Back to Journals » Journal of Inflammation Research » Volume 15
The Neutrophil/Lymphocyte Ratio is an Independent Predictor of All-Cause Mortality in Patients with Idiopathic Hypereosinophilic Syndrome
Authors Xue J, Jiang J , Liu Y
Received 10 January 2022
Accepted for publication 7 March 2022
Published 15 March 2022 Volume 2022:15 Pages 1899—1906
DOI https://doi.org/10.2147/JIR.S357758
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Junshuai Xue,* Jianjun Jiang,* Yang Liu
Department of General Surgery, Vascular Surgery, Shandong University Qilu Hospital, Jinan City, Shandong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yang Liu, Department of General Surgery, Vascular Surgery, Shandong University Qilu Hospital, No. 107, Road Wen Hua Xi, Jinan City, Shandong Province, 250012, People’s Republic of China, Tel +86 18560088317, Email [email protected]
Background: Idiopathic hypereosinophilic syndrome (IHES) often causes inflammatory damage to multiple organs. However, whether immune/inflammatory indicators and other factors are associated with mortality in patients with IHES remains unclear.
Patients and Methods: The clinical data and follow-up results of 167 patients with IHES were retrospectively analyzed using Cox regression analysis and receiver operating characteristic curve (ROC).
Results: Of 167 patients, 120 were men (71.9%) and 47 were women (28.1%). The median age was 52 (36.0, 68.0) years. The median follow-up period was 42.8 (18.5, 75.1) months, during which all-cause mortality occurred in 26 patients (15.6%). Age (HR: 1.041, 95% CI: 1.015– 1.068; p = 0.002), lymphocyte counts (109/L, HR: 0.866, 95% CI: 0.816– 0.907; p = 0.013), platelet counts (109/L, HR: 0.994, 95% CI: 0.989– 0.999; p = 0.012) and NLR (HR: 1.161, 95% CI: 1.054– 1.280; p = 0.003) were independent risk factors for all-cause mortality. There was no relationship between PLR, and SII and all-cause mortality (p = 0.181 and 0.202, respectively). ROC analysis showed that the AUCs of age, lymphocyte count (109/L), platelet count (109/L) and NLR were 0.712 (95% CI: 0.601– 0.824), 0.584 (95% CI: 0.448– 0.719), 0.686 (95% CI: 0.560– 0.812), and 0.797 (95% CI: 0.695– 0.899), respectively, with sensitivities of 0.5, 0.462, 0.769, and 0.792, respectively, and specificities of 0.765, 0.745, 0.617, and 0.845, respectively. Kaplan–Meier analysis (Log rank test) showed that patients with age ≥ 73.5 years, lymphocyte count (109/L) < 1.45, platelet count (109/L) < 225 and NLR ≥ 2.54 had high mortality. Patients with high NLR (≥ 2.54) usually have multiorgan involvement, with cardiac involvement and skin involvement being the most common. Patients with NLR ≥ 2.54 had significantly higher absolute eosinophil counts (p = 0.047) and percentages (p = 0.041).
Conclusion: We identified NLR for the first time as an independent predictive factor for all-cause mortality in patients with IHES, necessitating its further application in clinical practice.
Keywords: idiopathic hypereosinophilic syndrome, mortality, risk variable, neutrophil/lymphocyte ratio
Introduction
Idiopathic hypereosinophilic syndrome (IHES) is a clinical syndrome that is characterized by increased eosinophil counts in the peripheral blood and bone marrow and functional impairment caused by eosinophil infiltration in multiple organ tissues, including cardiac, skin, pulmonary, gastrointestinal, and cerebral tissues.1–3 The risk factors for all-cause mortality in patients with IHES are unclear, and few investigations have evaluated the associated risk factors in a large number of patients with IHES. Immune/inflammatory response plays an important role in the development of cancer and venous thromboembolism (VTE). Recent studies have demonstrated the diagnostic and predictive value of neutrophil/lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune/inflammatory index (SII).4–6 However, it is unclear whether these indicators are associated with the mortality in patients with IHES.
