Back to Journals » Therapeutics and Clinical Risk Management » Volume 12
The Oxfordshire Community Stroke Project classification system predicts clinical outcomes following intravenous thrombolysis: a prospective cohort study
Authors Yang Y, Wang A, Zhao X , Wang C, Liu L , Zheng H, Wang Y, Cao Y, Wang Y
Received 24 February 2016
Accepted for publication 28 April 2016
Published 29 June 2016 Volume 2016:12 Pages 1049—1056
DOI https://doi.org/10.2147/TCRM.S107053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deyun Wang
Yuling Yang,1,* Anxin Wang,2–6,* Xingquan Zhao,2–5 Chunxue Wang,2–5 Liping Liu,2–5 Huaguang Zheng,2–5 Yongjun Wang,2–5 Yibin Cao,7 Yilong Wang2–5
On behalf of the Thrombolysis Implementation and Monitoring of Acute Ischemic Stroke in China (TIMS-China) investigators
1Graduate School, North China University of Science and Technology, Tangshan, 2Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, 3China National Clinical Research Center for Neurological Diseases, 4Center of Stroke, Beijing Institute for Brain Disorders, 5Beijing Key Laboratory of Translational Medicine for Cerebrovascular Disease, 6Department of Epidemiology and Health Statistics, School of Public Health, Capital Medical University, Beijing, 7Department of Neurology, Tangshan Gongren Hospital, Tangshan, People’s Republic of China
*These authors contributed equally to this work
Background: The Oxfordshire Community Stroke Project (OCSP) classification system is a simple stroke classification system that can be used to predict clinical outcomes. In this study, we compare the safety and efficacy of intravenous thrombolysis in Chinese stroke patients categorized using the OCSP classification system.
Patients and methods: We collected data from the Thrombolysis Implementation and Monitoring of Acute Ischemic Stroke in China registry. A total of 1,115 patients treated with intravenous thrombolysis with alteplase within 4.5 hours of stroke onset were included. Symptomatic intracranial hemorrhage (SICH), mortality, and 90-day functional outcomes were compared between the stroke patients with different stroke subtypes.
Results: Of the 1,115 patients included in the cohort, 197 (17.67%) were classified with total anterior circulation infarct (TACI), 700 (62.78%) with partial anterior circulation infarct, 153 (13.72%) with posterior circulation infarct, and 65 (5.83%) with lacunar infarct. After multivariable adjustment, compared to the patients with non-TACI, those with TACI had a significantly increased risk of SICH (odds ratio [OR] 8.80; 95% confidence interval [CI] 2.84–27.25, P<0.001), higher mortality (OR 5.24; 95% CI 3.19–8.62; P<0.001), and poor functional independence (OR 0.38; 95% CI 0.26–0.56; P<0.001) at 3-month follow-up.
Conclusion: After thrombolysis, the patients with TACI exhibited greater SICH, a higher mortality rate, and worse 3-month clinical outcomes compared with the patients with non-TACI. The OCSP classification system may help clinicians predict the safety and efficacy of thrombolysis.
Keywords: acute ischemic stroke, intravenous thrombolysis, OCSP classification, outcome, symptomatic intracranial hemorrhage
Introduction
Intravenous (IV) thrombolytic administration of recombinant tissue plasminogen activator (rtPA) remains the principal therapy for acute ischemic stroke (AIS) patients in the early hours after stroke onset.1,2 However, which patients are most likely to benefit from thrombolytic treatment remains unclear. Careful selection of patients who are suitable for thrombolytic treatment is very important, because it can maximize the benefits and reduce the risk of symptomatic intracranial hemorrhage (SICH) as far as possible. The factors associated with outcome include age, National Institutes of Health Stroke Scale (NIHSS) score, glucose, systolic blood pressure, prior antiplatelet use (aspirin or aspirin plus clopidogrel), history of atrial fibrillation, history of hypertension, weight, time to treatment, renal impairment, congestive heart failure, and early ischemic changes on pretreatment brain imaging. Several risk score models, such as Safe Implementation of Thrombolysis in Stroke (SITS), Stroke-TPI, MSS, Hemorrhage after Thrombolysis (HAT), and iScore, include various combinations of these factors.1,3–8 Although these models appear to be good, there may be room for further improvement.
