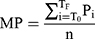Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 13
The Optimal Haemoglobin Target in Dialysis Patients May Be Determined by Its Contrasting Effects on Arterial Stiffness and Pressure Pulsatility
Authors Hsu HC, Robinson C, Norton GR, Woodiwiss AJ, Dessein PH
Received 3 October 2020
Accepted for publication 28 November 2020
Published 30 December 2020 Volume 2020:13 Pages 385—395
DOI https://doi.org/10.2147/IJNRD.S285168
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Hon-Chun Hsu,1,2 Chanel Robinson,1 Gavin R Norton,1 Angela J Woodiwiss,1 Patrick H Dessein1,3,4
1Cardiovascular Pathophysiology and Genomics Research Unit, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; 2Nephrology Unit, Milpark Hospital, Johannesburg, South Africa; 3Internal Medicine Department, University of the Witwatersrand, Johannesburg, South Africa; 4Free University and University Hospital, Brussels, Belgium
Correspondence: Patrick H Dessein 80 Scholtz Road, Norwood, Johannesburg 2117, South Africa
Email [email protected]
Introduction: It remains unclear why the optimal haemoglobin target is lower in patients with chronic kidney disease (CKD) than in non-CKD persons. Arteriosclerosis and consequent impaired arterial function comprise a central cardiovascular risk mechanism in CKD. We hypothesized that the optimal haemoglobin target depends on its opposing effects on arterial stiffness and pressure pulsatility in CKD.
Methods: Arterial stiffness (aortic pulse wave velocity), wave reflection (augmentation index, reflected wave pressure and reflection magnitude), and pressure pulsatility (central systolic and pulse pressure, peripheral pulse pressure, pressure amplification and forward wave pressure) were assessed in 48 dialysis patients.
Results: In established confounder and diabetes adjusted linear regression models, haemoglobin levels were directly associated with arterial stiffness (partial R=0.366, p=0.03) and inversely with central systolic pressure (partial R=− 0.344, p=0.04), central pulse pressure (partial R=− 0.403, p=0.01), peripheral pulse pressure (partial R=− 0.521, p=0.001) and forward wave pressure (partial R=− 0.544, p=0.001). The presence of heart failure and use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers and erythropoietin stimulating agents did not materially alter these relationships upon further adjustment for the respective characteristics in the models, and in sensitivity analyses. In receiver operator characteristic curve analysis, the optimal haemoglobin concentration cut-off values in predicting arterial stiffness and increased central pulse pressure were remarkably similar at 10.95 g/dl and 10.85 g/dl, respectively, and with clinically useful sensitivities, specificities and positive and negative predictive values. In logistic regression models, a haemoglobin value of > 10.9 mg/dl was associated with both arterial stiffness (> 10 m/sec; OR (95% CI) = 10.48 (1.57– 70.08), p=0.02) and normal central pulse pressure (> 50 mmHg; OR (95% CI) = 7.55 (1.58– 36.03), p=0.01).
Conclusion: This study suggests that the optimal haemoglobin target in dialysis patients is ∼ 11g/dl and determined by its differential and contrasting effects on arterial stiffness and pressure pulsatility.