In this study, we aimed to determine whether these immune/inflammatory indicators and other potential variables are associated with mortality in patients with IHES and whether they can serve as convenient predictors in clinical practice.
Patients and Methods
This was a retrospective, single-center study of patients with IHES admitted to Shandong University Qilu Hospital from January 1, 2011, to December 31, 2020. A total of 167 patients with IHES were included in this study. The patients’ clinical data were obtained from the electronic medical record system of Shandong University Qilu Hospital. The study protocols complied with the principles of the World Medical Association Declaration of Helsinki and its amendments. This study was approved by the Ethics Committee of Shandong University Qilu Hospital. The need for written informed consent was waived because this was a retrospective study based on previous medical record data and did not involve human specimens. The personal identifiers of all patients were removed. All the data were anonymized and maintained with confidentiality.
The patients’ clinical characteristics were recorded, including baseline clinical data, routine blood examination results, comorbidities (hypertension and diabetes mellitus), and the organs involved. Blood examination results were also analyzed, such as eosinophil count, eosinophil percentage, white blood cell count, neutrophil count, lymphocyte count, and platelet count. NLR was defined as neutrophil count (109/L)/lymphocyte count (109/L). PLR was defined as platelet count (109/L)/lymphocyte count (109/L). The systemic immune/inflammatory index (SII) was defined as platelet count (109/L) × neutrophil count (109/L)/lymphocyte count (109/L). All patients were followed up to record the all-cause mortality event.
Statistical Analysis
Statistical analysis was performed using R statistical software (version 4.0.3). For continuous variables, data were expressed as the median (p25, p75) and analyzed using an independent Student’s t-test or the Mann–Whitney test, as appropriate. Categorical variables were expressed as the number of cases and percentages (%) and were analyzed using the χ2 test or Fisher’s exact test. Multivariate Cox regression analysis was conducted to identify the risk variables related to all-cause mortality. The results were expressed as hazard ratios (HR) with 95% confidence intervals (95% CI). Receiver operating characteristic curve (ROC) was used to evaluate the predictive capability of independent risk variables, and the optimal cutoff value, sensitivity, and specificity. A Cox proportional hazards regression curve based on the identified risk variables was constructed, and the number of people at risk at each time point was determined. Survival curves were described by Kaplan-Meier analysis based on the cutoff values and compared using the Log rank test. p<0.05 was considered statistically significant.
Results
A total of 167 consecutive patients (120 men [71.9%] and 47 women [28.1%]) who met the criteria were included in this study, with a median age of 52 (36, 68) years. Of these 167 patients, 104 (62.3%) had cardiac involvement, 65 (39%) had skin involvement, 27 (16%) had pulmonary involvement, 32 (19%) had gastrointestinal involvement, and 13 (8%) had cerebral involvement. The last follow-up was in May 2021, with a median follow-up period of 42.8 (18.5, 75.1) months. During the follow-up period, all-cause mortality occurred in 26 patients (15.6%). The detailed clinical characteristics are presented in Table 1.
 |
Table 1 Basic Clinical and Laboratory Characteristics in Patients with IHES |
Multivariate Cox regression analysis identified age (HR:1.041, 95% CI:1.015–1.068; p=0.002), lymphocyte count (HR:0.866, 95% CI:0.816–0.907; p=0.013), platelet count (HR:0.994, 95% CI:0.989–0.999; p=0.012) and NLR (HR:1.161, 95% CI:1.054–1.280; p=0.003) as independent risk variables (Table 2). There was no relationship between cardiac involvement, eosinophil count, or eosinophil percentage and all-cause mortality (p=0.066, 0.871, and 0.153, respectively). The AUCs, sensitivities, specificities and cutoff values of age, lymphocyte count, platelet count and NLR are presented in Table 2. ROC analysis indicated that NLR had better predictive capability for all-cause mortality than the other variables in patients with IHES. The Cox proportional hazards regression curve is shown in Figure 1.
