Back to Journals » Drug Design, Development and Therapy » Volume 16
The Novel Compounds with Biological Activity Derived from Soil Fungi in the Past Decade
Authors Zhang D, Li S, Fan M , Zhao C
Received 20 June 2022
Accepted for publication 17 September 2022
Published 12 October 2022 Volume 2022:16 Pages 3493—3555
DOI https://doi.org/10.2147/DDDT.S377921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Danyu Zhang,1 Shoujie Li,1 Mohan Fan,2 Changqi Zhao1
1Gene Engineering and Biotechnology Beijing Key Laboratory, College of Life Science, Beijing Normal University, Beijing, People’s Republic of China; 2Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
Correspondence: Changqi Zhao, Tel +86-5880-5046, Email [email protected]
Abstract: The secondary metabolites isolated from soil fungi have received more and more attention, especially new compounds that exhibited good biological activities. In this review, a total of 546 new compounds are included in the relevant literature since 2011. The new compounds are isolated from soil fungi, We divided these compounds into seven categories, including alkaloids, terpenoids, steroids, ketones, phenylpropanoids, quinones, esters, lactones, etc. In addition, the biological activities and structure–activity relationships of these compounds have also been fully discussed. The activities of these compounds are roughly divided into eight categories, including anticancer activity, antimicrobial activity, anti-inflammatory activity, antioxidant activity, antiviral activity, antimalarial activity, immunosuppressive activity and other activities. Since natural products are an important source of new drugs, this review may have a positive guiding effect on drug screening.
Keywords: soil fungi, new compounds, chemical structure, biological activity
Introduction
During the period of 1981 to 2019, almost 60% of the clinically approved drugs were derived directly or indirectly from natural products.1 Nature can breed many species, including prokaryotes and eukaryotes. Among them, fungi play an important role in the ecosystems of plants, animals and humans.2 Soil is an excellent natural habitat for microorganisms and has a large number of microorganisms.3 Soil represents a very heterogeneous environment for its microbiota. Among the soil inhabitants, bacteria and fungi are important organisms as they are involved in key biogeochemical cycling processes.4 Terrestrial microorganisms have long been regarded as profile producers of specialised metabolites with unique structures or new modes of action, and particularly in recent years, the discovery of new active natural products from soil-derived microorganisms has increased rapidly, contributing significantly to drug development.5 Since Alexander Fleming discovered penicillin in 1928, fungi have become a considerable resource for novel compounds and new drugs.6 The secondary metabolites of fungi are a rich source of small molecule compound libraries, and some metabolites have significant biological activities,7 such as the antibacterial agent penicillins, immunosuppressive drug cyclosporin A, antifungal drug echinocandin B, and cholesterol-lowering agent lovastatin.8 Fungi are an ideal source for obtaining novel skeletons through large-scale culture: compared with plants, fungi can proliferate rapidly from small amounts of spores to a mass of branching hyphaes, which are more environmentally friendly than collecting plant materials; compared with bacteria, fungi can be cultured in a solid medium for a longer time.9 Therefore, soil-derived fungi are receiving continuous attention as the source of biologically active secondary metabolites. Since 2011, a large number of new compounds have been obtained from soil-derived fungi, but few people have summarized and analyzed these compounds. This review summarizes the compounds derived from soil fungi from 2011 to 2022, and classifies their structures and activities. Importantly, we made statistical analysis of these new compounds, readers can clearly understand which genus of soil fungi has great potential to produce new compounds. In addition, through the analysis of activity data, readers can clearly understand which kind of structural compounds account for the majority of compounds with certain activities, which kind of structural compounds account for the majority of compounds with certain activity. By summarizing the structure and activity of the new compound, a total of 546 compounds were isolated from the metabolites of soil fungi during the decade of 2011–2022, mainly including alkaloids, terpenes, steroids, ketones, phenylpropanoids, quinones, esters and lactones, of which ketones (31.9%), terpenoids and steroids (22.2%) are the main structural types. Among the 546 compounds, 279 compounds (approximately 51%) have good biological activities, including anticancer, antimicrobial, anti-inflammatory, antioxidant, antiviral, antimalaria, and immunosuppressive activities. Among them, anticancer and antimicrobial activities accounted for the highest proportion (Figure 1).
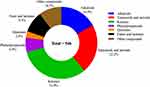 |
Figure 1 The percentage of various compounds. |
In the author’s personal view, this review can provide a positive guidance for the screening of new drugs. This is also the significance of writing this review. In addition, we also analyzed the habitat sources of these soil fungi, which provides some directions for researchers who want to obtain new compounds from soil fungi from special habitats.
Structure Types of New Compounds Derived from Soil Fungi
Alkaloids
Indole alkaloids
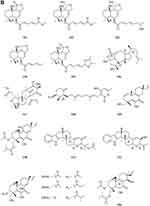
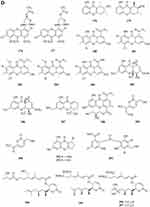
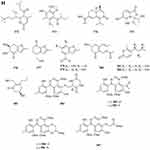
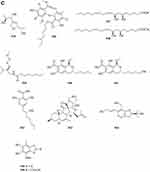
A quinazolinone-containing indole derivative, named pseudofischerine 1 (Figure 2A), was isolated from the fungus Neosartorya pseudofischeri S.W. Peterson.10 Two new compounds, named peneciraistins E 2 and F 3 (Figure 2A), were isolated from saline soil-derived fungus Penicillium raistrickii. Compounds 2 and 3 are the first naturally occurring 3-indoleformic acid derivatives.11 A new indole alkaloid named exopisiod 4 (Figure 2A) was obtained from the fermentation products of Exophiala pisciphila PHF-9.12 Waikialoid A 5 (Figure 2A) was isolated from Aspergillus sp.13 Effusin A 6 and dihydrocryptoechinulin D 7 (Figure 2A), two new spiropolyketide-diketopiperazines were isolated from the fungus Aspergillus effuses H1-1. Effusin A 6 possessed an unprecedented 3′,3a′,5′,6′-tetrahydrospiro[piperazine-2,2′-pyrano[2,3,4-de]chromene] skeleton.14 One new prenylated indole diketopiperazine alkaloid, named dihydroneochinulin B 8 (Figure 2A), was isolated from the mangrove rhizosphere soil-derived fungus, Aspergillus effuses H1-1.15 A new aszonalenin analogue 9c (Figure 2A) was isolated from the culture of the soil fungus Neosartorya fischeri (KUFC 6344).16 Six new indole-diterpenoids 10–15 (Figure 2A and B) were isolated from the fermentation broth of an aciduric fungal strain Penicillium camemberti OUCMDZ-1492 grown at pH 5.0.17 Asperdiazapinones A-F 16–21 (Figure 2B) were isolated from the mycelial extract of the soil fungus Aspergillus sp. PSU-RSPG185.18 Mangrovamides A-C 22–24 (Figure 2B), featured a bicyclo [2.2.2] diazaoctane core and possessed a novel γ-methyl proline and isoprene derived dimethyl γ-pyrone functionalities hitherto unknown among the family of paraherquamides, were isolated from the fungus Penicillium sp. SYFz-1.19 Rubrumazines A-C 25–27 (Figure 2B) were isolated and identified from a culture extract of Eurotium rubrum MA-150, a fungus obtained from mangrove-derived rhizospheric soil collected from the Andaman Sea coastline, Thailand.20 Three new indolyl diketopiperazine derivatives, penillines A 28 and B 30 (Figure 2B), isopenilline A 29 (Figure 2B), were isolated from the Antarctic soil-derived fungus Penicillium sp. SCSIO 05705.21 Cultivation and fractionation of secondary metabolites from Aspergillus kumbius revealed a unique chemotype comprising three new bis-indolyl benzenoids, kumbicins A-C 31–33 (Figure 2B and C).22 Two new bisindolylbenzenoid alkaloids asterriquinols E 34 and F 35 (Figure 2C) were isolated from the fermentation products of the fungus Aspergillus sp. CBS-P-2.23 Cyclopiamines C 36 and D 37 (Figure 2C) were obtained from Penicillium sp. CML 3020, a fungus sourced from an Atlantic Forest soil sample.24 Tolypocladins A-J 38–47 (Figure 2C and D) have been isolated from a culture of a mine-soil-derived fungus, Tolypocladium sp. XL115.25 Two novel cytochalasan alkaloids, chaetomadrasins A 48 and B 49 (Figure 2D), were isolated from the solid-state fermented culture of desert soil-derived Chaetomium madrasense 375.26 Paraherquamide J 50 (Figure 2D) was isolated from the fermentation product of the fungus Penicillium janthinellum HK1-6.27 Two new compounds tryptoquivalines W 51 and X 52 (Figure 2D) were isolated from a Hawaiian soil fungal strain Aspergillus terreus FS107.28 Three new cytochalasins Z24, Z25, Z26 (53–55, resp.) (Figure 2D) were isolated from the fungus Eutypella sp. D-1 which was isolated from the soil of high latitude of the Arctic.29 Purification of an extract from the broth of the soil fungus Aspergillus sp. PSU-RSPG185 resulted in the isolation of one new cytochalasin, aspergilluchalasin 56 (Figure 2D).30 Two new alkaloids, iizukines C 57 and D 58 (Figure 2D), were isolated from the culture of Aspergillus iizukae.31 Chemical screening of culture medium from the soil fungus Stachybotrys sp. resulted in the isolation of the three new phenylspirodrimanes MBJ-0030 59 (Figure 2D), MBJ-0031 60 and MBJ-0032 61 (Figure 2D).32 Pycnidiophorones A-D 62–65 (Figure 2E) were isolated from cultures of the wetland-soil-derived fungus Pycnidiophora dispersa.33 Clonorosins A 66 and B 67 (Figure 2E), two novel indole alkaloids featuring unprecedented 6/5/6/6/5 and 6/5/5 cores, were isolated from the soil-derived fungus Clonostachys rosea YRS-06.34
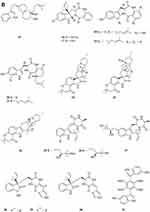
Figure 2 Continued. Figure 2 Continued. Figure 2 Continued. Figure 2 Continued. Figure 2 Continued. Figure 2 Alkaloids 1–92. (A) Alkaloids 1–14. (B) Alkaloids 15–31. (C) Alkaloids 32–46. (D) Alkaloids 47–61. (E) Alkaloids 62–79. (F) Alkaloids 80–92.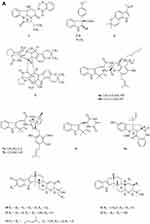
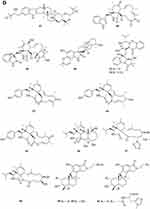
Other Alkaloids
Gymnastatins T-Y 68–73 (Figure 2E) and dankastatin D 74 (Figure 2E), were isolated from the soil fungus Gymnascella dankaliensis through fermentation on solid rice medium following addition of NaBr.35 Two new threonine-containing metabolites, N-[4-hydroxy-3-prenyl-benzoyl]-L-threonine 75 (Figure 2E) and N-[2,2-dimethyl-2H-chromene- 6-carbonyl]-L-threonine 76 (Figure 2E), were isolated from the fermentation broth of the soil fungus Curvularia inaequalis strain HS-FG-257.36 A new isoquinolinone alkaloid, (5S)-3,4,5,7-tetramethyl-5,8-dihydroxyl-6(5H)- isoquinolinone 77 (Figure 2E) was isolated from Penicillium sp. H9318.37 Compound 78 (Figure 2E), named 3,8-Diacetyl-4-(3-methoxy-4,5-methylenedioxy)benzyl-7-phenyl-6-oxa-3,8-diazabicyclo[3.2.1]octane, was isolated from the fungus Neosartorya pseudofischeri S.W. Peterson.10 In search for antibiotics from soil and endophytic fungi, the secondary metabolites of Fusarium avenaceum SF-1502. An alkaloid, fusaravenin 79 (Figure 2E), representing a new naphthoisoxazole formic acid connected with a morpholino carbon frame, the first example of a natural naphthoisoxazole type zwitter-ionic alkaloid, was characterized.38 (–) Benzomalvins E 80 (Figure 2F) was isolated from solid cultures of a interrhizospheric fungus Penicillium sp. SYPF 8411.39 A new tryptoquivaline analog, tryptoquivaline V 81 (Figure 2F) and a new brasiliamide analog, brasiliamide G 82 (Figure 2F), were isolated from the fungus Neosartorya pseudofischeri.40 A new compound, talarodone A 83 (Figure 2F) in co-culture of Talaromyces pinophilus and Paraphaeosphaeria sp. isolated from soil collected in Miyazaki Prefecture, Japan.41 One new pyrrolidine derivative, asperidine A 84 (Figure 2F), and two new piperidine derivatives, asperidines B 85 and C 86 (Figure 2F), were isolated from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178. Compound 86 possessed an unprecedented 7-oxa-1-azabicyclo[3.2.1]octane skeleton with four chiral centers.42 A new alkaloid, named iizukine E 87 (Figure 2F) was isolated from the culture of Aspergillus iizukae.31 In order to investigate bioactive metabolites from aciduric fungi, Aspergillus sp. OUCMDZ-1914 was isolated from the mangrove soil in Wenchang Hainan, China. The strain was fermented under low pH and the products were extracted and the extract was purified by column chromatography over silica gel, Sephadex LH-20 and semi-preparative HPLC. The structures were identified by means of NMR, MS, UV, IR and X-ray single crystal diffraction. As a result, a new compound, methyl (2Z,3E,5E,7E,9E)-4,10-dimethyl-11-(2,5-dioxopyrrolidin-3-yl)-2-(2-hydroxyethylidene)-11-oxoundeca-3,5,7,9-tetraenoate 88 (Figure 2F) was obtained.43 A new γ-lactam, virgaricin B 89 (Figure 2F), was isolated from a fermentation broth of the fungus Virgaria boninensis FKI-4958.44 From this active Paecilomyces lilacinus extract, a novel pyridone alkaloid, named Paecilomide 90 (Figure 2F), was isolated and its structure was elucidated by modern nuclear magnetic resonance techniques and mass spectrometric analyses.45 The soil-derived fungus Clonostachys rosea. Fermentation of the fungus on white beans instead of rice afforded a new γ-lactam 91 (Figure 2F), named Clonostalactam.46 A new nitrogen-containing compound, trichothioneic acid 92 (Figure 2F), was discovered from the metabolites of fungal strain Trichoderma virens FKI-7573.47
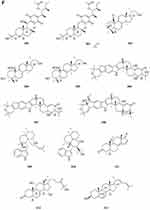
Terpenoids and Steroids
Terpenoids
Sesquiterpene
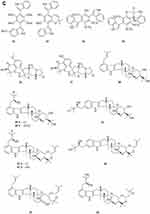
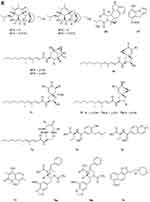
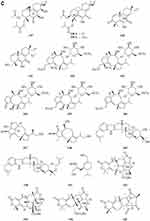
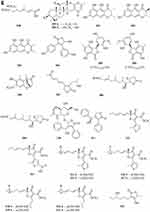
Two new drimane sesquiterpenoids, fudecadiones A 93 and B 94 (Figure 3A), were isolated from the soil fungus Penicillium sp. BCC 17468.48 Two new trichothecenes, named 8α-hydroxyroridin H 95 and myrothecin A 96 (Figure 3A), were isolated from the fermentation broth of a halotolerant fungus Myrothecium sp. GS-17, which was separated from the soil sample of a salina. Among them, compound 96 represents a class of rare trichothecenes with the linkage from C-13 to C-10, missing the epoxide group normally observed at C-12, C-13.49 Penicibilaenes A 97 and B 98 (Figure 3A) were characterized from Penicillium bilaiae MA-267, a fungus obtained from the rhizospheric soil of the mangrove plant Lumnitzera racemosa.50 Two eremophilane sesquiterpenes, penicilleremophilanes A 99 and B 100 (Figure 3A) were isolated from the soil fungus Penicillium copticola PSURSPG138.51 A new sesquiterpenoid derivative, named aspergiketone 101 (Figure 3A), was isolated from the coastal saline soil fungus Aspergillus fumigatus.52 Ochraceopones A-E 102–106 (Figure 3A) were isolated from an Antarctic soil-derived fungus, Aspergillus ochraceopetaliformis SCSIO 05702. Ochraceopones A-D 102–105 are the first examples of α-pyrone merosesquiterpenoids possessing a linear tetracyclic carbon skeleton.53 Four new 12.8-Eudesmanolides 107–110 (Figure 3A) were isolated from a mangrove rhizosphere-derived fungus Eutypella sp. 1–15.54 A new acorane sesquiterpene, 3β-hydroxy-β-acorenol 111 (Figure 3A), has been discovered from the green Chinese onion-derived fungus F.proliferatum AF-04. and a sesquiterpenoid 112 (Figure 3A), cyclonerotriol B, was isolated from the extracts of the soil fungus F. avenaceum SF-1502.38 Three new sesquiterpenes Trichodermapenes A-C 113–115 (Figure 3A) were isolated from the soil-derived fungus Trichoderma reesei PSU-SPSF013.55 Chemical investigation of the Dictyosporium digitatum fungus resulted in the identification of three undescribed compounds 116–118 (Figure 3A). which were named Dictyosporin A 116, Dictyosporin B 117, Dictyosporin C 118 respectively.56 Two new sesquiterpenes, trichocitrinovirenes A 119 and B 120 (Figure 3A), were isolated from the soil-derived fungus Trichoderma citrinoviride PSU-SPSF346.57 Five new sesquiterpenoids (121–125) (Figure 3B), one new ophiobolin sesterterpenoid (126) (Figure 3B), and one new 3.5-dimethylorsellinic acid (DMOA)-based meroterpenoid (127) (Figure 3B), were isolated and characterized from fungus Aspergillus calidoustus, which was separated from the wetland soil collected at Dianchi Lake, Yunnan Province.58
Figure 3 Continued. Figure 3 Continued. Figure 3 Continued. Figure 3 Continued. Figure 3 Continued. Figure 3 Terpenoids and steroids 93–213. (A) Terpenoids 93–120. (B) Terpenoids 121–136. (C) Terpenoids 137–155. (D) Terpenoids 156–179. (E) Terpenoids 180–200. (F) Terpenoids and steroids 201–213.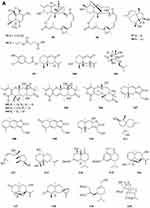
Diterpene