The stroke site and the size of the infarct may help to estimate the outcomes of intravenous thrombolysis (IVT) because posterior circulation stroke may be related to a lower risk of SICH following IVT.9,10 This is reflected in many clinical trials investigating thrombolytic therapy with rtPA in AIS. The concept was first described as part of the HAT score.6 In addition, Breuer et al11 published an observational study demonstrating differences in the complications and outcomes of IVT between patients with posterior circulation stroke and those with anterior circulation stroke. However, the use of neuroimaging to determine the site and size of the stroke may not be timely in emergency settings.
The Oxfordshire Community Stroke Project (OCSP)12 classification system is a simple clinical classification method that predicts the site and size of the infarct on cerebral tomography (CT) in AIS patients. It is based on clinical syndromes alone and easy to perform. Some trials have shown that OCSP classification is useful in predicting clinical outcomes.12,13 Moreover, Mead et al14 showed that OCSP classification showed good inter-rater reliability. Good inter-rater reliability was also reflected in the studies of Al-Buhairi et al15 and Wardlaw et al.16 Therefore, we conducted this prospective cohort study to compare the clinical efficacy and safety of IVT between different subtypes of stroke classified according to the OCSP system.
Patients and methods
Patients and study design
We collected data from the Thrombolysis Implementation and Monitoring of Acute Ischemic Stroke in China (TIMS-China) registry, a national prospective stroke registry of AIS patients who received IVT with rtPA within 4.5 hours of symptom onset.17 Consecutive patients were recruited from 67 centers in the People’s Republic of china between May 2007 and April 2012. We included patients according to the following criteria: 1) age between 18 years and 80 years; 2) clinical diagnosis of stroke; 3) CT or magnetic resonance imaging (MRI)-based exclusion of hemorrhage or other non-ischemic disease; and 4) the absence of contraindications for IVT therapy. Written informed consent for the thrombolytic treatment was obtained from the patients or their family in all cases. The protocol of TIMS-China was approved by the Ethics Committee of Beijing Tiantan Hospital of the Capital Medical University, in compliance with the Declaration of Helsinki. After the ethical approval of Tiantan Hospital was obtained and approved by the other 67 participating hospitals, the ethical approval took effect automatically in each center. The registry was regularly monitored by the quality monitoring committee of TIMS-China.
Data including sex, age, vascular risk factors such as atrial fibrillation, hypertension, diabetes, hyperlipidemia, smoking, and current medications (including anticoagulants/antiplatelets), NIHSS score upon admission, CT or MRI scans of the brain, and IVT information were recorded, and all patients were followed up for 3 months.
OCSP classification
The ischemic stroke patients included in our study were examined by two neurologists who independently looked at the history, conducted a physical examination, and assessed the patient’s symptoms before the administration of IV rtPA therapy. According to the OCSP classification system,12 the two examiners, who were blinded to the neuroimaging findings, independently categorized the patients into groups corresponding to the different subtypes of stroke. In cases of inter-rater disagreement, a third neurologist arbitrated the discrepancies. The patients were classified as having total anterior circulation infarct (TACI), partial anterior circulation infarct (PACI), posterior circulation infarct (POCI), or lacunar infarct (LACI) based on their maximum neurological defects.
Clinical outcomes
The primary efficacy appraisal indicator was the modified Rankin scale (mRS) score obtained at clinical follow-up or by telephone interview. The self-independence outcome was defined as an mRS score of 0–2 at 3 months.18,19 The main security evaluation index was the occurrence of SICH. SICH was assessed on follow-up MRI or CT at 24–36 hours after IVT in relation to neurologic worsening (NIHSS score ≥4) after treatment. We focused on the definition according to the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST).1 Other related SICH criteria, such as SICH based on NINDS and European Cooperative Acute Stroke Study (ECASS-2) criteria,20 are also presented here. As another main security evaluation index, all-cause mortality was recorded as well.