 |
Table 2 ROC Analysis Results for Risk Variables |
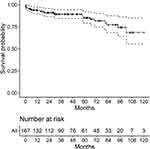 |
Figure 1 Cox proportional hazards regression curve. |
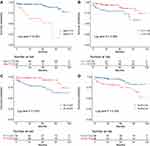
As shown in Figure 2, Kaplan–Meier analysis (Log rank test) based on the cutoff values showed significant differences in all-cause mortality between age≥73.5 and <73.5 years (A), lymphocyte count (LY)≥1.45 and <1.45 (B), platelet count (PLT)≥225 and <225 (C), and NLR≥2.54 and <2.54 (D) groups. The mortality rate was much higher in patients with older age (≥73.5), low lymphocyte count (<1.45), low platelet count (<225) and high NLR (≥2.54).
There were more elderly patients, patients with hypertension, and smokers in the high NLR (≥2.54) group than in the low NLR group (<2.54; Table 3). Patients with IHES with high NLR (≥2.54) were much more likely to have more than two organs involved, of which cardiac involvement and skin involvement were the most common. In contrast, patients with IHES with low NLR (<2.54) usually presented with none or one organ involvement. Patients with high NLR (≥2.54) had significantly higher absolute eosinophil count (p=0.047) and eosinophil percentage (p=0.041). However, there was no significant difference in PLR (p=0.086) and SII (p=0.111) between the high NLR (≥2.54) and low NLR (<2.54) groups.
 |
Table 3 Comparison Between Patients with High and Low NLR |
Discussion
IHES is a benign blood disorder that is characterized by excess eosinophils in the peripheral blood and is the most serious eosinophil-related disease, often results in damage to various organs, including the skin, lungs, and cardiovascular and gastrointestinal systems.7 The vascular system, including the cardiovascular, peripheral, and visceral vessels, is often involved in eosinophilia. Previous studies reported thrombosis at various sites, such as cardiac mural thrombosis, intracardiac thrombosis, cerebral arterial thrombosis, inferior vena cava thrombosis, superior vena cava thrombosis, portal vein thrombosis, hepatic vein thrombosis, mesenteric vein thrombosis, lower limb deep vein thrombosis, and pulmonary embolism.1,8–12 Approximately 40–84% of patients with eosinophilia have cardiovascular involvement, and 4–41% have vascular thrombosis, including pulmonary embolism and peripheral arterial thrombosis, with 5–10% of patients dying from this complication.1–3 Previous studies showed that vascular system involvement is a major cause of death in patients with eosinophilia.1,13 Although the proportion of cardiac involvement was high (62%) in our cohort, Cox regression analysis showed that cardiac involvement was not significantly different between the survivor and mortality groups. We identified age and NLR as risk variables associated with all-cause mortality and lymphocytes and platelets as protective variables, providing new clues for clinical practice.
Eosinophilia has been confirmed to result in damage to the vascular wall, which in turn can cause vascular disease.1 Peak absolute eosinophil count in peripheral blood was found to be an independent risk variable for pulmonary embolism in patients with eosinophilia.14 An increased eosinophil count was associated with an increased risk of venous thrombosis.15 Persistent eosinophilia was the only variable associated with a shorter time to recurrence of venous thromboembolic (VTE).16 Although eosinophil count was not associated with mortality independently in this cohort, patients with IHES with high NLR (≥2.54) had significantly higher absolute eosinophil count (p=0.047) and eosinophil percentage (p=0.041).
An increasing number of studies have shown that internal immune/inflammatory responses play an important role in the development of VTE, asthma, and cancers. Indicators, such as NLR and PLR, have diagnostic and predictive capability for VTE.4–6 Hossein Esmaeilzadeh et al identified NLRs in 211 pediatric patients with more severe asthma exacerbation in hospitalized children compared with that in non-hospitalized patients.17 NLR can serve as and indicator of the severity of asthma exacerbation. SII provides a comprehensive picture of the balance between host immunity and inflammatory status and is associated with survival in patients with cancers.18–20 This study is the first to report NLR as an independent predictor of all-cause mortality with good capability in patients with IHES. In contrast, PLR and SII were not associated with mortality. Therefore, NLR may be a feasible and reliable blood indicator to predict mortality in patients with IHES and merits further clinical application. Considering the high absolute eosinophil count in patients with high NLR, the interaction between neutrophils and lymphocytes and eosinophils may have a substantial impact on the progression of IHES, and further in vitro and in vivo experiments are needed to explore the underlying mechanisms.