Keywords: haemoglobin target, dialysis, arterial stiffness, pressure pulsatility
Introduction
Arteriosclerosis encompasses hypertrophy and fibrosis of the medial layer in large arteries.1 Chronic kidney disease (CKD) is characterized by marked premature arteriosclerosis that causes impaired arterial function.2–4 Arterial stiffness results in an accelerated forward wave, which increases central systolic blood pressure and enhances wave reflection that occurs at bifurcations and due to changes in composition and progressive narrowing of the vessels along the arterial tree. The increased reflected wave arrives earlier at the heart, ie in systole rather than diastole, and thereby reduces diastolic blood pressure and coronary perfusion. The increased forward and reflected waves enhance pressure pulsatility and decrease pulse pressure amplification, which increases transmission of pulsatile pressure into the microcirculation leading to CKD progression. Increased arterial stiffness, wave reflection and pressure pulsatility thereby each contribute to cardiovascular event rates including heart failure, arrhythmias, stroke and myocardial infarction in CKD.3,5,6
Accelerated arteriosclerosis in CKD is mediated by adverse traditional as well as non-traditional or renal impairment specific cardiovascular risk factors.2–7
In observational studies, anaemia is associated with CVD in CKD.8 However, the optimal haemoglobin target in CKD patients is reportedly substantially lower in CKD compared to non-CKD individuals.9,10 Indeed, treatment with erythropoietin stimulating agents in CKD patients is associated increased CVD events when a haemoglobin level of ~13 g/dl is targeted.11,12 In this regard, nitric oxide reduces arterial stiffness and peripheral vascular resistance13 whereas haemoglobin is a potent nitric oxide scavenger.14 High haemoglobin levels are associated with arterial stiffness in non-CKD persons.15–17 Other investigations in persons with and without CKD indicate that anaemia increases pressure pulsatility.18,19 In the present study, we therefore hypothesized that the optimal haemoglobin target in CKD may depend on its contrasting effects on arterial stiffness and pressure pulsatility in CKD. We assessed the independent relationships of haemoglobin levels with arterial function measures including pulse wave velocity, wave reflection and pressure pulsatility in a cohort of dialysis patients.
Patients and Methods
Patients
Forty-eight dialysis patients were enrolled at the Milpark Hospital in Johannesburg, South Africa. Patients with infection or/and active cancer were excluded. This study was performed according to the Helsinki Declaration of 1975 as revised in 2013 and was approved by the University of the Witwatersrand Human (Medical) research Ethics Committee (protocol number: M15-08-43) in Johannesburg, South Africa. Written informed consent was obtained in each patient prior to participation.
Methods
The recorded characteristics included demographic features, lifestyle factors, anthropometric measures, traditional and non-traditional cardiovascular risk factors, the presence of established cardiovascular disease including heart failure, arterial function and other hemodynamic characteristics that included systemic vascular resistance, stroke volume and work, cardiac output and left ventricular mass index. Data recording was performed on the day prior to undergoing dialysis, which was applied thrice weekly in each of these patients.
Cardiovascular Risk Factors
We recorded traditional and non-traditional or renal cardiovascular risk factors as previously reported20 and given in the online Supplementary Material. For the present study, high phosphate was considered present when the phosphate concentration was >1.42 mmol/l or/and phosphate lowering drugs including calcium carbonate or sevelamer therapy in 44 and 1 patients, respectively, was employed. Mean arterial blood pressure for the peripheral waveform was determined electronically by the SphygmoCor device (see below) and using the formula
where T0=start of the waveform; TF=end of waveform; Pi=pressure points and n=number of pressure points.
Established Cardiovascular Disease
Ischemic heart disease, heart failure and cerebrovascular and peripheral arterial disease that was confirmed by a cardiologist, neurologist and vascular surgeon, respectively, comprised recorded established cardiovascular disease.
Arterial Function
Applanation tonometry and SphygmoCor software were used to determine central hemodynamic features as previously reported20 and given in the online Supplementary Material. We assessed aortic pulse wave velocity, augmentation index, reflected wave pressure and reflection magnitude, central systolic and pulse pressure, peripheral pulse pressure, pressure amplification and forward wave pressure.
Other Hemodynamic Parameters
Echocardiography was performed as recommended by the American Society of Echocardiography convention and using a Sonosite M-Turbo ultrasound (SonoSite® Inc., Bothell, WA, USA).21 We assessed stroke volume, cardiac output and left ventricular mass index. Further details are given in the online Supplementary Material. Systemic vascular resistance was calculated from mean arterial pressure and cardiac output according to the equation mean arterial pressure=systemic vascular resistance x cardiac output assuming that right atrial pressure=0 mmHg. Stroke work was calculated as stroke volume x systolic blood pressure x 0.0144, and expressed in gram-meters/beat.
Data Analysis
Statistical analysis was performed on IBM SPSS statistics program (version 23.0 IBM, USA) and significance was set at p ≤0.05. Results are expressed as mean (SD) or median (interquartile range, IQR) for continuous variables and percentages for categorical variables. Logarithmic transformation was applied when non-normally distributed data were analysed in multivariable regression models.