A new meroditerpene sartorypyrone A 128 (Figure 3B) was isolated from the culture of the soil fungus Neosartorya fischeri (KUFC 6344). Interestingly, sartorypyrone A 128, which possesses a monocyclic diterpene core.16 Libertellenones G 129 and H 130 (Figure 3B) were isolated from the fungus Eutypella sp. D-1 isolated from the soil of high latitude of Arctic.59 Compounds 131 and 132 (Figure 3B) were isolated from the fermentation broth and the mycelia of the soil fungus Penicillium sp. CM-7.60 Libertellenones O-S 133–137 (Figure 3B and C), eutypellenones A 138 and B 139, libertellenones M 140 and N 141 (Figure 3C), were isolated from the culture of Eutypella sp. D-1 obtained from high-latitude soil of the Arctic. Structurally, compounds 133–137 possess a cyclopropyl-fused pimarane diterpene moiety, whereas compounds 138 and 139 share an unusual cyclobutyl-fused pimarane diterpene skeleton.61,62 Five new diterpenoid glycosides, dongtingnoids A-E 142–146 (Figure 3C), two new diterpenoid aglycones, dongtingnoids F 147 and G 148 (Figure 3C), were isolated from the fungus Penicillium sp. DT10, which was derived from wetland soil from Dongting Lake.63 Tolypocladins K 149 and L 150 (Figure 3C), were isolated from the solid fermentation culture of a mine soil-derived fungus Tolypocladium sp. XL115.64
Other Terpenoids
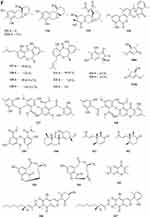
One new monoterpene named Trichodermanene 151 (Figure 3C) was isolated from the soil-derived fungus Trichoderma reesei PSU-SPSF013.55 Three new meroterpenoid derivatives, 4.25-dehydrominiolutelide B 152 (Figure 3C), 4,25-dehydro-22- deoxyminiolutelide B 153 and isominiolutelide A 154 (Figure 3C), were isolated from the shaken culture of the fungus Penicillium sp. MA-37.65 Purpurogenolides A-E 155–159 (Figure 3C and D), were isolated from the solid substrate fermentation cultures of the fungus Penicillium purpurogenum MHz 111.66 A search for cytotoxic agents from cultures of the Penicillium sp., isolated from the rhizosphere soil of Penicillium sp., led to the isolation of four new hybrid polyketide-terpenoid metabolites 160–163 (Figure 3D).67 Terretonin M 164 (Figure 3D), a new highly oxygenated tetracyclic meroterpenoid, was isolated from the thermophilic fungus Aspergillus terreus TM8.68 Nine novel polyketide−terpenoid hybrids 165–173 (Figure 3D), characterized by a 1-alkylated-3,5-dihydroxyphenyl derivative coupled with a modified farnesyl pyrophosphate (FPP) unit, were isolated from a soil-derived fungus Bipolaris zeicola. Compound 173 represents the first example of meroterpenoid having an unusual thiazol-2(3H)-one moiety.69 Four dimeric acremines, bisacremines A-D 175–178 (Figure 3D), with a novel carbon skeleton and a new monomer, acremine T 174 (Figure 3D), were obtained from cultures of the soil-derived fungus Acremonium persicinum SC0105.5 Three dimeric acremines, bisacremines E-G 179–181 (Figure 3D and E), with an unusual carbon skeleton were isolated from cultures of the soil-derived fungus Acremonium persicinum SC0105.70 By employing a large-scale culture approach, five undescribed compounds, namely asperanstinoids A–E 182–186 (Figure 3E), were obtained from fungus Aspergillus calidoustus, which was isolated from the wetland soil collected at Dianchi Lake, Yunnan Province.71 Ten new meroterpenoids, bipolaquinones A−J 187–196 (Figure 3E), were isolated and identified from the fermented rice cultures of a soil-derived fungus, Bipolaris zeicola.72 Six new meroterpenoids, bipolarinoids A—F 197–202 (Figure 3E and F), were isolated from a soil-derived fungus Bipolaris zeicola.73 Three nortriterpenoids aspergorakhins B–D 203–205 (Figure 3F) were obtained from Aspergillus gorakhpurensis F07ZB1707, which was fermented by a salt-containing medium.6 Encindolenes D-H 206–210 (Figure 3F), were isolated from the fungus Penicillium sp. HFF16 from the rhizosphere soil of Cynanchum bungei Decne.74 Figure 4 Continued. Figure 4 Continued. Figure 4 Continued. Figure 4 Continued. Figure 4 Continued. Figure 4 Continued. Figure 4 Continued. Figure 4 Ketones 214–387. (A) Ketones 214–238. (B) Ketones 239–256. (C) Ketones 257–275. (D) Ketones 276–297. (E) Ketones 298–322. (F) Ketones 323–347. (G) Ketones 348–371. (H) Ketones 372–387.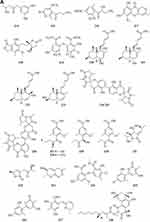
Steroids
Compound 211 (Figure 3F) was isolated from a soil fungus Curvularia borreriae (Pleosporaceae) strain HS-FG-237.75 One new steroid 212 (Figure 3F) was discovered in the extract of a soil-derived fungus Aspergillus flavus JDW-1.76 Aspergorakhin A 213 (Figure 3F) was obtained from the extract of soil-derived fungus Aspergillus gorakhpurensis F07ZB1707.6
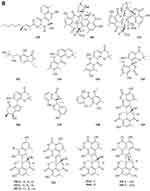
Ketones
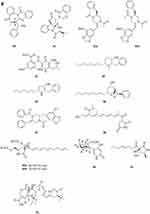
Polyketides
A new polyketide compound 214 (Figure 4A) was characterized from the ethyl acetate extract of a soil-derived fungal strain, Exophiala pisciphila PHF-9.77 Pyrenocine J 215 and pyrenochaetic acid D 216 (Figure 4A) were obtained from the fermentation broth of Curvularia affinis strain HS-FG-196.78 Phytochemical investigation of the soil microfungus Eupenicillium parvum led to the isolation of two new compounds: a chromone derivative euparvione 217 and a new mycophenolic derivative euparvilactone 218 (Figure 4A).79 A new polyketide penicillither 219 (Figure 4A), was isolated from the soil fungus Penicillium sp. PSU-RSPG9.80 Tanzawaic acids I-L 220–223 (Figure 4A), were isolated from the culture filtrates of the fungal strain Penicillium sp. IBWF104-06 isolated from a soil sample.81 Four new pyrenochaetic acid derivatives pyrenochaetic acids E-H 227–230 (Figure 4A), three new secalonic acid analogues Blennolides H-J 224–226 (Figure 4A) were purified from an Alternaria sp. isolate obtained from a Hawaiian soil sample.82 Three new polyketides, namely, asperochrins A-C 231–233 (Figure 4A), were isolated from Aspergillus ochraceus MA-15, a fungus obtained from the rhizospheric soil of marine mangrove plant Bruguiera gymnorrhiza.83 A new diphenyl derivative, named iizukine A 234 (Figure 4A) was isolated from coastal saline soil-derived fungus Aspergillus iizukae.84 Three new pyran rings containing polyketides, penicipyrans A-B 235–236 (Figure 4A), and penicipyran E 237 (Figure 4A) were isolated from the saline soil-derived Penicillium raistrickii.85 Cultivation of the mangrove rhizosphere soil-derived fungus Penicillium janthinellum HK1-6 with NaBr led to the isolation of two new tricyclic polyketides, penijanthinones A 238 (Figure 4A) and B 239 (Figure 4B).86 Two novel oxaphenalenone dimers, talaroketals A 240 and B 241 (Figure 4B), were isolated from the soil fungus Talaromyces stipitatus. Compound 240 features a rare benzannulated 5.6-spiroketal ring system within the dimeric bis(oxaphenalenone) skeleton, while the parent compound 241 harbors a fused bicyclic furano-pyran moiety.87 Aspergorakhins E–J, L (242–247, 248) (Figure 4B) were obtained from the extract of soil-derived fungus Aspergillus gorakhpurensis F07ZB1707.6 Six new polyketide-derived oxaphenalenone dimers, talaromycesone C 249 and macrosporusones A-E 250–254 (Figure 4B), were isolated from the mycelium of the fungus Talaromyces macrosporus KKU-1NK8.88 Four new oxaphenalenone dimers (bacillisporins I 255 and J 256 (Figure 4B), duclauxamides B 257 and C 258 (Figure 4C)) were isolated from the soil fungus Talaromyces bacillisporus BCC17645. Duclauxamides B and C were the rare N-containing oxaphenalenone dimers isolated from this fungus.89 New polyketide-derived oligophenalenone dimers, 9a-epi-bacillisporin E 259 and bacillisporins F-H 260–263 (Figure 4C), were isolated from the fungus Talaromyces stipitatus.90 A new chromanone derivative Aspergone 264 (Figure 4C) was isolated from Aspergillus sp. SCSIO41002.91 A novel compound 7-methoxy-2,2-dimethyl-4-octa-4′,6′-dienyl-2H-napthalene-1-one 265 (Figure 4C) was isolated from Penicillium sp.92 Aspereusins C-E 266–268 (Figure 4C) were obtained from the fermentation product of Aspergillus terreus YIM PH30711.93
Flavones
One new xanthone, penicillixanthone 269 (Figure 4C), was isolated from the soil fungus Penicillium sp. PSU-RSPG9.80 Castochrin 270 (Figure 4C), a new dimer of sulochrin linked by thioether bonds and a new secalonic acid analogues (–)-Blennolide G 271 (Figure 4C) were purified from an Alternaria sp. isolate obtained from a Hawaiian soil sample.82 One new xanthone, penicillone C 272 (Figure 4C), is isolated from the soil fungus Penicillium citrinum PSU-RSPG95.94 Compound 273 (Figure 4C) was found from the fermentation product of Penicillium sp., HT-28 by various spectroscopic techniques.95 In order to investigate new bioactive compounds, a piece of fungi Aspergillus aculeatus was obtained from the Jinyun mountain soil in Chongqing, China. A new compound 2-(2’,4’,6’-Trihydroxyphenyl)-(7-hydroxy-5-methyl) chromone 274 (Figure 4C) was obtained.96 A new xanthone (penicillanthone, 275) (Figure 4C) was isolated from the soil-derived fungus Penicillium aculeatum PSU-RSPG105.97 Two new sterigmatocystin derivatives, oxisterigmatocystins E 276 and F 277 (Figure 4D), were isolated from the fungus Botryotrichum piluliferum.98 Penixanthones A 278 and B 279 (Figure 4D), were isolated from the ethyl acetate extract of a culture of the fungus Penicillium sp. SYFz-1.99 Five new 280–284 (Figure 4D) xanthones were isolated from the coastal saline soil-derived Aspergillus iizukae by application of an OSMAC (one strain many compounds) approach.100 Three new blennolide derivatives, blennolides L-N 285–287 (Figure 4D), were isolated from the soil-derived fungus Trichoderma asperellum PSU-PSF14.101 A new metabolite, wentixanthone A 288 (Figure 4D), was obtained from the fungus Aspergillus wentii, isolated from soil of the hypersaline lake El Hamra in Wadi El-Natrun, Egypt.102
Other ketones
Fusarpyrones A 289 and B 290 (Figure 4D), two new pyrone derivatives, were isolated from the soil fungus Fusarium solani PSU-RSPG37.103 Two new benzopyranones, named coniochaetones E 291 and F 292 (Figure 4D), and one new benzophenone, penicillanone 293 (Figure 4D), are isolated from the soil fungus Penicillium citrinum PSU-RSPG95.94 Five new secondary metabolites 294–297 (Figure 4D) and 298 (Figure 4E) of Cephalotrichum microsporum were isolated, which were described, respectively, as 8′-O-(3R-Hydroxy-butyryl)-rasfonin 294, Cemironin A 295, Cemironin B 296, Cemironin C 297, 4-Methyl-8,10-dihydroxy-caprylic acid 298.104 Two new meroditerpene pyrones, chevalone F 299 and 11-hydroxychevalone E 300 (Figure 4E), were isolated from the fungus Neosartorya pseudofischeri.40 Peninaphones A-C 301–303 (Figure 4E), were isolated from mangrove rhizosphere soil-derived fungus Penicillium sp. HK1-22.105 The fungus, Aspergillus nidulans BF0142, was isolated from hot spring-derived soil collected at Hell Valley in Noboribetsu, Hokkaido, Japan. A new furanone compound designated helvafuranone 304 (Figure 4E) was isolated from a culture broth of A. nidulans BF0142.106 Enantiomers of a 2-benzofuran-1(3H)-one derivative [(–)-1 and (+)-1] described as (±)-europhenol A 305 (Figure 4E), was isolated and identified from the culture extract of Eurotium rubrum MA–150, a fungus obtained from the mangrove-derived rhizospheric soil.107 Chromatographic separation of the broth extract of the soil-derived fungus Aspergillus sclerotiorum PSURSPG178 resulted in isolation of a furanone derivative, aspersclerotiorone E 306 (Figure 4E).108 Three new secondary metabolites identified as harzianone 307 (Figure 4E), harzianol 308 (Figure 4E), harzianol acid 309 (Figure 4E) were isolated from a culture of Cephalotrichum microsporum.109 Two new aromatic butenolides, gotjawaside 310 and gotjawalide 311 (Figure 4E), possessing the 4-benzyl-3-phenylbutenolide motif, were isolated from a soil ascomycete Auxarthron sp. KCB15F070.110 Ten novel furancarboxylic acids including four pairs of epimers (314, 315; 316, 317; 318, 319; 320, 321) (Figure 4E), were isolated from the fermentation of the soil-derived fungus Penicillium sp. sb62. Compounds 312–317 represent the first class of natural furancarboxylic acids featuring a thiophene moiety.111 We present a new furanone derivative from a white, woolly and fast-growing soil fungus, Penicillium sp. FG9RK, isolated from a soil sample collected from Agulu in Anambra state, Nigeria. The compound was identified as a new natural product, R-hexitronic acid 322 (Figure 4E).112 Three new compounds, named peneciraistins A-C 323–325 (Figure 4F), were isolated from saline soil-derived fungus Penicillium raistrickii. Among them, compounds 323 and 324 are rare benzannulate 6.6-spitoketals.11 Sporulosol 326 (Figure 4F), a new ketal, has been isolated from the liquid fermentation cultures of a wetland-soil-derived fungus, Paraconiothyrium sporulosum.113 Three pairs of new isopentenyl dibenzo[b, e]oxepinone enantiomers, (+)-(5S)-arugosin K 327, (-)-(5R)-arugosin K 328, (+)-(5S)-arugosin L 329, (-)-(5R)-arugosin L 330, (+)-(5S)-arugosin M 331, (-)-(5R)-arugosin M 332, and a new isopentenyl dibenzo[b, e]oxepinone, arugosin N 333 (Figure 4F), were isolated from a wetland soil-derived fungus Talaromyces flavus.114 Two new indanones, asperunguisones A 334 and B 335 (Figure 4F), were isolated from the soil-derived fungus Aspergillus unguis PSU-RSPG204.115 A pair of enantiomeric cyclopentenones (336a new and 336b new natural) were isolated from Aspergillus sclerotiorum.116 Polluxochrin 337 and dioschrin 338 (Figure 4F), two new dimers of sulochrin linked by thioether bonds, and an additional new asterric acid analogue Dimethylamide asterrate 339 (Figure 4F), were purified from an Alternaria sp. isolate obtained from a Hawaiian soil sample.82 Two new cyclohexanone derivatives, nectriatones A 340 and B 341 (Figure 4F), and one new natural product, nectriatone C 342 (Figure 4F), were isolated from the culture of Nectria sp. B-13 obtained from high-latitude soil of the Arctic.117 Two new curvularin derivatives, curvulopyran 343 and ent-curvulone A 344 (Figure 4F), were isolated from a culture broth of the soil-derived fungus Aspergillus polyporicola PSU-RSPG187.118 7-methoxy-2,3,6-trimethylchromone 345 was obtained from the ethyl acetate extract of a soil-derived fungal strain, Exophiala pisciphila PHF-9.77 Cultivation of the mangrove rhizosphere soil-derived fungus Penicillium janthinellum HK1-6 with NaBr led to the isolation of two new brominated azaphilones, penicilones G 346 and H 347 (Figure 4F).86 As part of the ongoing search for antibiotics from fungi obtained from soil samples, the secondary metabolites of Clonostachys rosea YRS-06 were investigated. Through efficient bioassay-guided isolation, three new bisorbicillinoids possessing open-ended cage structures, tetrahydrotrichodimer ether 348 (Figure 4G) and dihydrotrichodimer ether A 349 and B 350 (Figure 4G), were obtained. Compounds 348–350 are rare bisorbicillinoids with a γ-pyrone moiety.119 Isochaetomium A2 351 (Figure 4G), a new bis(naphthodihydropyran-4-one), was isolated from the solid-state fermented rice culture of Chaetomium microcephalum.120 Wentiphenone A 352 (Figure 4G), was obtained from the fungus Aspergillus wentii.102 Four new azaphilones, penicilones A-D 353–356 (Figure 4G), were isolated from the mangrove rhizosphere soil-derived fungus Penicillium janthinellum HK1-6.121 Six new azaphilone derivatives, talaraculones A-F 357–362 (Figure 4G), were obtained from the saline soil-derived fungus Talaromyces aculeatus.122 One new double bond isomer of asteltoxin, isoasteltoxin 363 (Figure 4G), were isolated from an Antarctic soil-derived fungus, Aspergillus ochraceopetaliformis SCSIO 05702.53 Pang et al found curvularone A 364 and 4-hydroxyradianthin 365 (Figure 4G) from Curvularia inaequalis strain HS-FG-257.123 A new compound 1-(2′,4′-dihydroxy-5′-methyl-3′-methylsulfanylmethylphenyl)-ethanone 366 (Figure 4G) was isolated from the ethyl acetate extract of the fermentation broth of the fungus Penicillium crustosum YN-HT-15 isolated from the red soil in Yunnan Province, China.124 A new compound (E)-4-hydroxy-3-[(4-hydroxy-3-methylbut-2-en-1-yl)oxy] benzoic acid 367 (Figure 4G) was obtained from Aspergillus aculeatus.96 Two new metabolites, talaflavuols B 368 and C 369 (Figure 4G) were isolated from the wetland soil-derived fungus Talaromyces flavus BYD07-13.125 Aspereusin B 370 (Figure 4G) was isolated from the culture of Aspergillus terreus YIM PH30711.93 Compounds Pyrenochaetayu 371 and compound Galinsogisoliyu 372 (Figure 4G and H) were successfully separated from the fermentation broth of Seltsamia galinsogisoli sp. nov.126 A novel compound 1-(4-hydroxy-2,6-dimethoxy-3,5-dimethylphenyl)-2-methyl-1-butanone 373 (Figure 4H) was obtained from the soil-derived fungus Aspergillus sp. isolated from the rhizospheric soil of Phoenix dactylifera (Date palm tree).127 Chemical investigation of the Dictyosporium digitatum fungus resulted in the identification of nine undescribed compounds 374–382 (Figure 4H): Dictyosporin D 374, Dictyophthalide A 375, Dictyofuran A 376, Dictyofuran B 377, Dictyofuran C 378, Dictyofuran D 379, Dictyosporone A 380, Xylariolide E 381, Xylariolide F 382.56 One cyclohexanone derivative aspergorakhin K 383 (Figure 4H) was obtained from Aspergillus gorakhpurensis F07ZB1707.6 Yanchao Xu et al fermented Aspergillus fumigatus GZWMJZ-152 in a rice solid medium and isolated four new sulfur-containing benzophenones, sulfurasperines A−D 384–387 (Figure 4H).128