Eosinophils are a promising new target for preventing and treating atherosclerosis and thrombosis. Marx et al demonstrated that eosinophils promote atherosclerotic plaque formation by increasing the exposure of von Willebrand factors on endothelial cells and by enhancing platelet adhesion. Their interaction with platelets also induces eosinophil extracellular traps (EETs) formation, leading to thrombosis.21 Cugno et al found that eosinophils in human blood contain varying amounts of tissue factors. Patients with eosinophilia have higher tissue factor gene expression than healthy people, which may lead to an increased risk of thrombosis.22 Mukai et al found that eosinophil granule protein-major basic protein (MBP) inhibits the function of thrombomodulin (TM) as a cofactor for thrombin-catalyzed activation of protein C.23 In patients with eosinophilia, this may lead to an increased risk of thrombosis, because the function of TM as a cofactor in the anticoagulant system is inhibited.
In conclusion, eosinophils may be involved in concurrent vascular disease through various pathways, including interactions with platelets, release of tissue factors, and formation of extracellular traps. Although statistical analysis revealed that eosinophil count was not related to mortality, the cited studies still indicated that restoring abnormally elevated eosinophils was particularly important in the clinical treatment of patients with IHES, which may prevent the onset of all-cause mortality events.
This study had the following limitations. It was a single-center retrospective study. Given the low prevalence of IHES itself, the number of cases included in the study is therefore small, and the results may be biased. Therefore, multicenter studies with more cases are needed to validate our preliminary findings. Animal and cellular studies are required to further explore the specific mechanisms by which the interaction between neutrophils, lymphocytes, and eosinophils affects long-term mortality.
Conclusion
This study identified NLR for the first time as an independent predictive factor associated with all-cause mortality in patients with IHES. Patients with high NLR had a significantly higher mortality rate, absolute eosinophil count, and more organ involvement. Further prospective cohort studies are needed to validate these preliminary findings.
Abbreviations
AUC, area under the ROC curve; CI, confidence interval; EETs, eosinophil extracellular traps; HR, hazard ratio; MBP, major basic protein; NLR, neutrophil/lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ROC, receiver operating characteristic curve; SII, systemic immune/inflammatory index; TM¸ thrombomodulin; VTE, venous thromboembolism.
Acknowledgments
We thank Liwen Bianji (Edanz) for editing the English text of a draft of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This report is supported by National Natural Science Foundation of China (Grant No.82000451).
Disclosure
Junshuai Xue and Jianjun Jiang are co-first authors for this study. The authors declare that there is no conflict of interest regarding the study.