The associations of lifestyle factors, anthropometric measures and major traditional and non-traditional/renal cardiovascular risk factors with arterial function parameters were first assessed in established confounder20,22 including age, sex, race, weight, height, heart rate and mean arterial blood pressure adjusted linear regression models.
Subsequently, the independent associations of recorded cardiovascular risk factors with arterial function parameters were assessed by entering established confounders together with those that were related to arterial function in the previous analysis into single models.
As heart failure23 and treatment with angiotensin converting enzyme inhibitors or angiotensin blockers24 as well erythropoietin stimulating agents25 can also impact arterial function, the potential influence of the respective factors was assessed in separate models and sensitivity analyses.
The above mentioned analyses revealed that haemoglobin concentrations were directly associated with arterial stiffness and inversely related to pressure pulsatility. We therefore investigated the respective relationships further by performing receiver operator characteristic (ROC) curve analysis.
Finally, we assessed bivariate associations among haemoglobin concentrations and other hemodynamic characteristics by determining Pearson correlation coefficients.
Results
Patient Characteristics
The recorded characteristics are given in Table 1. The mean (SD) age was 55.8 (14.3) years and 22 (45.8%) were women. More than half of the study participants (56.3%) were of black population origin. Hypertension, dyslipidemia and diabetes were present in 44 (91.7%), 30 (71.4%) and 19 (39.6%), respectively. Angiotensin converting enzyme inhibitors or angiotensin receptor blockers and erythropoietin stimulating agents were used in 37 (77.1%) and 43 (89.6%) of the patients, respectively. Among those with cardiovascular disease (n=14 (29.6%)), 7 (14.6%) had heart failure. The pulse wave velocity and left ventricular mass index were both large with a median (interquartile range) and mean (SD) value of 11.2 (7.8–14.9) and 103.4 (51.0), respectively.
 |
Table 1 Recorded Patient Characteristics in 48 Dialysis Patients |
Associations of Haemoglobin Concentrations with Arterial Function
As given in Table 2, in age, sex, race, weight, height, mean blood pressure and heart rate adjusted linear regression models, haemoglobin levels were directly associated with pulse wave velocity (p=0.05) and inversely with central systolic blood pressure (p=0.03), central pulse pressure (p=0.01), peripheral pulse pressure (p=0.001) and forward wave pressure (p=0.001) (model 1 in Table 2).
 |
Table 2 Associations of Haemoglobin Concentrations with Arterial Function in 48 Dialysis Patients |
Apart from diabetes that was associated with pulse wave velocity (p=0.01), augmentation index (p=0.01), reflected wave pressure (p=0.02), reflection magnitude (p=0.02) and central pulse pressure (p=0.02), and heart rate and mean blood pressure for which data are not shown as these characteristics were included as established confounders in the models, none of the other traditional and non-traditional/renal risk factors as given in Table 1 were associated with arterial function parameters. Model 2 in Table 2 shows that upon additional adjustment for diabetes, haemoglobin concentrations remained associated with pulse wave velocity (p=0.03), central systolic blood pressure (p=0.04), central pulse pressure (p=0.01), peripheral pulse pressure (p=0.001) and forward wave pressure (p=0.001).
When we additionally adjusted for the presence of heart failure (model 3 in Table 2), the associations of haemoglobin concentrations with arterial function remained consistent (p=0.03, p=0.04, p=0.01, p=0.001 and p=0.002 for pulse wave velocity, central systolic blood pressure, central pulse pressure, peripheral pulse pressure and forward wave pressure, respectively.
As shown in model 4 in Table 2, upon additional adjustment for the use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, haemoglobin concentrations remained associated with pulse wave velocity (p=0.03), peripheral pulse pressure (p=0.002) and forward wave pressure (p=0.005) but their relationships with central systolic blood pressure and central pulse pressure no longer reached significance (p=0.1 and p=0.06, respectively.
As given in model 5 in Table 2, when we additionally adjusted for treatment with erythropoietin stimulating agents, haemoglobin levels remained associated with pulse wave velocity (p=0.03), central systolic blood pressure (p=0.04), central pulse pressure (p=0.01), peripheral pulse pressure (p=0.001) and forward wave pressure (p=0.001).
