0.280) as well as vancomycin central volume of distribution and weight (r2 =0.275) were observed as poorly correlated. The probability of target attainments, achieving the target AUC24/MIC ≥400, with different MIC values of vancomycin for MRSA using different vancomycin dosage regimens were summarized and presented in Figure 5.
Discussion
Vancomycin PK analyses were conducted by using the noncompartment and compartment models. The majority of the vancomycin concentration–time profiles in patients with septic shock were best fit to the two-compartment model. It was found that the clearance values in the studied patients were higher when compared with the patients without septic shock during the initial phase. The values of vancomycin volume of distribution observed in this study were similar to the result in non-septic shock patients. Patients with septic shock seemed to clear vancomycin faster than the critically ill patients. However, no difference was detected in the distribution of vancomycin in both populations.
In septic shock patients, the values of vancomycin volume of distribution from our noncompartmental and compartmental models were similar to the ones previously reported in non-septic shock phase. According to non-compartment analysis, the Vss value (78.73±21.78 L) was similar to the Vss value estimated by Matzke et al5 (63.38±23.52 L), which was conducted in non-septic shock patients. When using two-compartment model, the estimated mean Vc value (Table 3) was 8.34±4.36 L,which was similar to the values (Vc of 12±4 L) reported by Polard et al.6 However, Purwonugroho et al7 reported higher values of Vc (36.11 L) in Thai adult patients. One-third of patients in Purwonugroho et al7 study were in ICU. Similarly, the mean volume of peripheral compartment (Vp = 30.99±7.84 L) in the present study was similar to Polard et al6 (39±12 L), but less than Purwonugroho et al7 (44.2 L).
The difference in the Vc and Vp values between the present study and Purwonugroho et al7 may result from the different time in obtaining vancomycin serum concentration. Purwonugroho et al7 obtained vancomycin Vc and Vp at steady state, while the present study investigated vancomycin PK at non-steady state condition (during the first 12 h of vancomycin therapy). The Vc and Vp on the first day of therapy and at steady state might be different due to the following explanations.6 During the acute phase of septic shock, the capillary permeability increased which resulted in extravascular fluid sequestration. In addition, most patients received vigorous fluid resuscitation. However, in the initial phase of septic shock, the fluid leak and fluid resuscitation may not be accumulated long enough to increase the volume of distribution of vancomycin. In the later phase of septic shock which may reflect the vancomycin serum concentration at steady state, the large amounts of fluids leaking into the interstitium led to prolonged water accumulation. Therefore, the higher value of vancomycin Vd was observed in this phase. This hypothesis is supported by Polard et al6 whose study observed an increase of vancomycin Vp at steady state (53±38 L) when compared with the first day of therapy (39±12 L).
The values of vancomycin CL presented in this study (non-compartment model; CLss =6.05±1.06 L/h and two-compartment model; CL =3.70±1.25 L/h) were higher than the result obtained from other studies conducted in Thai patients without septic shock. The values of vancomycin CLss from the studies by Jaruratanasirikul et al,8 using non-compartment model and Purwonugroho et al, using two-compartment model were 1.46±0.88 L/h and 1.54 L/h, respectively. Furthermore, the estimated value of CL in the present study was higher than the result in critically ill patients by Mangin et al9 (CL = 1.55 L/h). However, Polard et al6 reported a similar result as the present study (vancomycin CL of 8.58 L/h) in a population of critically ill patients during the first dose of vancomycin administration. In addition, Polard et al6 reported a significant decrease (~30%) in the mean value of vancomycin clearance at steady state.
The higher values of vancomycin clearance may result from the compensation of non-renal clearance during acute kidney injury and the effect of inotropic drugs. First, the majority of patients with septic shock had acute kidney injury. During acute kidney injury, non-renal clearance of vancomycin may increase. Our hypothesis is supported by a study of vancomycin PK in critically ill patients with acute kidney injury. The result of that study suggested that substantial portion of the non-renal clearance of vancomycin is preserved during the early phase of acute kidney injury.10 Second, inotropic and vasoactive drugs, such as dopamine, dobutamine, and norepinephrine, used during the initial phase of septic shock may play an important role in enhancing vancomycin clearance by increasing cardiac output.11 Most patients in the study received these agents.