References
1. Ogbogu PU, Rosing DR, Horne MR. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27(3):457–475. doi:10.1016/j.iac.2007.07.001
2. Weller PF, Bubley GJ. The idiopathic hypereosinophilic syndrome. Blood. 1994;83(10):2759–2779. doi:10.1182/blood.V83.10.2759.2759
3. Kanno H, Ouchi N, Sato M, et al. Hypereosinophilia with systemic thrombophlebitis. Hum Pathol. 2005;36(5):585–589. doi:10.1016/j.humpath.2005.03.017
4. Barker T, Rogers VE, Henriksen VT, et al. Is there a link between the neutrophil/lymphocyte ratio and venous thromboembolic events after knee arthroplasty? A pilot study. J Orthop Traumatol. 2016;17(2):163–168. doi:10.1007/s10195-015-0378-3
5. Ming L, Jiang Z, Ma J, et al. Platelet-to-lymphocyte ratio, neutrophil/lymphocyte ratio, and platelet indices in patients with acute deep vein thrombosis. Vasa. 2018;47(2):143–147. doi:10.1024/0301-1526/a000683
6. Zhu X, Yao Y, Yao C, et al. Predictive value of lymphocyte to monocyte ratio and monocyte to high-density lipoprotein ratio for acute deep vein thrombosis after total joint arthroplasty: a retrospective study. J Orthop Surg Res. 2018;13(1):211. doi:10.1186/s13018-018-0910-2
7. Gotlib J. World Health Organization-defined eosinophilic disorders: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90(11):1077–1089. doi:10.1002/ajh.24196
8. Lin J, Huang X, Zhou W, et al. Thrombosis in the portal venous system caused by hypereosinophilic syndrome: a case report. Medicine. 2018;97(48):e13425. doi:10.1097/MD.0000000000013425
9. Boussir H, Ghalem A, Ismaili N, et al. Eosinophilic myocarditis and hypereosinophilic syndrome. J Saudi Heart Assoc. 2017;29(3):211–213. doi:10.1016/j.jsha.2016.11.001
10. Fujita K, Ishimaru H, Hatta K, et al. Hypereosinophilic syndrome as a cause of fatal thrombosis: two case reports with histological study. J Thromb Thrombolysis. 2015;40(2):255–259. doi:10.1007/s11239-014-1151-9
11. Zakhama L, Slama I, Boussabah E, et al. Recurrent native and prosthetic mitral valve thrombosis in idiopathic hypereosinophilic syndrome. J Heart Valve Dis. 2014;23(2):168–170.
12. Todd S, Hemmaway C, Nagy Z. Catastrophic thrombosis in idiopathic hypereosinophilic syndrome. Br J Haematol. 2014;165(4):425. doi:10.1111/bjh.12729
13. Klion AD, Bochner BS, Gleich GJ, et al. Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006;117(6):1292–1302. doi:10.1016/j.jaci.2006.02.042
14. Liu Y, Meng X, Feng J, et al. Hypereosinophilia with concurrent venous thromboembolism: clinical features, potential risk variables, and short-term outcomes in a Chinese cohort. Sci Rep. 2020;10(1):8359. doi:10.1038/s41598-020-65128-4
15. Reau V, Vallee A, Terrier B, et al. Venous thrombosis and predictors of relapse in eosinophil-related diseases. Sci Rep. 2021;11(1):6388. doi:10.1038/s41598-021-85852-9
16. Kushnir M, Cohen HW, Billett HH. Persistent neutrophilia is a marker for an increased risk of venous thrombosis. J Thromb Thrombolysis. 2016;42(4):545–551. doi:10.1007/s11239-016-1398-4
17. Esmaeilzadeh H, Nouri F, Nabavizadeh SH, et al. Can eosinophilia and neutrophil-lymphocyte ratio predict hospitalization in asthma exacerbation? Allergy Asthma Clin Immunol. 2021;17(1):16. doi:10.1186/s13223-021-00512-x
18. Wang BL, Tian L, Gao XH, et al. Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clin Chem Lab Med. 2016;54(12):1963–1969. doi:10.1515/cclm-2015-1191
19. Dolan RD, McSorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing Surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119(1):40–51. doi:10.1038/s41416-018-0095-9
20. Cannon NA, Meyer J, Iyengar P, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic variables after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol. 2015;10(2):280–285. doi:10.1097/JTO.0000000000000399
21. Marx C, Novotny J, Salbeck D, et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood. 2019;134(21):1859–1872. doi:10.1182/blood.2019000518
22. Cugno M, Marzano AV, Lorini M, et al. Enhanced tissue variable expression by blood eosinophils from patients with hypereosinophilia: a possible link with thrombosis. PLoS One. 2014;9(11):e111862. doi:10.1371/journal.pone.0111862
23. Mukai HY, Ninomiya H, Ohtani K, et al. Major basic protein binding to thrombomodulin potentially contributes to the thrombosis in patients with eosinophilia. Br J Haematol. 1995;90(4):892–899. doi:10.1111/j.1365-2141.1995.tb05211.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.