Associations of Haemoglobin Concentrations with Arterial Function in Sensitivity Analyses
To further assess whether the presence of heart failure and angiotensin converting enzyme inhibitor or angiotensin receptor blocker and erythropoietin stimulating agent therapy could influence the impact of haemoglobin concentrations on arterial function, we performed sensitivity analyses. These results are shown in Table 3.
 |
Table 3 Associations of Haemoglobin Concentrations with Arterial Function in Sensitivity Analyses Among Patients Without Heart Failure and on ACEI or ARB and ESA Agent Therapy |
In patients without heart failure (n=41), haemoglobin levels were associated with pulse wave velocity (p=0.01), central pulse pressure (p=0.02), peripheral pulse pressure (p=0.004) and forward wave pressure (p=0.004) whereas their relationship with central systolic blood pressure did not reach significance (p=0.07). Haemoglobin concentrations were further directly associated with reflection magnitude (p=0.04). When the mean arterial pressure was replaced by systemic vascular resistance in the respective model, the association of haemoglobin levels with reflection magnitude was not materially altered (partial R=0.334, p=0.08).
Among patients on angiotensin converting enzyme inhibitor or angiotensin receptor blocker therapy, haemoglobin levels were associated with central pulse pressure (p=0.03), peripheral pulse pressure (0.002) and forward wave pressure (p=0.003) but their relationship with pulse wave velocity and central systolic blood pressure did not reach significance (p=0.07 and p=0.09, respectively).
In patients treated with erythropoietin stimulating agents, haemoglobin concentrations were associated with pulse wave velocity (p=0.03), central pulse pressure (p=0.02), peripheral pulse pressure (p=0.004) and forward wave pressure (p=0.002) whereas their relationship with central systolic blood pressure did not reach significance (p=0.1). Haemoglobin levels were further associated with augmentation index (p=0.05) and reflection magnitude (p=0.03). When the mean arterial pressure was replaced by systemic vascular resistance in the respective model, the association of haemoglobin levels with reflection magnitude was not materially altered (partial R=0.317, p=0.1).
Haemoglobin Concentrations, Arterial Stiffness and Increased Central Pulse Pressure
Arterial stiffness (pulse wave velocity >10 m/sec)26,27 and increased pulse pressure (>50 mmHg)28 were recorded in 46.3% and 28.7% of the patients, respectively. Given the independent associations of haemoglobin concentrations with arterial stiffening and low pressure pulsatility among these patients (Table 2), we determined the accuracy of haemoglobin concentrations in predicting arterial stiffness and a normal central pulse pressure in ROC curve analysis. This is shown in Figure 1. The area under the curve (AUC) of the ROC curve was associated with arterial stiffness (Figure 1A; AUC=0.670) and normal pulse pressure (Figure 1B; AUC=0.717). To estimate the optimal cut-off values for haemoglobin concentrations in determining arterial function, we calculated the Youden index. The optimal haemoglobin concentration cut-off value in predicting the presence of arterial stiffness was 10.95 g/dl with a corresponding sensitivity, specificity, and positive and negative predictive value as determined by applying Bayes’ theorem of 45.7%, 77.8%, 84.8% and 60.9%, respectively; the optimal haemoglobin concentration cut-off value in predicting the presence of a normal pulse pressure was 10.85 g/dl with a corresponding sensitivity, specificity, and positive and negative predictive value of 72.7%, 81.8%, 77.1% and 78.0%, respectively.
A haemoglobin value of >10.9 mg/dl was associated with arterial stiffness (OR (95% CI) = 4.77 (1.23–18.53), p=0.02) and normal central pulse pressure (OR (95% CI) = 7.88 (1.96–31.57), p=0.004). In established confounder adjusted logistic regression models, a haemoglobin value of >10.9 mg/dl remained associated with arterial stiffness (OR (95% CI) = 10.48 (1.57–70.08), p=0.02) and normal central pulse pressure (OR (95% CI) = 7.55 (1.58–36.03), p=0.01).