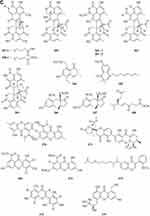
Phenylpropanoids
Eight new polyphenols namely spiromastols A-F 388–393 and spiromastols J-K 394–395 (Figure 5A) were obtained from the fermentation broth of Spiromastix sp. MCCC 3A00308.129 Three new tetralol analogs, myrochromanols A-C 396–398 (Figure 5A), were isolated from a soil fungus Myrothecium verrucaria HL-P-1.130 Peneciraistin D 399 (Figure 5A), which belong isocoumarin, was isolated from saline soil-derived fungus Penicillium raistrickii.11 Talacoumarins A 400 and B 401 (Figure 5A), were isolated from the ethyl acetate extract of the wetland soil-derived fungus Talaromyces flavus BYD07-13.131 Compounds 402–404 (Figure 5A) were isolated from the fermentation broth of a deep sea-derived fungus Spiromastix sp. MCCC 3A00308.129 Eight new isocoumarin glycosides 405–412 (Figure 5A) were obtained from the solid culture of the wetland soil-derived fungus Metarhizium anisopliae (No. DTH12-10).132 Two new isocoumarin derivatives, talaisocoumarins A 413 and B 414 (Figure 5A), and one new related metabolite, talaflavuol A 415 (Figure 5A) were isolated from the wetland soil-derived fungus Talaromyces flavus BYD07-13.125 Penicipyrans C 416 and D 417 (Figure 5A), were isolated from Penicillium raistrickii.85 In research on novel secondary metabolites from microorganisms, two new 418–419 (Figure 5B) were derived from soil fungus Hypoxylon sp. The two new compounds are assigned as (3R, 4R)-4,8-Dihydroxy-5-(hydroxymethyl)-3-methylisochroman-1-one 418 and (3R, 4S)-4,8-dihydroxy-5-(hydroxymethyl)-3-methylisochroman-1-one 419.133 Two new epimeric dihalogenated diaporthins, (9R*)-8-methyl-9,11-dichlorodiaporthin 420 and (9S*)-8-methyl-9,11-dichlorodiaporthin 421 (Figure 5B), have been isolated from the soil fungus Hamigera fusca NRRL 35721.134 Chemical characterization of ethyl acetate extract of Exophiala sp. has afforded the isolation of three compounds including a new isocoumarin named exophiarin 422 (Figure 5B).135
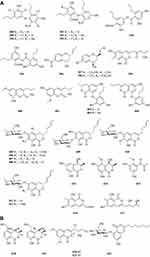 |
Figure 5 Phenylpropanoids 388–422. (A) Phenylpropanoids 388–417. (B) Phenylpropanoids 418–422. |
Quinones
Two new benzoquinones, citriquinones A 423 and B 424 (Figure 6), were isolated from Penicillium citrinum.136 Penicilliquinone 425 (Figure 6) was isolated from the fermentation product of Penicillium sp. PSU-RSPG9.80 Tansakul et al investigated secondary metabolites produced by Penicillium herquei PSU-RSPG93 isolated from soil collected from the Plant Genetic Conservation Project under the Royal Initiation of Her Royal Highness Princess Maha Chakri Sirindhorn at Ratchaprapa Dam in Suratthani province, Thailand. They obtained one new phenalenone derivative, peniciherqueinone 426 (Figure 6).137 Two new phenalenones, aspergillussanones A 427 and B 428 (Figure 6) were obtained from the mycelial extract of the soil fungus Aspergillus sp. PSU-RSPG185.30 A new naphthoquinone, solaninaphthoquione 429 (Figure 6), was isolated from the soil fungus Fusarium solani PSU-RSPG227.138 Cultivation and fractionation of secondary metabolites from Aspergillus kumbius revealed a new bis-indolyl benzoquinone, kumbicin D 430 (Figure 6).22 Membrane active compound PA3-d10 431 (Figure 6) produced by Aspergillus flavus strain demonstrated antimicrobial activities against bacteria and yeast strains.139 The diastereomeric mixtures of the bianthrones wentibianthrone A (432a, b) (Figure 6) and wentibianthrone B (433a, b) (Figure 6), as well as (10R,10′S)-wentibianthrone C (434a) and (10R,10′R)-wentibianthrone C (434b) (Figure 6) were obtained from the fungus Aspergillus wentii.102 The study of a Hawaiian volcanic soil-associated fungal strain Penicillium herquei FT729 led to the isolation of one unprecedented benzoquinone-chromanone, herqueilenone A 435 (Figure 6) Herqueilenone A 435 contains a chroman-4-one core flanked by a tetrahydrofuran and a benzoquinone with an acetophenone moiety.140 Three unreported phenalenone derivatives 436–438 (Figure 6), named ent-12-methoxyisoherqueinone (436) (Figure 6), (−)-scleroamide (437) (Figure 6), and (+)-scleroamide (438) (Figure 6) were isolated from the Hawaiian soil fungus Penicillium herquei FT729, collected on the Big Island, Hawaii.141
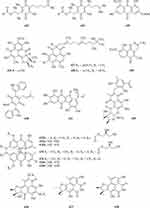 |
Figure 6 Quinones 423–438. |
Esters and Lactones
Esters
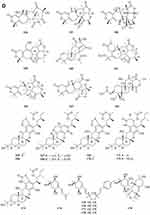
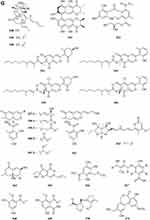
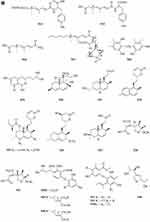
A new ester of 2.4-dihydroxy-6-methylbenzoic acid 439 (Figure 7A) named 3-Hydroxy-5-methylphenyl 2.4-dihydroxy-6-methylbenzoate was isolated from the fungus Neosartorya pseudofischeri S.W. Peterson.10 Six new polyesters, talapolyesters A-F 440–445 (Figure 7A), were isolated from the wetland soil-derived fungus Talaromyces flavus BYD07-13. All the polyesters are composed of (R)-2,4-dihydroxy-6-(2-hydroxypropyl)benzoic acid and (R)-3-hydroxybutyric acid/(S)-3,4-dihydroxybutyric acid residues.142 Liu et al obtained a new compound R-3-(3′-acetyl-2′,6′-dihydroxy-5′-methylphenyl)-2-methylpropionic acid methyl ester 446 (Figure 7A) isolated from the ethyl acetate extract of the fermentation broth of the fungus Penicillium crustosum YN-HT-15.124 Compound 4-(4- hydroxyphenethoxy)-4-oxobutanoic acid 447 (Figure 7A), was isolated from the soil fungus Fusarium solani PSU-RSPG227.138 Penicillithiophenols A 448 and B 449 (Figure 7A) were isolated from Penicillium copticola PSURSPG138.51 Figure 7 Continued. Figure 7 Continued. Figure 7 Esters and lactones 439–490. (A) Esters and lactones 439–453. (B) Esters and lactones 454–478. (C) Lactones 479–490.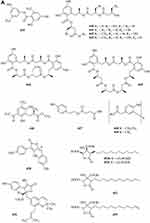
Lactones
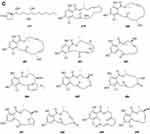
In search for anti-influenza virus inhibitors from marine-derived fungi, a strain identified as Aspergillus terreus Gwq-48, was isolated from a mangrove rhizosphere soil sample collected in the coast of Fujian province. The study of its chemical components led to the isolation of one new aspulvinone, isoaspulvinone E 450 (Figure 7A).143 One new γ-butyrolactone, aspergillulactone 451 (Figure 7A) was isolated from the mycelial extract of the soil fungus Aspergillus sp. PSU-RSPG185.30 Two new hydroxycitric acid lactone derivatives named cinatrins D 452 and E 453 (Figure 7A), were obtained from the fungus Virgaria boninensis FKI-4958.44 One phthalide (asperlide, 454) and one depsidone (aspersidone, 455) (Figure 7B) were obtained from the fungus Aspergillus unguis PSURSPG199.144 Penicimenolides A-E 456–460 (Figure 7B) and penicimenolide F 461 (Figure 7B) were isolated from the culture broth of a strain of Penicillium sp. (NO. SYP-F-7919).145 Chromatographic separation of the broth extract of the soil-derived fungus Aspergillus sclerotiorum PSURSPG178 resulted in isolation of four γ-butenolide-furanone dimers, aspersclerotiorones A-D 462–465 (Figure 7B), and two γ-butenolide derivatives, aspersclerotiorones F 466 and G 467 (Figure 7B).108 Zhou et al isolated and identified a new sesquiterpene lactone named eut-Guaiane sesquiterpene 468 (Figure 7B) from the fungus Eutypella sp. derived from the soil of high latitude of Arctic.146 New natural products, designated pochoniolides A and B 469–470 (Figure 7B), were isolated from the cultured broth of fungal strain FKI-7537 using a physicochemical screening methodology.147 Three new γ -hydroxyl butenolides named aspersclerolides A-C 471–473 (Figure 7B), a pair of new enantiomeric spiro-butenolides named (±)-aspersclerolide D 474 (Figure 7B), were isolated from Aspergillus sclerotiorum.116 Investigation of the soil-derived fungus Lasiodiplodia theobromae NSTRU-PN1.4 resulted in the isolation of two new dimeric c-lactones botryosphaerilactones D 475 and E 476 (Figure 7B).148 One new metabolite, therlanubutanolide A 477 (Figure 7C), was isolated from the YGP culture broth of Thermomyces lanuginosus.149 During screening for microbial regulators of bone metabolism, a new compound, 6-ethoxy-5,6-dihydropenillic acid 478 (Figure 7B), was isolated from the culture broth of the soil-derived fungus Penicillium sp. BF-0343.150 Twelve new resorcylic acid lactones (RALs) including three new 16-membered RALs 479–481 (Figure 7C), eight new 14-membered RALs 482−489 (Figure 7C), and one new 12-membered RAL 490 (Figure 7C), were identified from the fermentation of the soil-derived fungus Ilyonectria sp. sb65. These new compounds are assigned, respectively, as Ilyoresorcy A 479, Atrop-ilyoresorcy A 480 Ilyoresorcy B 481, Ilyoresorcy C 482, Ilyoresorcy D 483, Ilyoresorcy E 484, Ilyoresorcy F 485, Ilyoresorcy G 486, Ilyoresorcy H 487, Ilyoresorcy I 488, Ilyoresorcy J 489 and Ilyoresorcy K 490.151
Other Compounds
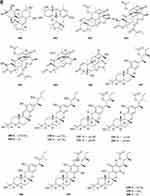
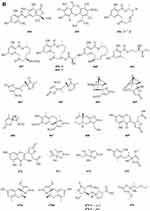
Prenylterphenyllins A-C 491–493 (Figure 8A) and prenylcandidusins A-C 495–497 (Figure 8A), and one new polyhydroxy-pterphenyl named 4”-dehydro-3-hydroxyterphenyllin 494 (Figure 8A), were obtained from Aspergillus taichungensis ZHN-7-07, a root soil fungus isolated from the mangrove plant Acrostichum aureum.152 A new benzoic acid derivative 4-hydroxy-3-(3-methyoxy-3-methylbutyl)-benzoic acid 498 (Figure 8A) was isolated from Curvularia inaequalis strain HS-FG-257.153 Eight new 2-pentenedioic acid derivatives 499–506 (Figure 8A) were isolated from a soil-derived fungus Gongronella butleri collected in Cameroon. The isolated compounds feature a 2-pentenedioic acid core structure substituted by a 2-alkyl chain that has even number of carbon atoms (C6, C8, and C10) with or without an oxygenated substituent.154 Peniciketals A-C 507–509 (Figure 8A), three new spiroketals with a benzo-fused 2.8-dioxabicyclo[3.3.1]nonane moiety, were isolated from the saline soil-derived fungus Penicillium raistrichii.155 Masaphy et al report on the purification and characterization of a novel anticandidal echinocandin – MIG0310 510 (Figure 8A) from Fusarium brachygibbosum strain MS-R1 and the elucidation of its molecular structure.156 Two new cyclic carbonate derivatives, aspergillusols A 511 and B 512 (Figure 8A) which contain an unusual cyclic carbonate functionality, and one new eutypinic acid derivative, aspergillusic acid 513 (Figure 8A) were found from Aspergillus sp. PSU-RSPG185.30 The soil fungus Gymnascella dankaliensis was collected in the vicinity of the Giza pyramids, Egypt. When grown on solid rice medium the fungus yielded four new compounds including 11′-carboxygymnastatin N 514, gymnastatin S 515, dankamide 516, and aranorosin-2-methylether 517 (Figure 8B).157 A new diphenyl derivative, named iizukine B 518 (Figure 8B), was isolated from coastal saline soil-derived fungus Aspergillus iizukae.84 A new compound namely (13-(3,3-dihydroxypropyl)-1,6-dihydroxy-3,4-dihydro-1H-isochromen-8(5H)-one 519 (Figure 8B) was isolated from an ethyl acetate extract of the borne fungi Sclerotium rolfsii. The new compound is also known as Sclerotium.158 A new lovastatin analogue versicorin 520 (Figure 8B), was isolated from mycelial solid cultures of Aspergillus versicolor SC0156. The new compound versicorin 520 possesses a hexahydro-2H-naphtho[1,8-bc]furan moiety, which is a rare type of the lovastatin-analogous compounds.159 Three new lovastatin analogues 521–523 (Figure 8B) were isolated from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178.160 Four previously undescribed metabolites including two lovastatin analogues, asterreusin A 524 and one unnamed compound 525 (Figure 8B), additionally aspereusin A 526 and epiaspereusin A 527 (Figure 8B) were isolated from the culture of Aspergillus terreus YIM PH30711.93 Three new diphenyl ether derivatives (penicillidic acids A-C, 528–530) (Figure 8B) were isolated from the soil-derived fungus Penicillium aculeatum PSU-RSPG105.97 Three new diphenyl ethers, aspergillusethers B-D 531–533 (Figure 8B), were isolated from the soil-derived fungus Aspergillus unguis PSU-RSPG204.115 Two new aspinotriol derivatives 534–535 (Figure 8B and C) determined as melleusin A 534, B 535 were isolated from a soil-borne fungus Aspergillus melleus.161 A new diphenyl ether 3-methylpentyl-2, 4-dichloroasterrate 536 (Figure 8C), was isolated from the metabolites of a wetland fungus Aspergillus flavipes. PJ03-11.162 Two new unsaturated fatty acids, 6R,8R-dihydroxy-9Z,12Z-octadecadienoic acid 537 (Figure 8C) and methyl-6R,8R-dihydroxy-9Z,12Z-octadecadienoate 538 (Figure 8C), were isolated from the mangrove rhizosphere soil-derived fungus Penicillium javanicum HK1-22.163 A new azo compound, penoxalin 539 (Figure 8C), a new isochroman carboxylic acid, penisochroman B 541 (Figure 8C), two new natural products, penisochroman A 540 (Figure 8C) and 2,6-dihydroxy-4-[(2R)-2-hydroxyheptyl] benzoic acid 542 (Figure 8C), were isolated from wetland soil fungus Penicillium oxalicum GY1.164 Fan et al report a new pentacyclic decalinoylspirotetramic acid derivative, pyrenosetin D 543 (Figure 8C), from an endophytic strain Pyrenochaetopsis FVE-087.165 One new benzofuranoid 544 (Figure 8C), was isolated and characterized from fungus Aspergillus calidoustus.58 Two new benzothiazoles (545 and 546) (Figure 8C) were isolated from the cave soil-derived fungus Aspergillus fumigatus GZWMJZ-152.128 Figure 8 Continued. Figure 8 Continued. Figure 8 Other compounds 491–546. (A) Other compounds 491–513. (B) Other compounds 514–534. (C) Other compounds 535–546.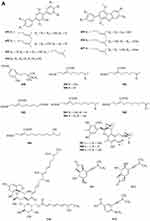
Biological Activity of New Compounds Derived from Soil Fungi
Anticancer Activities
The cytotoxicity of compounds 491, 494 and 496 was evaluated on HL-60, A-549, and P-388 cell lines using the SRB and MTT methods with adriamycin (ADM) as positive control Compound 491 exhibited moderate activities against all three cell lines (IC50 1.53–10.89 μM), whereas compounds 494 and 496 displayed moderate activities only against the P-388 cell line (IC50 of 2.70 and 1.57 μM, respectively).152 Compound 93 exhibited anticancer activity against MCF-7, KB, and NCI-H187, with IC50 values in the range of 12.56–24.91 μg/mL.48 Compound 214 exhibited moderate growth inhibition against A-549, Hela, PANC-28 and BEL-7402 cell lines with the IC50 values of 16.4, 23.4, 20.3, and 30.1 μg/mL, respectively. 10-hydroxycamptothecin was used as the positive control with the IC50 values of 0.5, 5.9, 10.6, and 4.6 μg/mL, respectively.77 The cytotoxic effects of compound peneciraistin C 325 were preliminarily evaluated against human lung adenocarcinoma (A549) and human breast adenocarcinoma (MCF-7) cell lines by the MTT method. Compound 325 showed comparatively significant cytotoxicities against A549 and MCF-7-60 cell lines with IC50 values of 3.2 and 7.6 μM, respectively.11 Pan et al report the anticancer mechanisms of Pe-C in a variety of lung cancer cells. The results showed that Pe-C induced caspase-independent autophagic cell death and elevated mitochondrial-derived reactive oxygen species levels, which indicated that Pe-C could be a potential drug candidate for therapy of lung cancer.166 Compound pyrenocine J 215 showed cytotoxic activity against the human hepatic cancer cell line HepG2 with an IC50 value of 28.5 μg/mL.78 Compound 4 showed cytotoxic activity against A-549, Hela, PANC-28 and BEL-7402 cell lines with IC50 values of 76.3, 77.2, 107.5 and 89.5 μmol·L−1, respectively.12 Compound 7 showed remarkable activities with IC50 values of 1.83 and 4.80 μM on P388 and HL-60 cells, respectively. The target of racemic 7 was also investigated and the (12R,28S,31S)-7 enantiomer showed selectivity against topoisomerase I.14 Compound 8 showed weak activity with IC50 values of 55.1 and 30.5 μM on BEL-7402 and A-549 cells, respectively.15 Compound 364 exhibited cytotoxic activity against the ACHN and HepG2 cell lines with IC50 values of 4.78 and 13.11 μg mL −1, respectively. The values of 365 were 54.18 and 52.07 μg mL −1.123 Sartorypyrone A 128 was evaluated for its capacity to inhibit the in vitro growth of MCF-7 (breast adenocarcinoma), NCI-H460 (nonsmall cell lung cancer) and A375-C5 (melanoma) cell lines, using the protein binding dye SRB method. Interestingly, sartorypyrone A 128, which possesses a monocyclic diterpene core, was more selective, exhibiting similar inhibitory activity to sartorypyrone B against A375-C5 (GI50=21.5±1.9 μM), but less active against MCF-7 (GI50=46.3±7.6 μM) and NCI-H460 (GI50=37.3±4.0 μM) cell lines.16 Citriquinone A 423 was assayed for cell migration inhibitory activity using human cancer cell line HEp 2. After growing cells in vitro in 96 well plates a scratch was made on wells with cells at the confluence stage. Citriquinone A 423 at a concentration of 0.5 mg/mL (dissolved in growth medium containing 1% DMSO) was introduced into wells and incubated for 24 h. Development of new cells to reduce the width of the scratch from its initial stage was determined qualitatively by comparing the width of the scratch after incubation in each well in the presence of test sample and control (1% DMSO in the growth medium) using microscopic images. It was revealed that 423 had a moderate