Statistical analysis
The chi-square (χ2) test was used to compare categorical variables. ANOVA or the Kruskal–Wallis test was used to compare means or medians for continuous variables. We further used chi-square (χ2) or Fisher’s exact test for post hoc multiple comparisons to compare any two OCSP subtypes for significant differences of different clinical outcomes. Statistical significance was defined as a P-value <0.008 (calculated as 0.05/6) for the post hoc multiple comparisons. The inter-rater reliability of the OCSP classification was assessed using unweighted kappa statistics. Univariable and multivariable logistic regression were used to analyze the odds ratios (ORs) and 95% confidence intervals (CIs) of the clinical outcomes among the different groups classified by the OCSP system. Because the number of SICH events collected in the PACI, POCI, and LACI groups was small, we called these three groups collectively as non-TACI group in the multivariable logistic regression. The interactions of baseline NIHSS score with OCSP classification system on clinical outcomes of IVT were analyzed by multivariate logistic regression modeling. The median NIHSS score (NIHSS =11) was used as the demarcation point of gradation in our analyses. In accordance with classic prediction models, such as HAT, IST-3, SITS, iScore, and so on, and combining significant (P<0.2) variables at baseline, all of the covariates that were adjusted for in the multivariable model were strictly selected. All analyses were performed with the SAS software version 9.4. Statistical significance was defined as a P-value <0.05 (two tailed).
Results
Baseline characteristics of study patients
Between May 2007 and April 2012, 1,440 consecutive AIS patients who received IVT were registered in TIMS-China. Of those, 325 patients were excluded because the thrombolysis took place outside the 4.5-hour time window (n=312) or because of incomplete data (n=13). Finally, a total of 1,115 patients were included in the study. Based on the OCSP criteria, the clinical stroke syndromes of all of the patients were as follows: 197 (17.67%) with TACI, 700 (62.78%) with PACI, 153 (13.72%) with POCI, and 65 (5.83%) with LACI (Figure 1). Inter-rater reliability for the first two examiners was excellent (κ=0.94, P<0.001).
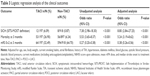
The baseline characteristics of the patients included in the study are summarized in Table 1. The patients in the TACI (23.35%; P=0.03) group had the highest proportion of atrial fibrillation history, and the patients in the POCI (8.25±3.07, P=0.01) group had the highest blood glucose levels. The baseline NIHSS scores were higher in the TACI (16.19±5.37) and POCI (14.73±11.37) groups than in the other two groups (P<0.001). The weights of the patients in the POCI (68.52±12.67; P=0.05) group were also higher than those in the other groups. Notably, the time to treatment (from onset to IV) was comparable among the groups. In addition, there were no significant differences in sex, rtPA dosage, medications, or other parameters (Table 1).
Symptomatic intracerebral hemorrhage
The risk of SICH was highest in the patients with TACI (6.09%; P<0.001) (Table 2). After adjusting for potential confounding factors (age, sex, body weight, current smoking habits, atrial fibrillation, history of transient ischemic attack, history of stroke, hypertension, diabetes mellitus, blood glucose, systolic blood pressure, diastolic blood pressure, current medications (including anticoagulants/antiplatelets), NIHSS score upon admission, mean rtPA dose, and median stroke onset to treatment time), the risk of SICH was significantly increased in the TACI group compared with the non-TACI group (OR 8.80; 95% CI 2.84–27.25; P<0.001) (Table 3).
Clinical outcomes
Figure 2 shows the clinical outcomes at 3 months. The functional independence rate (mRS 0–2) at 3 months was significantly lower in the TACI group compared with the other groups (32.49% vs 63.88%; P<0.001). Although patients with PACI tend to have roughly equivalent SICH rate compared to POCI, patients with POCI appear to have higher mortality than those with PACI (17.88% vs 4.26%; P<0.008) (Table 2). After multivariable adjustment, the patients in the TACI group exhibited a significantly lower rate of good functional outcomes (mRS 0–2) compared to the rates of those in the non-TACI group (OR 0.38; 95% CI 0.26–0.56; P<0.001) (Table 3).
Nearly 109 patients died within 90 days of IVT (Table 2). Of these, 53 (26.90%) had TACI, 29 (4.26%) had PACI, 27 (17.88%) had POCI, and 0 (0.00%) had LACI (Table 2). After multivariable adjustment, the TACI group showed a significantly higher mortality rate at 3 months compared to the non-TACI group (OR 5.24; 95% CI 3.19–8.62; P<0.001) (Table 3).