Associations Among Haemoglobin Levels and Other Haemodynamic Characteristics
Associations among haemoglobin concentrations and other hemodynamic characteristics are given in Table 4. Haemoglobin levels were directly associated with systemic vascular resistance (p=0.05) and inversely related to stroke volume (p=0.05), cardiac output (p=0.03) and stroke work (p=0.01). Systemic vascular resistance was inversely associated with stroke volume (p<0.0001), cardiac output (p<0.0001), stroke work and left ventricular mass index (p=0.01). Stroke volume was directly associated with cardiac output (p<0.0001), stroke work (p=0.01) and left ventricular mass index. Cardiac output was directly associated with stroke work (p<0.0001) and left ventricular mass index (p=0.005). Stroke work was directly associated with left ventricular mass index (p=0.02). Additionally, cardiac output was directly associated with pressure pulsatility measures including peripheral pulse pressure (R=0.340, p=0.02), central pulse pressure (R=0.299, p=0.05) and forward wave pressure (R=0.377, p=0.01), and tended to be directly associated with central systolic pressure (R=0.292, p=0.06).
 |
Table 4 Bivariate Associations Among Haemoglobin Concentrations and Hemodynamic Variables |
Discussion
To our knowledge, this is the first study that simultaneously assessed the potential impact of haemoglobin concentrations on pulse wave velocity, wave reflection and pressure pulsatility as arterial function markers, as well as other hemodynamic characteristics in dialysis patients. The most striking and novel finding in this study was that haemoglobin concentrations were associated not only with arterial stiffness but also simultaneously with low pressure pulsatility measures including central systolic blood pressure, central and peripheral pulse pressure as well as forward wave pressure. In ROC curve analysis, the optimal haemoglobin concentration cut-off values in predicting arterial stiffness and increased central pulse pressure were remarkably similar at 10.95 g/dl and 10.85 g/dl respectively, and with clinically useful sensitivities, specificities and positive and negative predictive values. A haemoglobin value of >10.9 mg/dl was independently and strongly associated with both arterial stiffness (OR (95% CI) = 10.48 (1.57–70.08), p=0.02) and normal central pulse pressure (OR (95% CI) = 7.55 (1.58–36.03), p=0.01).
Anaemia is a highly prevalent comorbidity in dialysis patients.29 Anaemia in CKD is mediated by multiple factors including reduced erythropoietin synthesis and release, iron deficiency and chronic inflammation.30 Anaemia causes reduced viscosity and increased nitric oxide production and activation mediated vasodilatation and decreased peripheral vascular resistance.18,31,32 Nitric oxide additionally reduces arterial stiffness, this to a larger extent than peripheral vascular resistance.13 Haemoglobin is a potent nitric oxide scavenger.14 The use of nitric oxide donors comprises a potential therapeutic intervention for arterial stiffness.33 The vascular effects of anaemia or low haemoglobin result in reduced cardiac afterload, increased venous return, preload, left ventricular filling pressure and end-diastolic volume with consequent enhanced stroke volume and work. Increased stroke volume translates into enhanced cardiac output that is associated with enhanced pressure pulsatility.34,35 Anaemia together with arterial stiffness thereby ultimately engender left ventricular hypertrophy and heart failure in CKD.18,19 Our findings of a direct and concurrent inverse relationship of haemoglobin concentrations with arterial stiffness and pressure pulsatility measures, respectively, as well as the associations among haemoglobin levels and hemodynamic measures including systemic vascular resistance, stroke volume, stroke work, left ventricular mass index, cardiac output and pressure pulsatility parameters are each in keeping with these reported hemodynamic effects of anaemia in persons with and without CKD.
Our results are also in line with reported data in population and non-dialysis patient studies. The Korea National Health and Nutrition Examination Survey 2010–2012 revealed a relationship of anaemia with pulse pressure.36 In the Chronic Renal Insufficiency Cohort Ancillary Study,37 haemoglobin concentrations were inversely associated with peripheral pulse pressure. A direct association of haemoglobin concentrations with arterial stiffness has also been reported in two population studies15,16 and patients with hypertension.17
In contrast to our findings, Schwartz and colleagues38 previously reported in an inverse association between haemoglobin levels and aortic pulse wave velocity. The mean age (SD) age was 64.4 (15.8) years in the Schwarz study compared to 56.4 (13.3) years in ours. Notably, a high aortic pulse wave velocity strongly predicts overall and cardiovascular mortality in end-stage renal disease among patients <60 years but loses its prognostic value in older patients.39 The potential impact of age on haemoglobin-arterial function relationships in CKD merits further study. Wave reflection and pressure pulsatility were not explored in the Schwartz study.