inhibitory effect on the growth/migration of the HEp2 cells when compared with the control.136 Compounds 444 and 445 were evaluated by MTT method for their cytotoxic activities against five tumor cell lines, HL-60, SMMC-7721, A-549, MCF-7, and SW480, with cisplatin and paclitaxel as the positive controls. They exhibited cytotoxicity against the tested tumor cell lines. The IC50 values of compound 444 are 14.81,18.39,17.66,14.59 and 26.62 μM respectively. The IC50 values of compound 445 are 13.62,15.74,11.09,15.96 and 15.54 μM, respectively.142 Peniciketals A-C 507–509 showed selective activities against HL-60 cells with IC50 values of 3.2, 6.7, and 4.5 μM, respectively (doxorubicin as a positive control (IC50s: 0.31, 0.085, and 0.23 μM, respectively)), while were not active on other cells (IC50 > 10 μM).155 Libertellenone H 130 showed cytotoxicity against seven human tumor cell lines: U251, SW-1990, SG7901, MCF-7, Huh-7, Hela and H460 cell lines at a range between 3.31 and 44.1 μM.59 Compound cytochalasin Z24 53 exhibited moderate cytotoxicity toward human breast cancer MCF-7 cell line with IC50 of 9.33 μM.29 Compound aspergillussanone A 427 exhibited weak activity toward KB and Vero cells with IC50 values of 48.4 and 34.2 μM, respectively.30 Compounds Polluxochrin 337, dioschrin 338 and Castochrin 270 showed weak mammalian cell cytotoxicity effects against pancreatic cancer cells (MIA PaCa-2) with IC50 values of 50.8, 30.3, and 29.3 μM, respectively.82 Bisacremines A-B 175–176 exhibited weak cytotoxicity against HeLa cells with IC50 values of 10.7±1.0 and 9.3±1.7 μM respectively. And 176 also showed modest activity against A549 and HepG2 cells with IC50 values of 41.3±4.1 and 31.1±2.1 μM respectively.5 Compound aranorosin-2-methylether 517 showed potent cytotoxicity against the murine lymphoma cell line L5178Y with IC50 values of 0.44 μM.157 Compound solaninaphthoquione 429 showed significant cytotoxic activity against breast cancer (MCF-7) cells and mild cytotoxic activity against oral human carcinoma (KB) cells (IC50 values of 21.3 and 22.6 μM, respectively) compared to standard compound.138 Iizukines A 234 and B 518 were tested their cytotoxicities against HL-60 (human promyelocytic leukemia), BEL-7402 (human hepatoma) and A-549 (human lung carcinoma) were tested by the MTT assay in vitro with 5-fluorouracil (5-FU) as positive control. The two compounds showed weak cytotoxic activities. IC50 values of compound 234 were 26.5, 32.7 and 18.2 μM respectively, and IC50 values of compound 518 were 48.7, 56.6 and 32.3 μM.84 Penicilleremophilane A 99 was obtained from Penicillium copticola PSURSPG138. It showed much weaker cytotoxic activity.51 Cytotoxicity studies of the compound 265 on cancer cell lines showed a valuable cytotoxic potential against all tested human cancer cell lines. Further, the compound induces apoptosis in lung cancer (A549) cells revealed by increase the distribution of nuclear DNA in Sub-G1 phase as observed in flow cytometry.92 For compound 519, in the present study rhodamine-123 exclusion screening test on human mdr1 gene transfected mouse gene transfected L5178 and L5178Y mouse T-cell lymphoma which showed excellent MDR reversing effect in a dose-dependent manner against mouse T-lymphoma cell line. Moreover, molecular docking studies of compound 519 also showed better results as compared with the standard.158 Kumbicin C 33 was found to inhibit the growth of mouse myeloma cells (IC50 0.74 μg mL−1).22 Penicimenolides B-D 457–459 exhibited potent cytotoxicity against the U937 and MCF-7 tumour cell lines and showed moderate cytotoxic activity against the SH-SY5Y and SW480 tumour cell lines. There is experimental evidence that compound 457 may act as a potential MEK/ERK inhibitor, it is still need further study in the future.145 Penicipyran E 237 showed cytotoxicity effects on HL-60 and K562 cell lines with IC50 values of 4.4 and 8.5 μM, respectively.85 Compounds 68–74 showed potent to moderate cytotoxicity against the L5178Y mouse lymphoma cell line with IC50 values ranging from 0.99 to 14.1 μM.35 Asterriquinol E 34 was bioassayed for its inhibitory effects on NO production induced by LPS in microglia cells with NG-monomethyl-L-arginine (L-NMMA) as a positive control (IC50 4.8 μM). The results showed that compound 34 showed moderate activity with IC50 49.7 μM. Cell viability was determined at the same time by the MTT method and only compound 1 exhibited cytotoxicity with an IC50 9.7 μM.23 Bacillisporin H 263 exhibited modest cytotoxicity against HeLa cells.90 Compound Aspergiketone 101 was cytotoxic towards HL-60 and A549 cell lines with IC50 values of 12.4 and 22.1 μM, respectively.52 Oxisterigmatocystins E and F (276 and 277, respectively) exhibited cytotoxicity against KB, MCF-7, and NCI-H187 cell lines (IC50 = 7.7–78.6 μM). However, compounds 276–277 showed cytotoxic effects against the Vero cell line (IC50 = 4.3–9.7 μM).98 Compound 107 displayed growth inhibitory effect against human Mantle cell lymphoma JEKO-1 and human hepatoma carcinoma HepG2 with IC50 values of 8.4 and 28.5 μM, respectively.54 Compound penixanthone A 278 had weak cytotoxicity against the tested cancer cell lines, H1975, MCF-7, K562, HL7702 at concentration of 30 μM.99 Compound 211 exhibited poor cytotoxicity toward HCT-116 cells (20.5% inhibition at the dose of 100 μM, IC50 >100 μM), while the IC50 value of the positive control (doxorubicin) was 0.365±0.023 μM.75 Compound 468 exhibited in vitro a little cytotoxicity towards SGC7901 cell line with IC50 value of 39.8 μM.146 Compound 160 showed potent cytotoxic capability against HL-60, THP-1 and Caco 2 cell with IC50 values of 3.4 μM, 4.3 μM, 10.5 μM, and compound 161 showed significant inhibiting activities against HL-60 cell line and THP-1 cell line (IC50 =7.9 μM, 11.3 μM, respectively), using 5-fluorouracil as the positive drug with IC50 values of 6.4 μM, 4.4 μM, 56.6 μM for HL-60, THP-1 and Caco 2 cells, respectively.67 The cytotoxic effects of 3-methylpentyl-2, 4-dichloroasterrate 536 was evaluated using the MTT method on HL-60, HCT116, PC-3, and HT-29 cancer cell lines. The results showed that its IC50 values were larger than 5-fluorouracil, smaller than 80 μM.162 Libertellenones O-S 133–137 and eutypellenones A and B (138 and 139) exhibited cytotoxicities against HeLa, MCF-7, HCT-116, PANC-1, and SW1990 cells, with IC50 values in the range of 0.8 to 29.4 μM. Compounds 138 and 139 could dose-dependently inhibit the activity of NF-κB and exhibited significantly inhibitory effects on nitric oxide production induced by lipopolysaccharide.61 (9R*)-8-methyl-9,11-dichlorodiaporthin 420 and (9S*)-8-methyl-9,11-dichlorodiaporthin 421, were evaluated the cytotoxicity against seven cancer cell lines: human-derived cell lines CCD25sk (human fibroblasts), SHSY5 (neuroblastoma), MiaPaca-2 (epithelial pancreas carcinoma), MCF-7 (breast adenocarcinoma), HepG2 (hepatocellular carcinoma), A2058 (epithelial melanoma), and A549 (lung carcinoma), exhibiting moderate activity against two of them. While both dichlorinated compounds 420–421 were active against neuroblastoma (SHSY5y cells: 420 CC50 = 39.2 μM, and 421 CC50 = 36.2 μM). These results correlate well with the cytotoxicity against HeLa cells recently reported for dichlorodiaporthin (CC50 = 9 μg/mL = 28 μM), a compound considered as a mycotoxin.134 Sporulosol 326 showed modest cytotoxicity toward the human tumor cell line T24, with an IC50 value of 18.2 μM.113 Libertellenone M 140 and libertellenone N 141 were tested for cytotoxic activities against HeLa, MCF-7, HCT-116, K562, and SW1990 cell lines. Compound 141 displayed potent cytotoxicity against K562 cells with IC50 value of 7.67 μM and moderate cytotoxicity against HeLa, MCF-7, and SW1990 cell lines with IC50 values of 30.06, 18.52, and 24.36 μM, respectively, and compound 140 showed weaker cytotoxic activity against these five tumor cell lines with IC50 values of 34.78, 32.20, 26.67, 40.85 and 24.36 μM, respectively.62 Compound iizukine C 57 exhibited cytotoxic effect towards HL-60 and A549 cell lines with IC50 values of 3.8 and 7.2 μM, respectively.31 Compounds 471 and 473 showed selective cytotoxicity against HL60 (IC50: 6.5 and 12.1 μM, respectively), A549 (IC50: 8.9 and 16.7 μM, respectively), and HL-7702 (IC50: 17.6 and 22.8 μM, respectively) cell lines.116 The cytotoxicities of Compound 212 was evaluated against A-549, Hela, HCT-116, MGC-803, and HO-8910 cell lines. Compound 212 exhibited various activities with the IC50 values 5.0 to 22.4 μM, respectively.76 Tolypocladin A 38 displayed weak cytotoxicity against A549, Huh7, LN229, MGC and MHCC97H, LOVO and MDA231 cell lines with IC50 values from 16.32 to 37.80 μM (positive control camptothecin: 0.32–31.8 nM) and suppressed the growth and viability of the HCC cells T1224 in the patient-derived organoids (PDOs) model.25 8′-O-(3R-Hydroxy-butyryl)-rasfonin 294 and Cemironin A 295 displayed significant cytotoxicity on five human cancer cell lines: 786-O, A549, HeLa and MCF-7 cell lines, with the IC50 values <20 μM, which are more effective than positive control 5-fluorouracil and could be considered to be potential as antitumor agents, in which they could significantly inhibit the cancer cells growth in a dose-dependent manner.104 Nectriatone A 340 showed cytotoxicities against SW1990, HCT-116, MCF-7, and K562 cells, with IC50 values in the range of 26.37–42.64 μM.117 Compound 251 also showed cytotoxicity against NCI-H187 cells with an IC50 value of 16.73 μM. Moreover, macrosporusones A-B 250–251 and macrosporusone D 253 exhibited cytotoxicity against Vero cells with IC50 values in the range of 13.74–69.77 μM.88 Compounds 48 and 49 showed moderate cytotoxicity against HepG2 human hepatocellular carcinoma cells with an IC50 of 8.7 and 19.4 μM, respectively. The IC50 value of the positive control (cis-platin) was 3.14 μM.26 Compound 542 displayed significant cytotoxicity against human esophageal carcinoma cells OE19 with an IC50 value of 5.50 μM.164 Compounds 255–258 had low cytotoxicity against both cancerous (MCF-7 and NCIeH187) and non-cancerous (Vero) cells.89 Compounds 62–65 showed moderate cytotoxicity against five tested human tumor cell lines: HeLa, PC-3, A549, HepG-2 and HL-60.33 Pyrenosetin D 543 showed toxicity towards both A-375 and HaCaT cells with IC50 values of 77.5 and 39.3 μM, respectively.165 Compounds 165,166, and 168 exhibited moderate cytotoxic activities with IC50 values ranging from 18.4 to 29.4 μM.69 The cytotoxicity assay revealed that asperanstinoid D 185, dehydroaustinol, and austin displayed considerable cytotoxicity against the HL-60 and SU-DHL-4 tumor cell lines with IC50 values ranging from 15.7 to 27.8 μM.71 Protein tyrosine phosphatases (PTPs) are signaling enzymes that regulate tyrosine phosphorylation. The disorder of PTPs could induce human diseases such as tumor, diabetes, autoimmune diabetes, and infectious diseases. Compounds 213, 203, and 383 showed inhibitory effect against PTPs including PTP1B, SHP1, and TCPTP in vitro. Among them, compound 213 might exhibit selective activities against PTP1B and SHP1 over TCPTP with IC50 values 0.57, 1.19, and 22.97 μM, respectively. Compounds 213 and 203 exhibited modest cytotoxicity against tumor cell lines A549, HeLa, Bel-7402, and SMMC-7721 with IC50 values in the range of 6.75–83.4 μM.6 The inhibitory activity of the isolated compounds (436–438) against indoleamine 2.3-dioxygenase 1 (IDO1) was assessed. Compounds 436 inhibited IDO1, with the IC50 value of 32.59 μM.141
Antimicrobial Activities
Compounds 95 and 96 were tested for antifungal activities. 95 and 96 were active against plant pathogenic fungi Rhizoctonia solani and Fusarium oxysporum using standard agar diffusion tests at 20 μg/disk.49 Compound 5 was tested for its abilities to inhibit both cell viability and biofilm formation of Candida albicans. It demonstrated dose-dependent activity in the biofilm inhibition assay with an IC50 value of 1.4 ± 0.2 μM.13 Citriquinone A 423 was evaluated for antibacterial activity against Bacillus sp. At a dose of 250 µg/disc using the Kirby-Bauer Disc Diffusion method. Amoxicillin 25 µg and a disc soaked with MeOH and dried completely served as positive and negative control, respectively. It was found that 423 had moderate antibacterial activity compared with amoxicillin.136 Compounds 97 and 98 exhibited selective activity against Colletotrichum gloeosporioides with MIC values of 1.0 and 0.125 μg/mL, respectively, the latter of which is better than that of the positive control, zeocin (MIC 0.25 μg/mL). This result indicated that compounds 97 and 98 are responsible for the activity against C. gloeosporioides during the preliminary assay of the crude extract, and acetylation of 4-OH likely enhanced the activity.50 Libertellenone G 129 exhibited antibacterial activity against Escherichia coli, Bacillus subtilis and Staphylococcus aureus.59 Novel echinocandin compound MIG0310 510 exhibiting anticandidal activity, MIG0310 showed activity against two ATCC strains and 10 clinical isolates of Candida albicans. It also showed activity against a clinical isolate of C. tropicalis, showing a potential inhibition of this particular Candida species as well. The anticandidal agent had MFC values (killing activity) similar to the MIC for C. albicans, reminiscent of other echinocandins used as drugs, which showed also kill at growth-inhibiting concentrations for most of Candida species isolates (Moore et al 2001).156 Isochaetomium A2 351 possessed significant antimicrobial activity against Escherichia coli 1.044, Staphylococcus aureus 1.252, and Bacillus subtilis 1.079.120 Minimum inhibitory concentration (MIC) of the active compound 273 ranged from 0.5 to 15 μg/mL. Viable cell count studies of the active compound 273 showed Staphylococcus aureus, Escherichia coli, Staphylococcus epidermidis, and Salmonella typhimurium 1 to be the most sensitive.95 Compounds Polluxochrin 337, dioschrin 338 and Castochrin 270 exhibited anti-MRSA activity, with MIC values of 4.1, 4.9, and 3.2 μM (2.9, 3.2, and 2.0 μg/mL), respectively, whereas the MIC for chloramphenicol was 5 μM (1.6 μg/mL).82 Compounds 388–390 exhibited potent antibacterial activities against all tested strains of bacteria, including Xanthomanes vesicatoria ATCC 11633, Pseudomonas lachrymans ATCC11921, Agrobacterium tumefaciens ATCC11158, Ralstonia solanacearum ATCC11696, Bacillus thuringiensis ATCC 10792, Staphylococcus aureus ATCC 25923 and Bacillus subtilis CMCC 63501, with minimal inhibitory concentration (MIC) values ranging from 0.25 to 4 μg/mL, while compounds 394–395 and 404 showed moderate inhibition with MIC values in the range of 8–64 μg/mL.129 The antibacterial activity of asperochrin A 231 was evaluated. Compound 231 showed inhibitory activity against aquatic pathogenic bacterial Aeromonas hydrophila, Vibrio anguillarum, and Vibrio harveyi. However, compound 231 displayed selective antibacterial activity against A. hydrophilia, V. anguillarum, and V. harveyi, with IC50 values ranging from 8.0 to 16.0 μg/mL.83 Compounds 452 and 453 were shown to be weakly active against the bacteria Bacillus subtilis KB 211 (ATCC 6633) with inhibition zones of 7.3 and 7.2 mm, respectively, as well as against E. coli KB 213 (NIHJ), with inhibition zones of 10.9 and 14.7 mm, respectively, at 100 μg per 6 mm disc. Compound 89 displayed only weak antibacterial activity against E. coli KB 213 (NIHJ) with an inhibition zone of 7.8 mm at 30 μg per 6 mm disc.44 Penicilleremophilane A 99 was approximately half as active against Plasmodium falciparum with the IC50 value of 3.45 μM.51 MIC of the compound 265 ranged from 0.5 to 15 µg/mL, which was found to be comparable with the standard antibiotics.92 Compound 349 (MIC 25 μg/mL) exhibited stronger antibacterial activity against E. coli than erythromycin, streptomycin, and ampicillin. Compound 350 (MIC 50 μg/mL) exhibited more prominent activity than streptomycin against Bacillus subtilis. Compound 350 (MIC 50 μg/mL) also displayed similar activity against Escherichia coli compared to erythromycin and ampicillin, and greater than streptomycin.119 Kumbicin C 33 was found to inhibit the growth of Gram-positive bacterium Bacillus subtilis (MIC 1.6 μg mL−1).22 Compound 405 showed antibacterial activity comparable with (Z)-4-bromo- 5-(bromomethylene)-2(5H)-furanone (BF), which may block the QS system of Pseudomonas aeruginosa with the mechanism of reducing biofilm formation and virulence factor secretion.132 Talaroketals A 240 and B 241 display modest antimicrobial activity against Staphylococcus aureus with an IC50 value around 50 μg mL−1 but no activity against the other bacterial strains:Staphylococcus haemolyticus, Escherichia coli and E. faecalis.87 The antimicrobial activity of bacillisporin H 263 was evaluated against a panel of human pathogenic bacteria: Escherichia coli (ATCC 8739), Staphylococcus aureus (ATCC 6538), Staphylococcus hemolyticus (MNHN26), and Enterococcus faecalis (CIP 103014). Also of note is the effect of compound 263 against Staphylococcus aureus with a MIC value of 5.0 µM.90 Compound 