The adjusted multivariable logistic regression model with interaction performed well; there were no significant interactions of baseline NIHSS score with OCSP classification system on the risk of SICH (P=0.51), mortality rate at 3 months (P=0.78), and the functional independence rate (P=0.69) (Table 4).
Discussion
This multicenter prospective study based on the TIMS-China registry demonstrated an unfavorable outcome following IVT in AIS patients with TACI compared to those in the non-TACI group. The patients with TACI had a higher risk of post-thrombolytic SICH and a higher mortality rate at 3 months, and they were less likely to be functionally independent. These findings are consistent with the results of Sung et al.21
Our study revealed that the OCSP clinical classification system identified two major groups (TACI and non-TACI). These two groups exhibited different risks of SICH following IVT, a finding that primarily reflects the different pathological and physiological mechanisms of the different types of stroke. TACI is most likely to develop into massive cerebral infarction; the majority of TACI cases are caused by proximal middle cerebral artery occlusion, while few are caused by internal carotid siphon occlusion. PACI can also involve the occlusion of the middle cerebral artery; however, the occlusion is most likely to be its distal end or branches, or the cortex which is of abundant collateral circulation. The anterior cerebral artery and its branches may also be responsible for PACI. Therefore, PACI usually results in small and medium infarctions. POCI is likely to include brainstem infarction, cerebellum infarction, or occipital lobe infarction caused by occlusion of the vertebral basilar artery and its branches. LACI includes lacunar infarctions in small perforating branches supplying the basal ganglia or pons. In conclusion, stroke symptoms were more severe in patients with TACI than in the other groups. CT and diffusion weighted imaging (DWI) studies have demonstrated that SICH is most likely to occur with extensive cerebral ischemia.9,22 Although POCI can also involve massive cerebral infarction, there are better collaterals in the territory affected by POCI than by TACI. Moreover, we observed that there were no significant interactions between the baseline NIHSS score and OCSP clinical classification system. These interactions indicated that the severity of stroke at baseline had no effect on the association between the OCSP classification system and clinical outcomes of IVT.
Another finding of our study was that patients with PACI tend to have roughly equivalent SICH rate compared to POCI, but patients with POCI appear to have higher mortality than patients with PACI, despite there being better collaterals in the territory affected by POCI. It could be due to the characteristic of the infarct location of POCI. The anatomic structure and physiological function of posterior circulation are very complicated. Brainstem is an important part of nerve activity of posterior circulation, and the ascending reticular activating system, the cranial nerve, and the nerve conduction bundle are mainly localized around this area. When cerebral circulation insufficiency and neurological impairment occur, there will be different but overlapping clinical manifestations, especially during multifocal cerebral infarction. As such, it is difficult for doctors to diagnose the infarct location accurately. Moreover, CT is not sensitive to the diagnosis of posterior circulation infarction. Therefore, it tends to delay treatment for POCI patients, which may lead to a higher mortality. Furthermore, posterior circulation contains important vital centers, especially the medulla oblongata. Infarction of these locations can cause sudden death in patients.23 In view of these reasons, patients with POCI appear have higher mortality compared to patients with PACI.
The OCSP clinical classification system is a simple and reliable method that can be used to categorize AIS patients at the bedside. Several studies have shown that OCSP classification is closely associated with the size and site of the infarct on neuroimaging findings.15,24 However, the size and site of ischemic brain tissue were also closely associated with the occurrence of SICH after IVT.25,26 Additionally, the OCSP classification assigned in the early hours of AIS correlated well with early ischemic changes on pretreatment CT,27 which has been shown to be one of the most important independent risk factors for SICH following IVT.28 Moreover, because baseline NIHSS score is a key predictor, models that predict post-thrombolysis SICH usually include high NIHSS scores at baseline.6,28,29 However, the NIHSS score exhibits a bias when forecasting the outcomes of POCI following IVT. Because it does not have such an assessment bias, OCSP clinical classification complements NIHSS score when predicting SICH risk. In general, OCSP classification may be another tool to help predict the safety and efficacy of IVT.