Partial correction of severe anaemia targeting a haemoglobin level of 11.0 g/dl with erythropoietin stimulating agents in dialysis patients improves quality of life and reduces hospitalization and the need for transfusion.40 However, recent meta-analyses have documented an increased risk of all-cause mortality, stroke, hypertension and vascular access thrombosis11,12 when a normal haemoglobin level of ~13 g/dl is targeted with erythropoietin stimulating agents in CKD patients. The reason(s) why the optimal haemoglobin target as relates to CVD risk is lower in CKD compared to non-CKD individuals and the potential involved mechanisms remain largely unknown. Postulates include highly prevalent underlying atherosclerotic disease in CKD, increased viscosity and platelet aggregation, endothelial damage, vasoconstriction and increased peripheral vascular resistance.30 Moreover, given the design of recent trials, the effect of a haemoglobin level of 11.5 to 13 g/dl on the vasculature in CKD patients is currently unknown.9,10 Accordingly, the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guideline and a commentary on a recent meta-analysis state that targeting a haemoglobin level of 11.5 to 13 g/dl may still need to be considered in some CKD patients.9,10 It is in this context that our findings in ROC curve analysis are particularly relevant as they indicate that a haemoglobin target of 10.9 g/dl in dialysis patients is associated with optimal arterial function comprising of a lower frequency of both arterial stiffness and increased pressure pulsatility. Taken together, the optimal haemoglobin target in CKD may be determined by its effects on arteriosclerosis, which is a key mechanism of increased CVD in CKD.2,3 Notably, our results also suggest that the haemoglobin level reached is more important than the intervention used to reach the respective level.
Heart failure is associated with reduced pulse wave velocity and wave reflection.23 Additionally, angiotensin converting enzyme inhibitors and angiotensin receptor blockers can improve arterial function,24 and erythropoietin stimulating agents can impair nitric oxide production.25 When we adjusted for these characteristics in separate models and performed sensitivity analyses, the partial R values for the haemoglobin-pulse wave velocity and haemoglobin-pressure pulsatility relations were materially unaltered. However, in sensitivity analyses, haemoglobin concentrations were additionally associated with reflection magnitude among patients without heart failure as well as in those using erythropoietin stimulating agents. Reduced nitric oxide activity is also implicated in increased wave reflection.41 Interestingly, when we replaced mean arterial pressure by systemic vascular resistance in the respective models, the results were also materially unaltered. This suggests that potential effect of haemoglobin concentrations on reflection magnitude in dialysis patients is located centrally rather than at the peripheral or arteriolar level and is therefore unlikely to improve upon treatment with vasodilators.
Our study has limitations. Its design was cross-sectional, which precludes determining cause-effect relations. The numbers of patients, particularly in sensitivity analyses, were small. However, our main conclusions originated in comprehensively adjusted multivariable regression models. The strength of our study is that we performed a detailed evaluation of aortic function using SphygmoCor and other hemodynamic characteristics.
In conclusion, this study suggests that the optimal haemoglobin target in dialysis patients is ~11g/dl and determined by its differential and contrasting effects on arterial stiffness and pressure pulsatility.
Ethics Statement
This study was performed according to the Helsinki Declaration of 1975 as revised in 2013 and was approved by the University of the Witwatersrand Human (Medical) research Ethics Committee (protocol number: M15-08-43) in Johannesburg, South Africa. Written informed consent was obtained in each patient prior to participation.
Acknowledgment
We thank Ms Noluntu Dlongolo for revising the manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The study was supported by the South African National Research Foundation.
Disclosure
Patrick H Dessein reports receiving grants from South African National Research Foundation, during the conduct of the study. The authors declare no other potential conflicts of interest.