533 exhibited moderate antifungal activity against Candida albicans (NCPF3153), flucytosine-resistant Cryptococcus neoformans (ATCC90113) and Penicillium marnefeii with the MIC values of 16, 8 and 16 μg/mL, respectively.115 Compound 107 exhibited inhibitory efficacy to Bacillus subtilis CMCC63501 and Bacillus pumilus CMCC63202 with IC50 value of 18.1 and 23.8 μM, respectively54 The antibacterial activities of Penicilones B-D 354–356 were evaluated with Gram-positive S. aureus (ATCC 43300), S. aureus (ATCC 33591), S. aureus (ATCC 29213), S. aureus (ATCC 25923), Enterococcus faecalis (ATCC 51299), E. faecium (ATCC 35667), and Gram-negative Escherichia coli (ATCC 25922). The results indicated that 354–356 showed significant antibacterial activities against the tested Gram-positive bacteria including both antibiotic-resistant and -susceptible strains with MIC values ranging from 3.13 to 12.5 μg/mL, while none of the tested compounds exhibited activity against E. coli.121 The antibacterial activities of talaraculone B 358 against three Gram-positive bacteria and three Gram-negative bacteria were tested. It showed selective activities against the pathogenic bacteria Vibrio anguillarum, with the same MIC values of 0.26 μg/mL, stronger than the positive control ciprofloxacin (MIC = 0.52 μg/mL).122 When the compound was 50 μg on the antibacterial paper (Ø 6 mm), compound 468 showed strong antibacterial activities against Escherichia coli, Bacillus subtilis and Staphylococcus aureus such as the positive control ampicillin.146 Compound 160 showed antibacterial activity toward Bacillus cereus (IC50=49 μg/mL, IC90 =111 μg/mL) and Bacillus subtilis (IC50=10 μg/mL, IC90=85 μg/mL) [191]. Libertellenone M 140 exhibited antibacterial activity against Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 27217), and Vibrio vulnificus (ATCC 27562) with MIC values of 32, 32, and 16 μg/mL, respectively.62 Compound 285 displayed antifungal activity against Cryptococcus neoformans ATCC90112 and ATCC90113 flucytosine-resistant with the MIC values of 128 and 64 μg/mL, respectively.101 11-Hydroxychevalone E 300 showed weak antibacterial activity against Escherichia coli and Salmonella enterica serovar Typhimurium., both with MIC 128 μg/mL.40 Membrane active compound PA3-d10 431 produced by Aspergillus flavus strain demonstrated antimicrobial activities against bacteria and yeast strains.139 Compounds 346 and 347 showed moderate to strong inhibitory activity against the tested Gram-positive bacteria. It is worth noting that 347 and 238 displayed potent antibacterial activities against methicillin-resistant Staphylococcus aureus ATCC 33591 with the same MIC value of 3.13 μg/mL, which was close to the positive control vancomycin (MIC 1.56 μg/mL).86 Compounds 372 and 371 both displayed antimicrobial activities against Staphylococcus aureus with MIC values of 25 and 75µg/mL, respectively. Moreover, morphological observation showed the coccoid cells of S. aureus to be swollen to a volume of 1.4 and 1.7-fold after treatment with compounds 371 and 372, respectively. Microbial filamentous temperature-sensitive protein Z (FtsZ) is a novel target for drug discovery, which plays a key role in cell division. The inhibitors of FtsZ prevent the cellular fusion of bacteria, which lead to apoptosis of bacteria. Molecular docking was carried out to investigate interactions of filamentous temperature-sensitive protein Z (FtsZ) with compounds 371 and 372. The results indicated that compounds 372 might form lower potential energies and more stable binding sites with the target protein FtsZ compared to compound 371 which validated the observed antimicrobial activities.126 MIC values of 200 μg/mL were obtained for compounds 472–473 towards Staphylococcus aureus and Escherichia coli using the micro-broth dilution method.116 Peninaphones A-C (301–303) showed antibacterial activity against Staphylococcus aureus. Compound 303 exhibited significant activity against the rice sheath blight pathogen Rhizoctonia solani.105 Compounds 308 and 309 displayed moderate inhibitory effects on Staphylococcus aureus and Bacillus cereus with an inhibition rate of more than 50%. Additionally, compounds 307–309 showed weak antibacterial effects on MR Staphylococcus aureus.109 Compounds 38 and 45 displayed significant antimicrobial activities. Compound 38 showed obvious inhibitory activities against seven pathogenic fungi (Alteranira f ragariae, Corynespora cassiicola, Alternaria alternata, Botrytis cinereal Pers., Cercospora personata, Verticillium dahliae Kleb, Sclerotinia sclerotiorum) with MIC values of 6.25–25 μg/mL (ketoconazole: 0.78–1.56 μg/mL), and compounds 38–47 except for 45 were active against A. fragariae with MIC values of 6.25–50 μg/mL (ketoconazole: 0.78 μg/mL). Compounds 38–47 were also evaluated for their antibacterial activity toward Gram-positive and Gram-negative human pathogenic bacterial strains. The results indicated that compound 45 was active against all tested bacteria and compound 38 was active against Bacillus cereus and methicillin-resistant Staphylococcus aureus. Moreover, compound 39 exhibited weak activity against methicillin-resistant S. aureus.25 Compound 373 exhibited potent antimicrobial activities against Staphylococcus aureus with MIC values of 2.3 μg mL−1 and significant growth inhibitions of 82.3 ± 3.3 against Candida albicans and of 79.2 ± 2.6 against Candida parapsilosis. Compound 373 further showed strong activity against the pathogenic bacteria Escherichia fergusonii with MIC of 3.1 μg mL−1.127 Compounds 115 and 151 displayed interesting antifungal activity against Cryptococcus neoformans ATCC90113 with the respective MIC values of 8 and 4 μg/mL. Moreover, only 115 was active against C. neoformans ATCC90112 with the MIC value of 32 μg/mL.55 Compounds 295–297 also displayed moderate inhibitory effects on MR Staphylococcus aureus, Staphylococcus aureus and Bacillus subtilis, which could be the major anti-bacterial constituents of Cephalotrichum microsporum.104 Tolypocladin K 149 displayed moderate antifungal activity against Sclerotinia sclerotiorun, Helminthosporium maydis, Botrytis cinereal Pers. and Colletotrichum acutatum Simmonds with an MIC value of 50 μg/mL.64 Duclauxamide B 257 showed anti-Mycobacterium tuberculosis activity with MIC value of 12.5 μg/mL and also had anti-Bacillus cereus and anti-Staphylococcus aureus with equal MIC values of 12.5 μg/mL.89 Compounds 312–321 showed antimicrobial inhibitory activities against Escherichia coli, Staphylococcus aureus, and Candida albicans with MIC values ranging from 0.9 to 7.0 μg/mL, from 1.7 to 3.5 μg/mL, and from 3.3 to 7.0 μg/mL, respectively.111 Compound 66 was active against Fusarium oxysporum with an MIC value of 50 μg/mL.34
Anti-Inflammatory Activities
In the in vitro anti-inflammation assay, bisacremine G 181 exhibited dose-dependent inhibitory effects on the production of TNF-α, IL-6, and nitric oxide (NO) in LPS-stimulated RAW 264.7 macrophages. At 50 μM, it inhibited TNF-α, IL-6, and NO production by 80.1%, 89.4%, and 55.7%, respectively. The inhibition was comparable to that of dexamethasone (inhibition rates at 50 μM: 78.0%, 92.6%, and 62.6%, respectively).70 Compounds 156–158 showed inhibitory activity against NO production in BV-2 microglial cells using the Griess assay with IC50 values of 30.0 ± 1.5, 15.5 ± 0.5 and 8.8 ± 0.1μM, respectively. Indomethacin was used as a positive control (IC50 = 34.5 ± 1.2 μM).66 Compounds 457–459 exhibited a significant inhibitory effect on the production of nitric oxide (NO) in murine macrophages (RAW 264.7) activated by lipopolysaccharide (LPS).145 Asterriquinol E 34 and asterriquinol F 35 have inhibitory effects on NO production induced by LPS in microglia cells with NG-monomethyl-L-arginine (L-NMMA) as a positive control (IC50 4.8 μM) with IC50 values of 11.3 μM and 49.7 μM respectively.23 Compared with dimethyl fumarate (DMF), a potent inflammation inhibitor, compound 211 exhibited weak anti-inflammatory activity in an ANA-1 murine macrophages model.75 There is experimental evidence that 138 and 139 could dose-dependently inhibit the activity of NF-κB and exhibited significantly inhibitory effects on nitric oxide (NO) production induced by lipopolysaccharide (LPS) in the murine RAW 264.7 macrophage cells.61 Myrochromanols A 396 and C 398 inhibited lipopolysaccharide (LPS)-induced NO production in BV2 cells with IC50 values of 26.04 and 25.80 μM, respectively.130
Antioxidant Activities
Compounds 323, 324 and 399 showed radical scavenging activity against DPPH with IC50 values of 38.9, 42.7, and 87.5 μM, respectively.11
(E)-4-hydroxy-3-[(4-hydroxy-3-methylbut-2-en-1-yl)oxy] benzoic acid 367 showed radical scavenging activity against DPPH with IC50 value of 0.51 mg/mL.96 Compound 305 exhibited potent DPPH radical scavenging activities with IC50 value of 1.23μg/mL.107 Pochoniolides A 469 and B 470 showed hydroxyl radical-scavenging and singlet oxygen-quenching activities. Quercetin, pochoniolides A 469 and B 470 all showed scavenging activity against ·OH as well as a quenching effect on O2. The detected values of ·OH and O2 were suppressed by 6.7% and 4.3% in quercetin, 7.9% and 3.1% in pochoniolide A, and 1.2% and 0.5% in pochoniolide B, with being assumed that 100% generation represented a negative control.147 Trichothioneic acid 92 exhibited hydroxyl radical-scavenging and singlet oxygen-quenching activities.47 Compounds 545–546 displayed radical-scavenging activity against 2,2-diphenyl-1-picrylhydrazyl free radicals with the IC50 values of 3.45 ± 0.02, 23.73 ± 0.08 μM, respectively. Compounds (±)-385, 387 exhibited potent antioxidant capacity with oxygen radical absorbance capacity values of 1.73 ± 0.13, 1.65 ± 0.03 μmol TE/μmol, respectively. Compounds (±)-385 and (±)-386 also exhibited protective effects on oxidative injury of PC12 cells induced by H2O2.128
Antiviral Activities
Isoaspulvinone E 450 showed significant anti-influenza A H1N1 virus activities, with IC50 value of 32.3 μg/mL.143 When compared with ribavirin (IC50 113.1 μM), compounds 10–12 and 14–15 exhibited significant protection against H1N1 virus-induced cytopathogenicity in MDCK cells with IC50 values of 28.3, 38.9, 32.2, 73.3, 34.1 μM, respectively.17 Ochraceopone A 102 and isoasteltoxin 363 exhibited antiviral activities against the H1N1 and H3N2 influenza viruses with IC50 values of >20.0/12.2 ± 4.10 and 0.23 ± 0.05/0.66 ± 0.09 μM, respectively. It was noteworthy that the selectivity indexes (SI) of anti-H1N1 activity of 363 was 2.35.53 Compounds 280 and 281 exhibited anti-H1N1 activity with IC50 values of 133.4, 44.6, respectively (ribavirin was used as the positive control, IC50 101.4 μM). They also showed a strong anti-HSV-1 activity with IC50 values of 55.5and 21.4 μM, respectively, compared with the positive control (acyclovir, IC50 150.2 μM). Compound 281 also possessed a strong anti-HSV-2 effect with IC50 value of 76.7 μM (acyclovir as the positive control, IC50 128.6 μM), respectively.100
Antimalarial Activities
Compound 17 was tested for antimalarial activity against the parasite Plasmodium falciparum (K1, multidrug resistant strain), and it showed weak antimalarial activity with an IC50 value of 16.7 μM.18 Solaninaphthoquione 429 displayed weak antimalarial activity (IC50 of 26.1 μM).138 Penicilleremophilane A 99 was approximately half as active against Plasmodium falciparum with the IC50 value of 3.45 μM.51 Oxisterigmatocystin E 276 showed antimalarial activity against Plasmodium falciparum with IC50 value of 7.9 μM.98 Macrosporusone B 251 exhibited antimalarial activity against Plasmodium falciparum with IC50 values of 10.28 μM.88
Immunosuppressive Activities
Isochaetomium A2 351, compound 351 showed obvious inhibitory effects on mouse spleen cell proliferation with successive IC50 values of 0.52 μM.120 Compounds 481 and 482 exhibited significant inhibition of ConA-induced T-cell proliferation, with IC50 values of 4.1 and 1.9 μM, and LPS-induced B-cell proliferation with IC50 values of 9.8 and 1.1 μM, respectively. Compounds 484, 485, and 488 exhibited selective immunosuppressive effects toward the LPS-induced B-cell proliferation with IC50 values ranging from 5.5 to 21.9 μM.151 The bioactivity assays revealed that compounds 165, 166, 169, 170, and 173 exhibited a significant immunosuppressive effect against concanavalin A (ConA)-induced T lymphocyte proliferation with IC50 values ranging from 5.6 to 8.8 μM.69 The immunosuppressive activity assay revealed that compounds 188, 189, and 193−196 showed significant inhibitory activity against concanavalin A (ConA)-induced T lymphocyte proliferation with IC50 values ranging from 4.1 to 9.4 μM.72 In the immunosuppressive activity assay, compounds 201–202 showed potent inhibitory activity against concanavalin A (ConA)-induced T lymphocyte proliferation with IC50 values of 78.3, 81.1 nmol/L, respectively, which provided promising leads for designing and developing new immunosuppressive agents to treat auto-immunological diseases.73
Other Activities
Paecilomide 90 was evaluated for acetylcholinesterase inhibition, presenting 57.5±5.50% of acetylcholinesterase inhibition.45 Compound 217 was evaluated using in vitro binding assays of opioid receptors (subtypes δ, κ, and μ) and cannabinoid receptors (CB1 and CB2). Compound 217 selectively inhibited 42% of the specific binding of [3H]-DAMGO to CHO-K1 cell membranes expressing human μ-opioid receptors at 10 μM.79 For antiacetylcholinesterase activity, Mangrovamide C 24 showed moderate acetylcholinesterase inhibitory effect with an IC50 value of 58.0 μM.19 Compounds 222 and 223 were found to inhibit the conidial germination in the rice blast fungus Magnaporthe oryzae at concentrations of 25 μg/mL, 50 μg/mL, respectively.81 Talacoumarins A 400 and B 401 had moderate anti-Aβ42 aggregation activity, with relative inhibitory rates of (49.33 ± 3.16)% and (44.99 ± 3.64)% [the positive control EGCG had a relative inhibitory rate of (67.23 ± 2.51)%] at the concentration of 100 μM, and this is the first report on the Aβ42 inhibitory aggregation activity of coumarins.131 In the brine shrimp lethality assay, rubrumazine B 26 exhibited potent activity, with LD50 value of 2.43 μM, Compounds 25, 27 displayed modest activities, with LD50 values of 29.8 and 16.5 μM.20 Compound 523 exhibited the most potent activity against HMG-CoA reductase, with an IC50 value of 387 μM. In addition, the present study indicated the direct interaction of compound 523 with HMG-CoA reductase.160 Talaraculones A and B (357 and 358) exhibited stronger inhibitory activity against α-glucosidase than the positive control acarbose (IC50 = 101.5 μM), with IC50 values of 78.6 and 22.9 μM, respectively.122 Aspereusin A 526 was active against acetylcholinesterase (AchE) with a ratio of 62% at the concentration of 50 μM.93 (–) Benzomalvins E 80 enhanced the cytotoxic capability of 5-fluorouracil against A549 on a different level.39 Compounds 142, 145, and 146 showed comparable seed-germination-promoting activities to that previously reported for the growth regulator cotylenin E.63 Exophiarin 422 has been evaluated by glucose uptake assay (GUA) using L6 skeletal muscle cells (myotubes), which takes up glucose for its metabolic activities through the glucose transporter protein, GLUT4. And exophiarin 422 with TPI-2 and TPI-5 have displayed moderate improvement in glucose uptake activity when tested in rat skeletal muscle cell line L6.135 Compound 478 dose-dependently inhibited bone morphogenetic protein–induced alkaline phosphatase activity in myoblasts with half-maximal inhibitory concentration values of 19.8 µM.150 Compounds 206–210 could inhibit the hepatic glucose production with EC50 values of 17.6, 30.1, 21.3, 9.6, and 9.9 μM, respectively, and decrease the cAMP contents in glucagon-induced HepG2 cells.74
Concluding Remarks and Discussion
Based on the above literature, as mentioned above, we analyzed the classification of these new compounds and the proportion of different types of these new compounds. From the analysis data, 279 of the 546 new compounds are active. In these activity categories, anticancer and antimicrobial active compounds accounted for a large proportion, 36.2% and 30.8%, respectively (Figure 9). From the strain sources of these new compounds, Aspergillus, Penicillium and Talaromyces accounted for the majority, accounting for about 56%. We also classified and analyzed the compounds from different strains, as illustrated in Figure 10. In addition, we also analyzed the habitats of these source strains. Compared with special habitats, such as alpinc, polar regions, oceans, etc., most of the strains are from non-extreme living environments (66.9%) and rhizosphere soil (21.8%) (Figure 11). We also made an interesting analysis to analyze the proportion of different compounds in the same activity, for example, among the 86 compounds with antimicrobial activity, there are 44 ketone compounds, accounting for about 51%. Among the compounds with immunosuppressive activity, terpenes and steroids accounted for about 68% (Figure 12).