Infectious Diseases Society of America (IDSA) guideline suggests that AUC24/MIC of 400 μg·h/mL is the PK/pharmacodynamic (PD) parameter that relates to good clinical and bacteriological outcomes for patients with MRSA infection.12 The present study observed the increase in vancomycin CL, but not for the volume of distribution in the patients with early phase of septic shock. By giving the loading dose of vancomycin, six out of ten patients in the study achieved the PK-PD target (AUC24 >400 μg·h/mL). However, four patients have subtherapeutic PK-PD target. Thus, to achieve an AUC/MIC24 target, it was suggested that the loading dose of 30 mg/kg, followed by a maintenance dose of 20 mg/kg every 8 h to compensate for the increase of vancomycin CL in the early phase of septic shock.
Limitations
There are some limitations found in this study that should be noted. First, due to the nature of PK studies, the study lacks an evaluation of patient outcomes. Second, the sample size may be insufficient. However, in the absence of data from a larger sample size, the study remains the only one conducted with patients with initial phase of septic shock.13 Third, the Cockcroft-Gault method used in estimating CLCr in this study is known to have certain limitations in severely ill patients.14,15 Fourth, the narrow range of patient weights and a lack of obese patients in this study which may limit its external validity. Finally, this study only evaluated the early phase of septic shock; hence, it should not be used in extrapolating parameters beyond this time frame.
Conclusion
In the early phase of septic shock, the value of total clearance of vancomycin increased while the values of central and peripheral compartment volume of distribution did not increase. According to the observations of this study (Figure 5), loading dose of 30 mg/kg vancomycin and subsequent dose of 20 mg/kg every 8 h to achieve AUC24/MIC >400 μg·h/mL on the first day of therapy were recommended. Finally, therapeutic drug monitoring is required for further dosage adjustments.
Acknowledgments
This study was sponsored by the Faculty of Medicine and Faculty of Pharmaceutical Sciences, Prince of Songkla University. The authors would like to thank the Certara Company for the Academic Licenses for the Phoenix WinNonlin Version 6.3, Phoenix NLME 1.2, and Pharsight Trial SimulatorTM Version 2.2.2, with special thanks to Dr Gary H Smith for English editing of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Vázquez M, Fagiolino P, Boronat A, Buroni M, Maldonado C. Therapeutic drug monitoring of vancomycin in severe sepsis and septic shock. Int J Clin Pharmacol Ther. 2008;46:140–145. | ||
Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37:840–851. | ||
del Mar Fernández de Gatta Garcia M, Revilla N, Calvo MV, Domínguez-Gil A, Sánchez Navarro A. Pharmacokinetic/pharmacodynamic analysis of vancomycin in ICU patients. Intensive Care Med. 2007;33:279–285. | ||
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. | ||
Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25:433–437. | ||
Polard E, Le Bouquin V, Le Corre P, et al. Non steady state and steady state PKS Bayesian forecasting and vancomycin pharmacokinetics in ICU adult patients. Ther Drug Monit. 1999;21:395–403. | ||
Purwonugroho TA, Chulavatnatol S, Preechagoon Y, Chindavijak B, Malathum K, Bunuparadah P. Population pharmacokinetics of vancomycin in Thai patients. Sci World J. 2012;2012:762649. | ||
Jaruratanasirikul S, Julamanee J, Sudsai T, Saengsuwan P, Jullangkoon M, Ingviya N, Jarumanokul R. Comparison of continuous infusion versus intermittent infusion of vancomycin in patients with methicillin-resistant Staphylococcus aureus. J Med Assoc Thai. 2010;93:172–176. | ||
Mangin O, Urien S, Mainardi JL, Fagon JY, Faisy C. Vancomycin pharmacokinetic and pharmacodynamic models for critically ill patients with post-sternotomy mediastinitis. Clin Pharmacokinet. 2014;53:849–861. | ||
Macias WL, Mueller BA, Scarim SK. Vancomycin pharmacokinetics in acute renal failure: preservation of nonrenal clearance. Clin Pharmacol Ther. 1991;50:688–694. | ||
Pea F, Porreca L, Baraldo M, Furlanut M. High vancomycin dosage regimens required by intensive care unit patients cotreated with drugs to improve haemodynamics following cardiac surgical procedures. J Antimicrob Chemother. 2000;45:329–335. | ||
Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98. | ||
Roberts JA, Kirkpatrick CMJ, Lipman J. Monte Carlo simulations: maximizing antibiotic pharmacokinetic data to optimize clinical practice for critically ill patients. J Antimicrob Chemother. 2011;66:227–231. | ||
Martin JH, Fay MF, Udy A, et al. Pitfalls of using estimations of glomerular filtration rate in an intensive care population. Intern Med J. 2011;41:537–543. | ||
Tanaka A, Suemaru K, Araki H. A new approach for evaluating renal function and its practical application. J Pharmacol Sci. 2007;105:1e5. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.