References
1. Moody WE, Edwards NC, Chue CD, Ferro CJ, Townsend JN. Arterial disease in chronic kidney disease. Heart. 2013;99(6):365–372. doi:10.1136/heartjnl-2012-302818
2. Zanoli L, Lentini P, Briet M, et al. Arterial stiffness in the heart of CKD. J Am Soc Nephrol. 2019;30(6):918–928. doi:10.1681/ASN.2019020117
3. London GM. Arterial stiffness in chronic kidney disease and end-stage renal disease. Blood Purif. 2018;45(1–3):154–158. doi:10.1159/000485146
4. Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103(7):987–992. doi:10.1161/01.CIR.103.7.987
5. Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82(4):388–400. doi:10.1038/ki.2012.131
6. Fernandez-Fresnedo G, Rodrigo E, de Francisco ALM, de Castro SS, Castaneda O, Arias M. Role of pulse pressure on cardiovascular risk in chronic kidney disease patients. J Am Soc Nephrol. 2006;17:S246–S249.
7. McIntyre NJ, Fluck RJ, McIntyre CW, Fakis A, Taal MW, Bochud M. Determinants of arterial stiffness in chronic kidney diseased stage 3. PLoS One. 2013;8(1):e55444. doi:10.1371/journal.pone.0055444
8. Major RW, Cheng MRI, Grant RA, et al. Cardiovascular disease risk factors in chronic kidney disease: a systematic review and meta-analysis. PLoS One. 2018;13:e0192895.
9. Wyatt CM, Drueke TB. Higher hemoglobin levels and quality of life in patients with advanced chronic kidney disease: no longer a moving target? Kidney Int. 2016;89(5):971–975. doi:10.1016/j.kint.2016.03.001
10. Kidney Disease: Improving. Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. 2012;2(Suppl):279–335.
11. Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369(9559):381–388. doi:10.1016/S0140-6736(07)60194-9
12. Palmer SC, Navaneethan SD, Craig JC, et al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Int Med. 2010;153(1):23–33. doi:10.7326/0003-4819-153-1-201007060-00252
13. Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of arterial stiffness. Hypertension. 2004;44(2):112–116. doi:10.1161/01.HYP.0000138068.03893.40
14. Donadee C, Raat NJH, Kanias T, Tejero J, Lee JS, Kelly EE. Nitric oxide scavenging by red cell microparticles and cell-free haemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465–476. doi:10.1161/CIRCULATIONAHA.110.008698
15. Zhang -Z-Z, Wang P, Kong X-L, Mao W-L, Cui M-Y. Association of haemoglobin with arterial stiffness evaluated by carotid-femoral pulse wave velocity among Chinese adults. Chronic Dis Transl Med. 2018;5(2):122–128.
16. Kawamoto R, Tabara Y, Kohara K, Miki T, Kusunoki T, Tatoh T. A slightly low haemoglobin level is beneficially associated with arterial stiffness in Japanese community-dwelling women. Clin Exp Hypertens. 2012;34(2):92–98. doi:10.3109/10641963.2011.618202
17. Chen H, Hua Q, Hou H. Association of haemoglobin with ambulatory arterial stiffness index in untreated essential hypertensive patients without anemia. Intern Med. 2011;50(22):2759–2765. doi:10.2169/internalmedicine.50.5832
18. Lullo LD, Gorini A, Russo D, Santoboni A, Ronco C. Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med. 2015;5(4):254–266. doi:10.1159/000435838
19. London GM, Gyerin AP, Marchais SJ, et al. Cardiac and arterial interactions in end-stage renal disease. Kidney Int. 1996;50(2):600–608. doi:10.1038/ki.1996.355
20. Gunter S, Robinson C, Woodiwiss AJ, et al. Arterial wave reflection and subclinical atherosclerosis in rheumatoid arthritis. Clin Exp Rheumatol. 2018;36:412–420.
21. Sahn DJ, DeMaria A, Kisslo J, Weyman A, American Society of echocardiography. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58(6):1072–1083. doi:10.1161/01.CIR.58.6.1072
22. Townsend RR. Arterial stiffness and chronic kidney disease: lessons from the chronic renal insufficiency cohort study. Curr Opin Nephrol Hypertens. 2015;24:47–53.