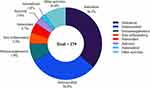 |
Figure 9 The percentage of compounds with various activities. |
 |
Figure 10 The number of various compounds from each genus. |
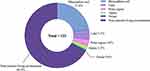 |
Figure 11 Proportion of strains in different living environments. |
 |
Figure 12 Number of different compounds with different activities. |
In this review, we summarized 546 new compounds derived from soil fungi, classified them according to their structures, and classified their activities, including anticancer, antimicrobial, anti-inflammatory, antioxidant, antiviral, antimalaria, and immunosuppressive activities. From the 546 new compounds from soil fungi and their activities summarized in this review since 2011, it is noteworthy that among these active compounds, anticancer and antimicrobial activities account for almost 51%. In order to facilitate readers’ understanding, we have made a table In this table, readers can more clearly and simply understand the microbial sources, biological activities, habitats of microbial sources, and relevant references of these new compounds. All information about the new compounds are briefly summarized in Table 1. It may provide a lot of help for future drug research and development. Microorganisms have provided abundant sources of natural products, which have been developed as commercial products for human medicine, animal health, and plant crop protection.167 Fungi are an ideal source for obtaining novel skeletons through large-scale culture: compared with plants, fungi can proliferate rapidly from small amounts of spores to a mass of branching hyphaes, which are more environmentally friendly than collecting plant materials; compared with bacteria, fungi can be cultured in a solid medium for a longer time. Specifically, a large-scale culture can produce unexpected rearrangements and combinatorial chemistry, which serve as “dark tunnels” to novel scaffolds.9 Artifacts are formed during isolation/purification of natural products. Factors such as pH, temperature, light, oxygen, humidity, and metal ions can lead to structural changes. Specific examples have been discussed in detail in a recent review on the formation of natural-product artifacts.168 Artifacts are often intentionally or unintentionally overlooked, but in large-scale culture it is an issue that cannot be ignored. Extensive separation of large-scale extracts exposes natural products to longer expose to solvents, heat, air, light, and pH variations during extraction, partitioning, chromatography and drying.9 The change of these external factors will inevitably affect the metabolism of microorganisms and the change of compound structure. Therefore, one problem arising from the use of large-scale cultivation of fungi is how to obtain stable access to those minor metabolites with novel skeletons. This may need to be solved in combination with chemical synthesis. Another problem is the habitat source of the strains. In this review, we analyzed the habitats of the strains involved. Although most of the strains come from non-extreme habitats, we still expect to obtain fungal strains from some extreme habitats. The extremobiosphere encompasses a broad range of biomes that include hyperarid deserts, deep-sea sediments and permafrost soils, as well as acid and high-temperature environments. Such extreme habitats are characterized by combinations of environmental variables, such as anoxia, aridity, extreme temperatures, low concentrations of organic matter, high salinity and intense irradiation. The search for new bioactive compounds from the extremobiosphere rests on the premise that harsh abiotic conditions select for novel microorganisms that express new chemistry.169 In order to adapt to extreme habitats, the strains are bound to adjust their metabolism, and new secondary metabolites will increase in this process, we may get some compounds with good biological activity.
 |
Table 1 Brief Summary of New Compounds |
Acknowledgments
We thank the authors of all the references cited herein for their valuable contributions. This work was supported by grants from the National Major Scientific and Technological Special Project for “Food Safety” (No. 2019YFC1604604).”
Disclosure
The authors report no conflicts of interest in this work.
References
1. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803. doi:10.1021/acs.jnatprod.9b01285
2. Bräse S, Encinas A, Keck J, Nising CF. Chemistry and biology of mycotoxins and related fungal metabolites. Chem Rev. 2009;109(9):3903–3990. doi:10.1021/cr050001f
3. Pettit RK. Soil DNA libraries for anticancer drug discovery. Cancer Chemother Pharmacol. 2004;54(1):1–6. doi:10.1007/s00280-004-0771-8
4. Nazir R, Warmink JA, Boersma H, van Elsas JD. Mechanisms that promote bacterial fitness in fungal-affected soil microhabitats. FEMS Microbiol Ecol. 2010;71(2):169–185. doi:10.1111/j.1574-6941.2009.00807.x
5. Wu P, Yao L, Xu L, Xue J, Wei X. Bisacremines A-D, dimeric acremines produced by a soil-derived Acremonium persicinum strain. J Nat Prod. 2015;78(9):2161–2166. doi:10.1021/np501037x
6. Ji Y, Zhou Q, Liu G, et al. New protein tyrosine phosphatase inhibitors from fungus Aspergillus gorakhpurensis F07ZB1707. RSC Adv. 2021;11(17):10144–10153. doi:10.1039/D1RA00788B
7. Keller NP, Turner G, Bennett JW. Fungal secondary metabolism - from biochemistry to genomics. Nat Rev Microbiol. 2005;3(12):937–947. doi:10.1038/nrmicro1286
8. Evidente A, Kornienko A, Cimmino A, et al. Fungal metabolites with anticancer activity. Nat Prod Rep. 2014;31(5):617–627. doi:10.1039/C3NP70078J
9. Hu Z, Ye Y, Zhang Y. Large-scale culture as a complementary and practical method for discovering natural products with novel skeletons. Nat Prod Rep. 2021;38(10):1775–1793. doi:10.1039/D0NP00069H
10. Eamvijarn A, Kijjoa A, Bruyere C, et al. Secondary metabolites from a culture of the fungus Neosartorya pseudofischeri and their in vitro cytostatic activity in human cancer cells. Planta Med. 2012;78(16):1767–1776. doi:10.1055/s-0032-1315301
11. Ma L, Liu W, Shen L, et al. Spiroketals, isocoumarin, and indoleformic acid derivatives from saline soil derived fungus Penicillium raistrickii. Tetrahedron. 2012;68(10):2276–2282. doi:10.1016/j.tet.2012.01.054
12. Wang C, Liu M, Wei J, Wang H, Lin X. Chemical metabolites from fungus PHF-9 and their anti-tumor activity. Chin J New Drugs. 2012;21(14):96–100.
13. Wang X, You J, King JB, Powell DR, Cichewicz RH. Waikialoid A suppresses hyphal morphogenesis and inhibits biofilm development in pathogenic Candida albicans. J Nat Prod. 2012;75(4):707–715. doi:10.1021/np2009994
14. Gao H, Liu W, Zhu T, et al. Diketopiperazine alkaloids from a mangrove rhizosphere soil derived fungus Aspergillus effuses H1-1. Org Biomol Chem. 2012;10(47):9501–9506. doi:10.1039/c2ob26757h
15. Gao H, Zhu T, Li D, Gu Q, Liu W. Prenylated indole diketopiperazine alkaloids from a mangrove rhizosphere soil derived fungus Aspergillus effuses H1-1. Arch Pharm Res. 2013;36(8):952–956. doi:10.1007/s12272-013-0107-5
16. Eamvijarn A, Gomes N, Dethoup T, et al. Bioactive meroditerpenes and indole alkaloids from the soil fungus Neosartorya fischeri (KUFC 6344), and the marine-derived fungi Neosartorya laciniosa (KUFC 7896) and Neosartorya tsunodae (KUFC 9213). Tetrahedron. 2013;69(40):8583–8591. doi:10.1016/j.tet.2013.07.078
17. Fan Y, Wang Y, Liu P, et al. Indole-diterpenoids with anti-H1N1 activity from the aciduric fungus Penicillium camemberti OUCMDZ-1492. J Nat Prod. 2013;76(7):1328–1336. doi:10.1021/np400304q
18. Rukachaisirikul V, Rungsaiwattana N, Klaiklay S, et al. Indole-benzodiazepine-2,5-dione derivatives from a soil fungus Aspergillus sp. PSU-RSPG185. Tetrahedron. 2013;69(52):11116–11121. doi:10.1016/j.tet.2013.11.009
19. Yang B, Dong J, Lin X, Zhou X, Zhang Y, Liu Y. New prenylated indole alkaloids from fungus Penicillium sp. derived of mangrove soil sample. Tetrahedron. 2014;70(25):3859–3863. doi:10.1016/j.tet.2014.04.043
20. Meng LH, Du FY, Li XM, et al. Indolediketopiperazines of the isoechinulin class from Eurotium rubrum MA-150, a fungus obtained from marine mangrove-derived rhizospheric soil. J Nat Prod. 2015;78(4):909–913. doi:10.1021/np5007839
21. Wang J, He W, Qin X, et al. Three new indolyl diketopiperazine metabolites from the Antarctic soil-derived fungus Penicillium sp. SCSIO 05705. RSC Adv. 2015;5(84):68736–68742. doi:10.1039/C5RA10828D
22. Lacey HJ, Vuong D, Pitt JI, Lacey E, Piggott AM. Kumbicins A–D: bis-indolyl benzenoids and benzoquinones from an Australian soil fungus, Aspergillus kumbius. Aust J Chem. 2016;69(2):152. doi:10.1071/CH15488
23. An X, Feng BM, Chen G, Chen SF, Wang HF, Pei YH. Two new asterriquinols from Aspergillus sp. CBS-P-2 with anti-inflammatory activity. J Asian Nat Prod Res. 2016;18(8):737–743. doi:10.1080/10286020.2016.1161613
24. Kildgaard S, de Medeiros LS, Phillips E, et al. Cyclopiamines C and D: epoxide spiroindolinone alkaloids from Penicillium sp. CML 3020. J Nat Prod. 2018;81(4):785–790. doi:10.1021/acs.jnatprod.7b00825
25. Xu LL, Hai P, Zhang SB, et al. Prenylated indole diterpene alkaloids from a mine-soil-derived Tolypocladium sp. J Nat Prod. 2019;82(2):221–231. doi:10.1021/acs.jnatprod.8b00589
26. Guo QF, Yin ZH, Zhang JJ, et al. Chaetomadrasins A and B, two new cytotoxic cytochalasans from desert soil-derived fungus Chaetomium madrasense 375. Molecules. 2019;24(18):3240. doi:10.3390/molecules24183240
27. Zheng YY, Shen NX, Liang ZY, Shen L, Chen M, Wang CY. Paraherquamide J, a new prenylated indole alkaloid from the marine-derived fungus Penicillium janthinellum HK1-6. Nat Prod Res. 2020;34(3):378–384. doi:10.1080/14786419.2018.1534105
28. Zaman KAU, Hu Z, Wu X, Cao S. Tryptoquivalines W and X, two new compounds from a Hawaiian fungal strain and their biological activities. Tetrahedron Lett. 2020;61(14):151730. doi:10.1016/j.tetlet.2020.151730
29. Liu J, Hu B, Gao Y, et al. Bioactive tyrosine-derived cytochalasins from fungus Eutypella sp. D-1. Chem Biodivers. 2014;11(5):800–806. doi:10.1002/cbdv.201300218
30. Rukachaisirikul V, Rungsaiwattana N, Klaiklay S, Phongpaichit S, Borwornwiriyapan K. Sakayaroj, J., g-Butyrolactone, cytochalasin, cyclic carbonate, eutypinic acid, and phenalenone derivatives from the soil fungus Aspergillus sp. PSU-RSPG185. J Nat Prod. 2014;77(11):2375–2382. doi:10.1021/np500324b
31. Kang HH, Zhong MJ, Ma LY, Rong XG, Liu DS, Liu WZ. Iizukines C-E from a saline soil fungus Aspergillus iizukae. Bioorg Chem. 2019;91:103167. doi:10.1016/j.bioorg.2019.103167
32. Kawahara T, Itoh M, Izumikawa M, et al. New phenylspirodrimane metabolites MBJ-0030, MBJ-0031, and MBJ-0032 isolated from the soil fungal strain Stachybotrys sp. f23793. Biosci Biotechnol Biochem. 2020;84(8):1570–1575. doi:10.1080/09168451.2020.1757402
33. Zhao C, Liu G, Liu X, Zhang L, Li L, Liu L. Pycnidiophorones A–D, four new cytochalasans from the wetland derived fungus Pycnidiophora dispersa. RSC Adv. 2020;10(66):40384–40390. doi:10.1039/D0RA08072A
34. Jiang CX, Yu B, Miao YM, et al. Indole Alkaloids from a Soil-Derived. Clonostachys Rosea J Nat Prod. 2021;84(9):2468–2474. doi:10.1021/acs.jnatprod.1c00457
35. Wang H, Dai H, Heering C, et al. Targeted solid phase fermentation of the soil dwelling fungus Gymnascella dankaliensis yields new brominated tyrosine-derived alkaloids. RSC Adv. 2016;6(85):81685–81693. doi:10.1039/C6RA14554J
36. Zhang SY, Li JS, Zhang H, et al. Two new threonine-containing metabolites from fungus Curvularia inaequalis strain HS-FG-257. Nat Prod Res. 2020;1:1–6.