Studies of the accuracy of the OCSP classification system have confirmed that it is accurate and effective. In combination with CT and MRI, Mead et al14 prospectively collected 1,012 patients with AIS and calculated the sensitivity, specificity, and the positive and negative predictive values of the OCSP classification system in predicting infarct area and size. The study showed that OCSP classification could correctly predict the location and size of the infarction in 75% of patients, and the best theoretical value was 84%. Moreover, Wardlaw et al16 showed that OCSP classification correctly predicted infarct size and location in 88% of patients, with 95% CI =77%–92%. Other studies have arrived at similar conclusions using neuroimaging and autopsy materials.30,31 However, it is not practical to require OCSP classification to have 100% accuracy, because even CT/MRI cannot reach absolute accuracy. Additionally, in the acute period of cerebral infarction, the validity of the TOAST classification, which ranged from 70.15% to 62% depending on neuroimaging and other devices used, was not higher than that of OCSP classification.32
However, some limitations of our study should be noted. First, the number of SICH events collected in our study was small, which decreases the statistical power and may not have been sufficient to enable adjustment for all of the potential confounds. Nevertheless, it did not prevent us from detecting a significant difference in clinical outcomes following IVT between the different types of OCSP classifications. Second, because we follow a hierarchical diagnosis and treatment policy in our country, many minor strokes were treated in community hospitals. Most centers in our study were tertiary hospitals, so the proportion of patients with LACI was low. This might have led to a selection bias in the study and, therefore, additional larger studies of the OCSP classifications are needed to assess the clinical implications of our findings.
Conclusion
In our study, we compared the clinical outcomes of different types of OCSP classifications following IVT in AIS patients. We found that patients with TACI exhibited a higher incidence of SICH, a higher mortality rate, and a worse clinical outcome at 3 months after IVT compared with those with non-TACI. We conclude that the OCSP classification system will help clinicians in predicting the safety and efficacy of IVT.
Acknowledgments
We gratefully appreciate the help from all participating hospitals, clinicians, statisticians, and imaging and laboratory technicians. This study was funded by the State Key Development Program of Basic Research of China (2009CB521905), the National Science and Technology Major Project of China (2011BAI08B02), and the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2013BAI09B03).
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Wahlgren N, Ahmed N, Davalos A, et al; SITS-MOST Investigators. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275–282. | ||
Wahlgren N, Ahmed N, Davalos A, et al; SITS Investigators. Thrombolysis with alteplase 3–4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372(9646):1303–1309. | ||
Mazya M, Egido JA, Ford GA, et al; SITS Investigators. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe implementation of treatments in stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. 2012;43(6):1524–1531. | ||
Kent DM, Selker HP, Ruthazer R, Bluhmki E, Hacke W. The stroke-thrombolytic predictive instrument: a predictive instrument for intravenous thrombolysis in acute ischemic stroke. Stroke. 2006;37(12):2957–2962. | ||
Cucchiara B, Tanne D, Levine SR, Demchuk AM, Kasner S. A risk score to predict intracranial hemorrhage after recombinant tissue plasminogen activator for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2008;17(6):331–333. | ||
Lou M, Safdar A, Mehdiratta M, et al. The HAT Score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology. 2008;71(18):1417–1423. | ||
Saposnik G, Fang J, Kapral MK, et al; Investigators of the Registry of the Canadian Stroke Network (RCSN); Stroke Outcomes Research Canada (SORCan) Working Group. The iScore predicts effectiveness of thrombolytic therapy for acute ischemic stroke. Stroke. 2012;43(5):1315–1322. | ||
Saposnik G, Guzik AK, Reeves M, Ovbiagele B, Johnston SC. Stroke prognostication using age and NIH Stroke Scale: SPAN-100. Neurology. 2013;80(1):21–28. | ||