23. Feola M, Testa M, Ferreri C, Rosso GL, Rossi A, Ruocco G. The analysis of arterial stiffness in heart failure patients in comparison with healthy subjects with cardiovascular risk factors. J Clin Med. 2019;8(10):1721. doi:10.3390/jcm8101721
24. Liu M, Li G-L, Li Y, Wang J-G. Effects of various antihypertensive drugs on arterial stiffness and wave reflections. Pulse. 2013;1(2):97–107. doi:10.1159/000354108
25. Scalera F, Kielstein JT, Martens-Lobenhoffer J, Postel SC, Tager M, Bode-Borger SM. Erythropoietin increases asymmetric dimethylarginine in endothelial cells: role of dimethylarginine dimethylaminohydrolase. Am J Soc Nephrol. 2005;16(4):892–898. doi:10.1681/ASN.2004090735
26. Esh H, Agabiti E, France MA, et al. 2018 ESC/ESH guidelines for the management of hypertension. Eur Heart J. 2018;39:3021–3104.
27. Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448. doi:10.1097/HJH.0b013e32834fa8b0
28. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of cardiology (ESC). J Hypertens. 2013;31:1281–1357.
29. Fishbane S, Spinowitz B. Update on anemia in ESRD and earlier stages of CKD: core curriculum 2018. Am J Kidney Dis. 2018;71(3):423–435. doi:10.1053/j.ajkd.2017.09.026
30. Fishbane S, Besarab A. Mechanisms of increased mortality risk with erythropoietin treatment to higher haemoglobin targets. Clin J Am Soc Nephrol. 2007;2(6):1274–1282. doi:10.2215/CJN.02380607
31. Ni Z, Morcos S, Vaziri ND. Up-regulation of renal and vascular nitric oxide synthase in iron-deficiency anaemia. Kidney Int. 1997;52(1):195–201. doi:10.1038/ki.1997.319
32. Metivier F, Marchais SJ, Guerin AP, Pannier B, London GM. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant. 2000;15(suppl_3):14–18. doi:10.1093/oxfordjournals.ndt.a027970
33. Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease. State-of-the-art review. J Am Coll Cardiol. 2019;9(9):1237–1263. doi:10.1016/j.jacc.2019.07.012
34. Fazelli N, Hahan J-O. Estimation of cardiac output and peripheral resistance using square-wave-approximated aortic flow signal. Front Physiol. 2012;3:298.
35. Papaioannou TG, Vardoulis O, Stergiopulos N. The systolic volume balance method for noninvasive estimation of cardiac output based on pressure wave analysis. Am J Physiol Heart Circ Physiol. 2012;302(10):H2064–H2073. doi:10.1152/ajpheart.00052.2012
36. Yoon H, Lee JH, Kim GS, et al. The relationship between anaemia and pulse pressure and hypertension: the Korea national health and nutrition examination survey 2010–2012. Clin Exp Hypertens. 2018;40(7):650–655. doi:10.1080/10641963.2017.1416123
37. Townsend RR, Chirinos JA, Parsa A, et al. Central pulse pressure in chronic kidney disease: a CRIC ancillary study. Hypertension. 2010;56(3):518–524. doi:10.1161/HYPERTENSIONAHA.110.153924
38. Schwartz CP, Koppelstaetter C, Amann E, Mayer G. Impact of anemia on aortic pulse wave velocity in hemodialysis patients. Kidney Blood Press Res. 2009;32:210–216.
39. Ferreira JP, Girerd N, Pannier B, Rossignol P, London GM. High pulse wave velocity defines a very high risk cohort of dialysis patients under age 60. Am J Nephrol. 2017;45(1):72–81. doi:10.1159/000453338
40. Jones M, Ibels L, Schenkel B, Zagari M. Impact of epoetin alfa on clinical end points in patients with chronic renal failure. Kidney Int. 2004;65(3):757–767. doi:10.1111/j.1523-1755.2004.00450.x
41. Weber T, Maas R, Auer J, et al. Arterial wave reflections and determinants of endothelial function. A hypothesis based on peripheral mode of action. Am J Hypertens. 2007;20(3):256–262. doi:10.1016/j.amjhyper.2006.09.009
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.