37. Yao G, Sebisubi F, Voo L, Ho C, Tan G, Chang L. Citrinin derivatives from the soil filamentous fungus Penicillium sp. H9318. J Braz Chem Soc. 2011;22(6):1125–1129. doi:10.1590/S0103-50532011000600018
38. Jiang CX, Li J, Zhang JM, et al. Isolation, identification, and activity evaluation of chemical constituents from soil fungus Fusarium avenaceum SF-1502 and endophytic fungus Fusarium proliferatum AF-04. J Agric Food Chem. 2019;67(7):1839–1846. doi:10.1021/acs.jafc.8b05576
39. Feng QM, Feng Y, Zhang TY, et al. (±) Benzomalvins E isolated from Penicillium sp. SYPF 8411 in the rhizosphere soil of Codonopsis clematidea. Nat Prod Res. 2020;34(13):1884–1890. doi:10.1080/14786419.2019.1569004
40. Paluka J, Kanokmedhakul K, Soytong M, Soytong K, Kanokmedhakul S. Meroditerpene pyrone, tryptoquivaline and brasiliamide derivatives from the fungus Neosartorya pseudofischeri. Fitoterapia. 2019;137:104257. doi:10.1016/j.fitote.2019.104257
41. Murakami S, Hayashi N, Inomata T, Kato H, Hitora Y, Tsukamoto S. Induction of secondary metabolite production by fungal co-culture of Talaromyces pinophilus and Paraphaeosphaeria sp. J Nat Med. 2020;74(3):545–549. doi:10.1007/s11418-020-01400-1
42. Phainuphong P, Rukachaisirikul V, Saithong S, et al. pyrrolidine and piperidine derivatives from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178. Bioorg Med Chem. 2018;26(15):4502–4508. doi:10.1016/j.bmc.2018.07.036
43. Lin Y, Wang L, Wang Y, Wang W, Hao J, Zhu W. Bioactive Natural Products of Aspergillus sp. OUCMDZ-1914, an Aciduric Fungus from the Mangrove Soils. Chin J Organic Chem. 2015;35(9):1955. doi:10.6023/cjoc201504007
44. Ishii T, Nonaka K, Sugawara A, et al. Cinatrins D and E, and virgaricin B, three novel compounds produced by a fungus, Virgaria boninensis FKI-4958. J Antibiot. 2015;68(10):633–637. doi:10.1038/ja.2015.45
45. Teles AP, Takahashi JA. Paecilomide, a new acetylcholinesterase inhibitor from Paecilomyces lilacinus. Microbiol Res. 2013;168(4):204–210. doi:10.1016/j.micres.2012.11.007
46. Abdel-Wahab NM, Harwoko H, Muller WEG, et al. Cyclic heptapeptides from the soil-derived fungus Clonostachys rosea. Bioorg Med Chem. 2019;27(17):3954–3959. doi:10.1016/j.bmc.2019.07.025
47. Miyano R, Matsuo H, Mokudai T, et al. Trichothioneic acid, a new antioxidant compound produced by the fungal strain Trichoderma virens FKI-7573. J Biosci Bioeng. 2020;129(4):508–513. doi:10.1016/j.jbiosc.2019.11.007
48. Pittayakhajonwut P, Dramae A, Intaraudom C, et al. Two new drimane sesquiterpenes, fudecadiones A and B, from the soil fungus Penicillium sp. Planta Med. 2011;77(1):74–76. doi:10.1055/s-0030-1250057
49. Zhang SY, Li ZL, Guan LP, et al. Structure determination of two new trichothecenes from a halotolerant fungus Myrothecium sp. GS-17 by NMR spectroscopy. Magnetic Resonance Chem. 2012;50(9):632–636. doi:10.1002/mrc.3845
50. Meng LH, Li XM, Liu Y, Wang BG. Penicibilaenes A and B, sesquiterpenes with a tricyclo[6.3.1.01,5]dodecane skeleton from the marine isolate of Penicillium bilaiae MA-267. Org Lett. 2014;16(23):6052–6055. doi:10.1021/ol503046u
51. Daengrot C, Rukachaisirikul V, Tansakul C, et al. Eremophilane sesquiterpenes and diphenyl thioethers from the soil fungus Penicillium copticola PSU-RSPG138. J Nat Prod. 2015;78(4):615–622. doi:10.1021/np5005328
52. Liu D, Huang Y, Li C, et al. A new sesquiterpenoid derivative from the coastal saline soil fungus Aspergillus fumigatus. Records Natural Products. 2016;10(6):708–713.
53. Wang J, Wei X, Qin X, et al. Antiviral merosesquiterpenoids produced by the Antarctic fungus Aspergillus ochraceopetaliformis SCSIO 05702. J Nat Prod. 2016;79(1):59–65. doi:10.1021/acs.jnatprod.5b00650
54. Wang Y, Wang Y, Wu AA, et al. New 12,8-Eudesmanolides from Eutypella sp. 1-15. J Antibiot (Tokyo). 2017;70(10):1029–1032. doi:10.1038/ja.2017.89
55. Rukachaisirikul V, Chinpha S, Phongpaichit S, Saikhwan N, Sakayaroj J, Preedanon S. Sesquiterpene and monoterpene derivatives from the soil-derived fungus Trichoderma reesei PSU-SPSF013. Phytochem Lett. 2019;30:124–129. doi:10.1016/j.phytol.2019.01.023
56. Tran TD, Wilson BAP, Henrich CJ, et al. Structure elucidation and absolute configuration of metabolites from the soil-derived fungus Dictyosporium digitatum using spectroscopic and computational methods. Phytochemistry. 2020;173:112278. doi:10.1016/j.phytochem.2020.112278
57. Yaosanit W, Rukachaisirikul V, Phongpaichit S, Preedanon S, Sakayaroj J. Sesquiterpenes from the soil-derived fungus Trichoderma citrinoviride PSU-SPSF346. Beilstein J Org Chem. 2022;18:479–485. doi:10.3762/bjoc.18.50
58. Li F, Mo S, Yin J, et al. Structurally diverse metabolites from a soil-derived fungus Aspergillus calidoustus. Bioorg Chem. 2022;127:105988. doi:10.1016/j.bioorg.2022.105988
59. Lu XL, Liu JT, Liu XY, et al. Pimarane diterpenes from the Arctic fungus Eutypella sp. D-1. J Antibiot. 2014;67(2):171–174. doi:10.1038/ja.2013.104
60. Zhang YH, Huang SD, Pan HQ, et al. Structure determination of two new indole-diterpenoids from Penicillium sp. CM-7 by NMR spectroscopy. Magnetic Resonance Chem. 2014;52(6):306–309. doi:10.1002/mrc.4065
61. Wang X, Sun K, Wang B. Bioactive pimarane diterpenes from the Arctic fungus Eutypella sp. D-1. Chem Biodivers. 2018;15(2):e1700501. doi:10.1002/cbdv.201700501
62. Yu H, Wang X, Zhang Y, et al. Libertellenones O–S and Eutypellenones A and B, pimarane diterpene derivatives from the Arctic fungus Eutypella sp. D-1. J Nat Prod. 2018;81(7):1553–1560. doi:10.1021/acs.jnatprod.8b00039
63. Bie Q, Chen C, Yu M, et al. Dongtingnoids A-G: fusicoccane diterpenoids from a Penicillium species. J Nat Prod. 2019;82(1):80–86. doi:10.1021/acs.jnatprod.8b00694
64. Xu LL, Pang XJ, Shi Q, Xian PJ, Tao YD, Yang XL. Two new prenylated indole diterpenoids from Tolypocladium sp. and their antimicrobial activities. Chem Biodivers. 2019;16(6):e1900116.
65. Zhang Y, Li XM, Shang Z, Li CS, Ji NY, Wang BG. Meroterpenoid and diphenyl ether derivatives from Penicillium sp. MA-37, a fungus isolated from marine mangrove rhizospheric soil. J Nat Prod. 2012;75(11):1888–1895. doi:10.1021/np300377b
66. Sun J, Zhu ZX, Song YL, et al. Nitric oxide inhibitory meroterpenoids from the fungus Penicillium purpurogenum MHZ 111. J Nat Prod. 2016;79(5):1415–1422. doi:10.1021/acs.jnatprod.6b00160
67. Feng Q, Yu Y, Tang M, et al. Four new hybrid polyketide-terpenoid metabolites from the Penicillium sp. SYPF7381 in the rhizosphere soil of Pulsatilla chinensis. Fitoterapia. 2018;125:249–257. doi:10.1016/j.fitote.2018.01.010
68. Shaaban M, El-Metwally MM, Abdel-Razek AA, Laatsch H, Terretonin M. A new meroterpenoid from the thermophilic Aspergillus terreus TM8 and revision of the absolute configuration of penisimplicins. Nat Prod Res. 2018;32(20):2437–2446. doi:10.1080/14786419.2017.1419230
69. Liu M, Zhang X, Shen L, et al. Bioactive polyketide-terpenoid hybrids from a soil-derived fungus Bipolaris zeicola. J Org Chem. 2020;1:54.
70. Wu P, Xue J, Yao L, Xu L, Li H, Wei X. Bisacremines E-G, three polycyclic dimeric acremines produced by Acremonium persicinum SC0105. Org Lett. 2015;17(19):4922–4925. doi:10.1021/acs.orglett.5b02536
71. Mo S, Yin J, Ye Z, et al. Asperanstinoids A-E: undescribed 3,5-dimethylorsellinic acid-based meroterpenoids from Aspergillus calidoustus. Phytochemistry. 2021;190:112892. doi:10.1016/j.phytochem.2021.112892
72. Liu M, Gu L, Shen L, et al. Bipolaquinones A-J, Immunosuppressive Meroterpenoids from a Soil-Derived. Bipolaris zeicola J Nat Prod. 2021;84(9):2427–2436. doi:10.1021/acs.jnatprod.1c00327
73. Liu M, Zhang X, Shen L, et al. Meroterpenoids with Potent Immunosuppressive Activity from Fungus Bipolaris zeicola. Chin Chem. 2021;39(9):7.
74. Xiao N, Xu Y, Zhang X, et al. Anti-Diabetic Indole-Terpenoids From Penicillium sp. HFF16 Isolated From the Rhizosphere Soil of Cynanchum bungei Decne. Front Chem. 2022;9:792810. doi:10.3389/fchem.2021.792810
75. Liu LF, Zhang H, Qi H, Wang XM, Wang JD, Tan GS. A new androstanoid metabolite from a soil fungus Curvularia borreriae strain HS-FG-237. Nat Prod Res. 2017;31(9):1080–1084. doi:10.1080/14786419.2016.1272115
76. Guo W, Liu W, Xiao F, et al. New cytotoxic steroid produced by the soil-derived fungus Aspergillus flavus JDW-1. Nat Prod Commun. 2019;14(5):1–9. doi:10.1177/1934578X19850376
77. Wang CC, Liu HZ, Liu M, Zhang YY, Li TT, Lin XK. Cytotoxic metabolites from the soil-derived fungus Exophiala pisciphila. Molecules. 2011;16(4):2796–2801. doi:10.3390/molecules16042796
78. Zhang H, Mao LL, Qian PT, Shan WG, Wang JD, Bai H. Two new metabolites from a soil fungus Curvularia affinis strain HS-FG-196. J Asian Nat Prod Res. 2012;14(11):1078–1083. doi:10.1080/10286020.2012.713351
79. Leon F, Gao J, Dale OR, et al. Secondary metabolites from Eupenicillium parvum and their in vitro binding affinity for human opioid and cannabinoid receptors. Planta Med. 2013;79(18):1756–1761. doi:10.1055/s-0033-1351099
80. Rukachaisirikul V, Satpradit S, Klaiklay S, Phongpaichit S, Borwornwiriyapan K, Sakayaroj J. Polyketide anthraquinone, diphenyl ether, and xanthone derivatives from the soil fungus Penicillium sp. PSU-RSPG99. Tetrahedron. 2014;70(34):5148–5152. doi:10.1016/j.tet.2014.05.105
81. Sandjo LP, Thines E, Opatz T, Schuffler A. Tanzawaic acids I-L: four new polyketides from Penicillium sp. IBWF104-06. Beilstein J Org Chem. 2014;10:251–258. doi:10.3762/bjoc.10.20
82. Cai S, King JB, Du L, Powell DR, Cichewicz RH. Bioactive sulfur-containing sulochrin dimers and other metabolites from an Alternaria sp. isolate from a Hawaiian soil sample. J Nat Prod. 2014;77(10):2280–2287. doi:10.1021/np5005449
83. Liu Y, Li X, Meng L, Wang B. Polyketides from the marine mangrove-derived fungus Aspergillus ochraceus MA-15 and their activity against aquatic pathogenic bacteria. Phytochem Lett. 2015;12:232–236. doi:10.1016/j.phytol.2015.04.009
84. Liu D, Yan L, Ma L, et al. Diphenyl derivatives from coastal saline soil fungus Aspergillus iizukae. Arch Pharm Res. 2015;38(6):1038–1043. doi:10.1007/s12272-014-0371-z
85. Ma LY, Liu DS, Li DG, et al. Pyran rings containing polyketides from Penicillium raistrickii. Mar Drugs. 2016;15(1):2. doi:10.3390/md15010002
86. Chen M, Zheng YY, Chen ZQ, et al. NaBr-induced production of brominated azaphilones and related tricyclic polyketides by the marine-derived fungus Penicillium janthinellum HK1-6. J Nat Prod. 2019;82(2):368–374. doi:10.1021/acs.jnatprod.8b00930
87. Zang Y, Genta-Jouve G, Retailleau P, et al. Talaroketals A and B, unusual bis(oxaphenalenone) spiro and fused ketals from the soil fungus Talaromyces stipitatus ATCC 10500. Org Biomol Chem. 2016;14(9):2691–2697. doi:10.1039/C5OB02657A
88. Chaiyosang B, Kanokmedhakul K, Sanmanoch W, et al. Bioactive oxaphenalenone dimers from the fungus Talaromyces macrosporus KKU-1NK8. Fitoterapia. 2019;134:429–434. doi:10.1016/j.fitote.2019.03.015
89. Dramae A, Intaraudom C, Bunbamrung N, Saortep W, Srichomthong K, Pittayakhajonwut P. Heptacyclic oligophenalenones from the soil fungus Talaromyces bacillisporus BCC17645. Tetrahedron. 2020;76(9):130980. doi:10.1016/j.tet.2020.130980
90. Zang Y, Genta-Jouve G, Escargueil AE, et al. Antimicrobial oligophenalenone dimers from the soil fungus Talaromyces stipitatus. J Nat Prod. 2016;79(12):2991–2996. doi:10.1021/acs.jnatprod.6b00458
91. Yang B, Tao H, Qin XC, et al. Aspergone, a new chromanone derivative from fungus Aspergillus sp. SCSIO41002 derived of mangrove soil sample. J Antibiotics. 2017;70(6):788–790. doi:10.1038/ja.2016.169
92. Kaur H, Onsare JG, Sharma V, Arora DS. Isolation, purification and characterization of novel antimicrobial compound 7-methoxy-2,2-dimethyl-4-octa-4’,6’-dienyl-2H-napthalene-1-one from Penicillium sp. and its cytotoxicity studies. AMB Express. 2015;5(1):1–13. doi:10.1186/s13568-015-0120-9
93. Wang Q, Yang YB, Yang XQ, et al. Lovastatin analogues and other metabolites from soil-derived Aspergillus terreus YIM PH30711. Phytochemistry. 2018;145(2018):146–152. doi:10.1016/j.phytochem.2017.11.006
94. Trisuwan K, Rukachaisirikul V, Borwornwiriyapan K, Phongpaichit S, Sakayaroj J. Benzopyranone, benzophenone, and xanthone derivatives from the soil fungus Penicillium citrinum PSU-RSPG95. Tetrahedron Lett. 2014;55(7):1336–1338. doi:10.1016/j.tetlet.2014.01.017
95. Kaur H, Arora DS, Sharma V. Isolation, purification, and characterization of antimicrobial compound 6-[1,2-dimethyl-6-(2-methyl-allyloxy)-hexyl]-3-(2-methoxy-phenyl)-chromen-4-one from Penicillium sp. HT-28. Appl Biochem Biotechnol. 2014;173(8):1963–1976. doi:10.1007/s12010-014-0979-y
96. Xu Y, Tian S, Yu H, Yang W, Zhu H. Two new compounds isolated from Aspergillus aculeatus. Chem J Chin Universities. 2015;36(6):1107–1111.