Pagola J, Ribo M, Alvarez-Sabin J, et al. Thrombolysis in anterior versus posterior circulation strokes: timing of recanalization, ischemic tolerance, and other differences. J Neuroimaging. 2011;21(2):108–112. | ||
Sarikaya H, Arnold M, Engelter ST, et al. Outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke. Stroke. 2011;42(9):2498–2502. | ||
Breuer L, Huttner HB, Jentsch K, et al. Intravenous thrombolysis in posterior cerebral artery infarctions. Cerebrovasc Dis. 2011;31(5):448–454. | ||
Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521–1526. | ||
Pittock SJ, Meldrum D, Hardiman O, Thornton J, Brennan P, Moroney JT. The Oxfordshire Community Stroke Project classification: correlation with imaging, associated complications, and prediction of outcome in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2003;12(1):1–7. | ||
Mead GE, Wardlaw JM, Dennis MS, Lewis SC, Warlow CP. Relationship between pattern of intracranial artery abnormalities on transcranial doppler and Oxfordshire Community Stroke Project clinical classification of ischemic stroke. Stroke. 2000;31(3):714–719. | ||
Al-Buhairi AR, Phillips SJ, Llewellyn G, Jan MM. Prediction of infarct topography using the Oxfordshire Community Stroke Project classification of stroke subtypes. J Stroke Cerebrovasc Dis. 1998;7(5):339–343. | ||
Wardlaw JM, Dennis MS, Lindley RI, Sellar RJ, Warlow CP. The validity of a simple clinical classification of acute ischaemic stroke. J Neurol. 1996;243(3):274–279. | ||
Liao XL, Wang CX, Wang YL, et al; Thrombolysis Implementation and Monitor of acute ischemic Stroke in China (TIMS-China) Investigators. Implementation and outcome of thrombolysis with alteplase 3 to 4.5 h after acute stroke in Chinese patients. CNS Neurosci Ther. 2013;19(1):43–47. | ||
Jenkinson D. ECASS-II: intravenous alteplase in acute ischaemic stroke. European Co-operative Acute Stroke Study-II. Lancet. 1999;353(9146):67–68. | ||
Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–1587. | ||
Gumbinger C, Gruschka P, Bottinger M, et al. Improved prediction of poor outcome after thrombolysis using conservative definitions of symptomatic hemorrhage. Stroke. 2012;43(1):240–242. | ||
Sung SF, Wu CS, Hsu YC, Tseng MC, Chen YW. Oxfordshire Community Stroke Project classification but not NIHSS predicts symptomatic intracerebral hemorrhage following thrombolysis. J Neurol Sci. 2013;324(1–2):65–69. | ||
Obach V, Oleaga L, Urra X, et al. Multimodal CT-assisted thrombolysis in patients with acute stroke: a cohort study. Stroke. 2011;42(4):1129–1131. | ||
Jaster JH. Unexpected sudden death after lateral medullary infarction. J Neurol Neurosurg Psychiatry. 2001;70(1):137. | ||
Mead GE, Lewis SC, Wardlaw JM, Dennis MS, Warlow CP. How well does the Oxfordshire community stroke project classification predict the site and size of the infarct on brain imaging? J Neurol Neurosurg Psychiatry. 2000;68(5):558–562. | ||
Dzialowski I, Hill MD, Coutts SB, et al. Extent of early ischemic changes on computed tomography (CT) before thrombolysis: prognostic value of the Alberta Stroke Program Early CT Score in ECASS II. Stroke. 2006;37(4):973–978. | ||
Singer OC, Humpich MC, Fiehler J, et al; MR Stroke Study Group Investigators. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2008;63(1):52–60. | ||
Kobayashi A, Wardlaw JM, Lindley RI, et al; IST-3 Collaborative Group. Oxfordshire community stroke project clinical stroke syndrome and appearances of tissue and vascular lesions on pretreatment ct in hyperacute ischemic stroke among the first 510 patients in the Third International Stroke Trial (IST-3). Stroke. 2009;40(3):743–748. | ||
Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24(1):1–10. | ||
Wahlgren N, Ahmed N, Eriksson N, et al; Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy Investigators. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST). Stroke. 2008;39(12):3316–3322. | ||
Lindley RI, Warlow CP, Wardlaw JM, Dennis MS, Slattery J, Sandercock PA. Interobserver reliability of a clinical classification of acute cerebral infarction. Stroke. 1993;24(12):1801–1804. | ||
Anderson CS, Taylor BV, Hankey GJ, Stewart-Wynne EG, Jamrozik KD. Validation of a clinical classification for subtypes of acute cerebral infarction. J Neurol Neurosurg Psychiatry. 1994;57(10):1173–1179. | ||
Lindgren A, Norrving B, Rudling O, Johansson BB. Comparison of clinical and neuroradiological findings in first-ever stroke. A population-based study. Stroke. 1994;25(7):1371–1377. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.