97. Daengrot C, Rukachaisirikul V, Tadpetch K, et al. Penicillanthone and penicillidic acids A–C from the soil-derived fungus Penicillium aculeatum PSU-RSPG105. RSC Adv. 2016;6(46):39700–39709. doi:10.1039/C6RA04401H
98. Rajachan OA, Kanokmedhakul K, Soytong K, Kanokmedhakul S. Mycotoxins from the fungus Botryotrichum piluliferum. J Agric Food Chem. 2017;65(7):1337–1341. doi:10.1021/acs.jafc.6b05522
99. Tao H, Wei X, Lin X, Zhou X, Dong J, Yang B. Penixanthones A and B, two new xanthone derivatives from fungus Penicillium sp. SYFz-1 derived of mangrove soil sample. Nat Prod Res. 2017;31(19):2218–2222. doi:10.1080/14786419.2017.1297442
100. Kang HH, Zhang HB, Zhong MJ, et al. Potential antiviral xanthones from a coastal saline soil fungus Aspergillus iizukae. Mar Drugs. 2018;16(11):449. doi:10.3390/md16110449
101. Maha A, Phainuphong P, Rukachaisirikul V, et al. Blennolide derivatives from the soil-derived fungus Trichoderma asperellum PSU-PSF14. Tetrahedron. 2018;74(39):5659–5664. doi:10.1016/j.tet.2018.07.041
102. Form IC, Bonus M, Gohlke H, Lin W, Daletos G, Proksch P. Xanthone, benzophenone and bianthrone derivatives from the hypersaline lake-derived fungus Aspergillus wentii. Bioorg Med Chem. 2019;27(20):115005. doi:10.1016/j.bmc.2019.07.021
103. Trisuwan K, Rukachaisirikul V, Borwornwiriyapan K, Phongpaichit S, Sakayaroj J. Pyrone derivatives from the soil fungus Fusarium solani PSU-RSPG37. Phytochem Lett. 2013;6(3):495–497. doi:10.1016/j.phytol.2013.06.008
104. Zhu H, Li D, Yan Q, et al. a-Pyrones, secondary metabolites from fungus Cephalotrichum microsporum and their bioactivities. Bioorg Chem. 2019;83:129–134. doi:10.1016/j.bioorg.2018.10.022
105. Zheng YY, Liang ZY, Shen NX, et al. New naphtho-g-pyrones isolated from marine-derived fungus Penicillium sp. HK1-22 and their antimicrobial activities. Mar Drugs. 2019;17(6):322. doi:10.3390/md17060322
106. Furukawa T, Fukuda T, Nagai K, Uchida R, Tomoda H. Helvafuranone produced by the fungus Aspergillus nidulans BF0142 isolated from hot spring-derived soil. Nat Prod Commun. 2016;11(7):1001–1003. doi:10.1177/1934578X1601100733
107. Meng LH, Mandi A, Li XM, Liu Y, Kurtan T, Wang BG. Isolation, stereochemical study, and antioxidant activity of benzofuranone derivatives from a mangrove-derived fungus Eurotium rubrum MA-150. Chirality. 2016;28(8):581–584. doi:10.1002/chir.22613
108. Phainuphong P, Rukachaisirikul V, Tadpetch K, et al. g-Butenolide and furanone derivatives from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178. Phytochemistry. 2017;137:165–173. doi:10.1016/j.phytochem.2017.02.008
109. An Y, Zhu H, Deng S, et al. α-Furanones, secondary metabolites from the fungus Cephalotrichum microsporum and their antibacterial activities. Phytochem Lett. 2019;30:58–61. doi:10.1016/j.phytol.2019.01.015
110. Kim J, Son S, Kim G, et al. Aromatic butenolides produced by a soil ascomycete Auxarthron sp. KCB15F070 derived from a volcanic island. Tetrahedron Lett. 2019;60(45):151227. doi:10.1016/j.tetlet.2019.151227
111. Chang JL, Xu HZ, Zhou J, et al. Antimicrobial furancarboxylic acids from a Penicillium sp. J Nat Prod. 2020;83(12):3606–3613. doi:10.1021/acs.jnatprod.0c00758
112. Ngwoke KG, El-Kashef DH, Daletos G, et al. R-Hexitronic acid, a new tetronic acid derivative isolated from a soil fungus FG9RK. Nat Prod Res. 2020;2:1–6.
113. Zhao C, Fu P, Zhang Y, Liu X, Ren F, Che Y. Sporulosol, a new ketal from the fungus Paraconiothyrium sporulosum. Molecules. 2018;23(6):1263. doi:10.3390/molecules23061263
114. Sun TY, Kuang RQ, Chen GD, et al. Three pairs of new isopentenyl dibenzo[b, e]oxepinone enantiomers from Talaromyces flavus, a wetland soil-derived fungus. Molecules. 2016;21(9):1184. doi:10.3390/molecules21091184
115. Phainuphong P, Rukachaisirikul V, Phongpaichit S, Preedanon S, Sakayaroj J. Diphenyl ethers and indanones from the soil-derived fungus Aspergillus unguis PSU-RSPG204. Tetrahedron. 2017;73(40):5920–5925. doi:10.1016/j.tet.2017.08.039
116. Ma LY, Zhang HB, Kang HH, et al. New butenolides and cyclopentenones from saline soil-derived fungus Aspergillus Sclerotiorum. Molecules. 2019;24(14):2642. doi:10.3390/molecules24142642
117. Yu HB, Jiao H, Zhu YP, Zhang JP, Lu XL, Liu XY. Bioactive metabolites from the Arctic fungus Nectria sp. B-13. J Asian Nat Prod Res. 2019;21(10):961–969. doi:10.1080/10286020.2018.1482880
118. Choochuay J, Xu X, Rukachaisirikul V, et al. Curvularin derivatives from the soil-derived fungus Aspergillus polyporicola PSU-RSPG187. Phytochem Lett. 2017;22:122–127. doi:10.1016/j.phytol.2017.09.011
119. Zhai MM, Qi FM, Li J, et al. Isolation of secondary metabolites from the soil-derived fungus Clonostachys rosea YRS-06, a biological control agent, and evaluation of antibacterial activity. J Agric Food Chem. 2016;64(11):2298–2306. doi:10.1021/acs.jafc.6b00556
120. Xu GB, Yang T, Bao JK, Fang DM, Li GY. Isochaetomium A2, a new bis(naphthodihydropyran-4-one) with antimicrobial and immunological activities from fungus Chaetomium microcephalum. Arch Pharm Res. 2014;37(5):575–579. doi:10.1007/s12272-013-0206-3
121. Chen M, Shen NX, Chen ZQ, Zhang FM, Chen Y, Penicilones A-D. Anti-MRSA azaphilones from the marine-derived fungus Penicillium janthinellum HK1-6. J Nat Prod. 2017;80(4):1081–1086. doi:10.1021/acs.jnatprod.6b01179
122. Ren J, Ding SS, Zhu A, Cao F, Zhu HJ. Bioactive azaphilone derivatives from the fungus Talaromyces aculeatus. J Nat Prod. 2017;80(8):2199–2203. doi:10.1021/acs.jnatprod.7b00032
123. Pang YW, Zhang LJ, Fang JS, et al. Two new antitumor constituents from a soil fungus Curvularia inaequalis (strain HS-FG-257). J Antibiot. 2013;66(5):287–289. doi:10.1038/ja.2012.128
124. Liu YP, Chen G, Wang HF, Zhang XL, Pei YH. Two new compounds from the fungus Penicillium crustosum YN-HT-15. J Asian Nat Prod Res. 2014;16(3):281–284. doi:10.1080/10286020.2013.867851
125. He J, Xu H, Yang L, et al. New isocoumarins and related metabolites from Talaromyces flavus. Nat Prod Commun. 2016;11(6):805–808. doi:10.1177/1934578X1601100627
126. Zhang TY, Wu YY, Zhang MY, et al. New antimicrobial compounds produced by Seltsamia galinsogisoli sp. nov., isolated from Galinsoga parviflora as potential inhibitors of FtsZ. Sci Rep. 2019;9(1):8319. doi:10.1038/s41598-019-44810-2
127. Orfali R, Perveen S. Secondary metabolites from the Aspergillus sp. in the rhizosphere soil of Phoenix dactylifera (Palm tree). BMC Chem. 2019;13(1):103. doi:10.1186/s13065-019-0624-5
128. Xu Y, Liu W, Wu D, et al. Sulfur-Containing Phenolic Compounds from the Cave Soil-Derived Aspergillus fumigatus GZWMJZ-152. J Nat Prod. 2022;85(2):433–440. doi:10.1021/acs.jnatprod.1c01158
129. Niu S, Liu D, Proksch P, Shao Z, Lin W. New polyphenols from a deep sea Spiromastix sp. fungus, and their antibacterial activities. Mar Drugs. 2015;13(4):2526–2540. doi:10.3390/md13042526
130. Liu L, Tang MX, Sang XN, et al. Three new tetralol analogs from soil-derived fungus Myrothecium verrucaria with anti-inflammatory activity. J Asian Nat Prod Res. 2019;21(1):33–42. doi:10.1080/10286020.2018.1439934
131. He JW, Qin DP, Gao H, et al. Two new coumarins from Talaromyces flavus. Molecules. 2014;19(12):20880–20887. doi:10.3390/molecules191220880
132. Tian JF, Li PJ, Li XX, et al. New antibacterial isocoumarin glycosides from a wetland soil derived fungal strain Metarhizium anisopliae. Bioorg Med Chem Lett. 2016;26(5):1391–1396. doi:10.1016/j.bmcl.2016.01.074
133. Zheng W, Ji YB, Li WL, et al. A pair of new isocoumarin epimers from soil fungus Hypoxylon sp. J Asian Nat Prod Res. 2017;19(10):993–999. doi:10.1080/10286020.2017.1347159
134. Almeida C, Perez-Victoria I, Gonzalez-Menendez V, et al. Non-geminal aliphatic dihalogenation pattern in dichlorinated diaporthins from Hamigera fusca NRRL 35721. J Nat Prod. 2018;81(6):1488–1492. doi:10.1021/acs.jnatprod.8b00041
135. Gohil AR, Deshmukh SK, Bhattacharya V, Lavhale R, Verekar S, Kate AS. Exophiarin, an isocoumarin from the fungus Exophiala sp. with antihyperglycemic activity. Nat Prod Res. 2019;2:1–9.
136. Ranji P, Wijeyaratne S, Jayawardana K, Gunaherath G. Citriquinones A and B, new benzoquinones from Penicillium citrinum. Nat Prod Commun. 2013;8(10):1431–1434. doi:10.1177/1934578X1300801024
137. Tansakul C, Rukachaisirikul V, Maha A, et al. A new phenalenone derivative from the soil fungus Penicillium herquei PSU-RSPG93. Nat Prod Res. 2014;28(20):1718–1724. doi:10.1080/14786419.2014.941363
138. Tadpetch K, Chukong C, Jeanmard L, et al. Cytotoxic naphthoquinone and a new succinate ester from the soil fungus Fusarium solani PSU-RSPG227. Phytochem Lett. 2015;11:106–110. doi:10.1016/j.phytol.2014.11.018
139. Azerang P, Khalaj V, Kobarfard F, Owlia P, Sardari S, Shahidi S. Molecular characterization of a fungus producing membrane active metabolite and analysis of the produced secondary metabolite. Iran Biomed J. 2019;23(2):121–128. doi:10.29252/ibj.23.2.121
140. Yu JS, Li C, Kwon M, et al. Herqueilenone A, a unique rearranged benzoquinone-chromanone from the Hawaiian volcanic soil-associated fungal strain Penicillium herquei FT729. Bioorg Chem. 2020;105(2):104397. doi:10.1016/j.bioorg.2020.104397
141. Yu JS, Jeong SY, Li C, et al. New phenalenone derivatives from the Hawaiian volcanic soil-associated fungus Penicillium herquei FT729 and their inhibitory effects on indoleamine 2,3-dioxygenase 1 (IDO1). Arch Pharm Res. 2022;45(2):105–113. doi:10.1007/s12272-022-01372-8
142. He J, Mu Z, Gao H, et al. New polyesters from Talaromyces flavus. Tetrahedron. 2014;70(29):4425–4430. doi:10.1016/j.tet.2014.02.060
143. Gao H, Guo W, Wang Q, et al. Aspulvinones from a mangrove rhizosphere soil-derived fungus Aspergillus terreus Gwq-48 with anti-influenza A viral (H1N1) activity. Bioorg Med Chem Lett. 2013;23(6):1776–1778. doi:10.1016/j.bmcl.2013.01.051
144. Klaiklay S, Rukachaisirikul V, Aungphao W, Phongpaichit S, Sakayaroj J. Depsidone and phthalide derivatives from the soil-derived fungus Aspergillus unguis PSU-RSPG199. Tetrahedron Lett. 2016;57(39):4348–4351. doi:10.1016/j.tetlet.2016.08.040
145. An YN, Zhang X, Zhang TY, et al. Penicimenolides A-F, resorcylic acid lactones from Penicillium sp., isolated from the rhizosphere soil of Panax notoginseng. Sci Rep. 2016;6:27396. doi:10.1038/srep27396
146. Zhou Y, Zhang YX, Zhang JP, et al. A new sesquiterpene lactone from fungus Eutypella sp. D-1. Nat Prod Res. 2017;31(14):1676–1681. doi:10.1080/14786419.2017.1286486
147. Miyano R, Matsuo H, Nonaka K, et al. Pochoniolides A and B, new antioxidants from the fungal strain Pochonia chlamydosporia var. spinulospora FKI-7537. J Biosci Bioeng. 2018;126(5):661–666. doi:10.1016/j.jbiosc.2018.05.003
148. Arunpanichlert J, Rukachaisirikul V, Chaiwarin T, et al. Dimeric g-lactone derivatives from the soil-derived fungus Lasiodiplodia theobromae NSTRU-PN1.4. Nat Prod Res. 2020;6:1–11.
149. Yang XY, Zhang JX, Ding QY, et al. Metabolites from two dominant thermophilic fungal species Thermomyces lanuginosus and Scytalidium thermophilum. Chem Biodivers. 2020;17:5.
150. Koyama N, Otoguro Y, Ohte S, Katagiri T, Tomoda H. Penicillic Acid Congener, a new inhibitor of BMP- induced alkaline phosphatase activity in myoblasts, produced by the fungus Penicillium sp. BF-0343. Nat Prod Commun. 2020;15(9):1–5. doi:10.1177/1934578X20942653
151. Zhou J, Gao Y, Chang JL, et al. Resorcylic acid lactones from an Ilyonectria sp. J Nat Prod. 2020;83(5):1505–1514. doi:10.1021/acs.jnatprod.9b01167
152. Cai S, Sun S, Zhou H, et al. Prenylated polyhydroxy-p-terphenyls from Aspergillus taichungensis ZHN-7-07. J Nat Prod. 2011;74(5):1106–1110. doi:10.1021/np2000478
153. Pang YW, Wang J, Xiang W, Liu Q, Wang X. A new benzoic acid derivative from a soil fungus Curvularia inaequalis strain HS-FG-257. Nat Product Res Dev. 2012;24(10):1331–1334.
154. Akone S, Rahn S, Henrich B, et al. 2-Pentenedioic acid derivatives from a soil-derived fungus Gongronella butleri. Phytochem Lett. 2014;10:184–188. doi:10.1016/j.phytol.2014.09.001
155. Liu WZ, Ma LY, Liu DS, et al. Peniciketals A-C, new spiroketals from saline soil derived Penicillium raistrichii. Org Lett. 2014;16(1):90–93. doi:10.1021/ol403076s
156. Masaphy S. A novel echinocandin MIG0310 with anticandida activity from newly isolated Fusarium sp. strain MS-R1. J Appl Microbiol. 2014;116(6):1458–1464. doi:10.1111/jam.12493
157. Hammerschmidt L, Aly AH, Abdel-Aziz M, et al. Cytotoxic acyl amides from the soil fungus Gymnascella dankaliensis. Bioorg. Med Chem. 2015;23(4):712–719. doi:10.1016/j.bmc.2014.12.068
158. Ahmad B, Rizwan M, Rauf A, et al. Isolation and structure elucidation, molecular docking studies of screlotiumol from soil borne fungi Sclerotium rolfsii and their reversal of multidrug resistance in mouse lymphoma cells. Asian Pacific J Cancer Prevention. 2016;17(4):2083–2087. doi:10.7314/APJCP.2016.17.4.2083
159. Fu Y, Wu P, Xue J, Wei X, Li H. Versicorin, a new lovastatin analogue from the fungus Aspergillus versicolor SC0156. Nat Prod Res. 2015;29(14):1363–1368.
160. Phainuphong P, Rukachaisirikul V, Saithong S, et al. Lovastatin analogues from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178. J Nat Prod. 2016;79(6):1500–1507. doi:10.1021/acs.jnatprod.5b00961
161. Wang Q, Yang X, Miao C, et al. A new pair of pentaketide diastereoisomers from Aspergillus melleus YIM PHI001. Records Natural Products. 2018;12(3):216–221. doi:10.25135/rnp.12.17.04.065
162. Liu F, Chen G, Zhang LH, et al. Isolation and structure elucidation of a new compound from the fungus Aspergillus flavipes PJ03-11. Nat Prod Res. 2018;32(1):30–35.
163. Liang ZY, Shen NX, Zheng YY, et al. Two new unsaturated fatty acids from the mangrove rhizosphere soil-derived fungus Penicillium javanicum HK1-22. Bioorg Chem. 2019;93:103331. doi:10.1016/j.bioorg.2019.103331
164. Ding T, Zhou Y, Qin JJ, Yang LJ, Zhang WD, Shen YH. Chemical constituents from wetland soil fungus Penicillium oxalicum GY1. Fitoterapia. 2020;142:104530. doi:10.1016/j.fitote.2020.104530
165. Fan B, Dewapriya P, Li F, et al. Pyrenosetin D, a new pentacyclic decalinoyltetramic acid derivative from the algicolous fungus Pyrenochaetopsis sp. FVE-087. Mar Drugs. 2020;18(6):281. doi:10.3390/md18060281
166. Pan X, Liu D, Wang J, et al. Peneciraistin C induces caspase-independent autophagic cell death through mitochondrial-derived reactive oxygen species production in lung cancer cells. Cancer Sci. 2013;104(11):1476–1482. doi:10.1111/cas.12253
167. Katz L, Baltz RH. Natural product discovery: past, present, and future. J Ind Microbiol Biotechnol. 2016;43(2–3):155–176. doi:10.1007/s10295-015-1723-5
168. Capon RJ. Extracting value: mechanistic insights into the formation of natural product artifacts - case studies in marine natural products. Nat Prod Rep. 2020;37(1):55–79. doi:10.1039/C9NP00013E
169. Okoro CK, Brown R, Jones AL, et al. Diversity of culturable actinomycetes in hyper-arid soils of the Atacama Desert, Chile. Antonie Van Leeuwenhoek. 2009;95(2):121–133. doi:10.1007/s10482-008-9295-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
