Back to Journals » Drug Design, Development and Therapy » Volume 13
The neuroprotective effect of bisperoxovandium (pyridin-2-squaramide) in intracerebral hemorrhage
Authors Liao XY, Lei Y, Chen SF, Cheng J, Zhao D, Zhang ZF , Han X, Zhang Y, Liao HB, Zhuang Y, Chen J, Zhou HB, Wan Q, Zou YY
Received 15 February 2019
Accepted for publication 18 April 2019
Published 13 June 2019 Volume 2019:13 Pages 1957—1967
DOI https://doi.org/10.2147/DDDT.S204956
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Xin-Yu Liao,1,* Yang Lei,2,* Song-Feng Chen,2 Jing Cheng,3 Dan Zhao,4 Zhi-Feng Zhang,2 Xin Han,5 Ya Zhang,2 Hua-Bao Liao,2 Yang Zhuang,2 Juan Chen,6 Hai-Bing Zhou,5 Qi Wan,7 Ying-Ying Zou,1
1Department of Pathology and Pathophysiology, Faculty of Basic Medical Sciences, Kunming Medical University, Kunming 650500, People’s Republic of China; 2Department of Physiology, School of Basic Medical Sciences, Wuhan University School of Medicine, Wuhan 430071, People’s Republic of China; 3Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan 430060, People’s Republic of China; 4Department of Physiology, School of Basic Medical Sciences, Hubei University of Medicine, Shiyan, Hubei 442000, People’s Republic of China; 5School of Pharmacy, Wuhan University, Wuhan 430071, People’s Republic of China; 6Department of Neurology, The Central Hospital of Wuhan, Tongji Medical College of Huazhong University of Science & Technology, Wuhan 430013, People’s Republic of China; 7Institute of Neuroregeneration & Neurorehabilitation, Collaborative Innovation Center for Brain Science, Department of Neurosurgery of the Affiliated Hospital, Qingdao University, Qingdao 266071, People’s Republic of China
*These authors contributed equally to this work
Background: The authors have recently designed a new compound bisperoxovandium (pyridin-2-squaramide) [bpV(pis)] and verified that bpV(pis) confers neuroprotection through suppressing PTEN and activating ERK1/2, respectively. Intracerebral hemorrhage (ICH) is the second most common cause of stroke and has severe clinical outcome. In this study, we investigate the effect of bpV(pis) in ICH model both in vivo and in vitro.
Materials and methods: The novel drug bpV(pis) was synthesized in the Faculty of Pharmacy, Wuhan University School of Medicine. An ICH model was generated on both SD rats and cells. bpV(pis) was injected into intracerebroventricular or culture media. Western blotting was applied to test the signal pathway. To determine the effect of bpV(pis) on PTEN inhibition and ERK1/2 activation, we measured the phosphorylation level of AKT (a direct downstream target of PTEN that negatively regulates AKT) and ERK1/2. FJC, MTT, and LDH were applied to measure the cell viability. Neurobehavioral tests were performed to measure the effect of bpV(pis).
Results: The in vivo results showed that intracerebroventricular administration of bpV(pis) significantly alleviates hematoma, the damage of brain–blood barrier and brain edema. The in vitro results demonstrated that bpV(pis) treatment reduces ICH-induced neuronal injury. Western blotting results identified that bpV(pis) exerts a neuroprotective effect by significantly increasing the phosphorylation level of AKT and ERK1/2 after experimental ICH. Neurobehavioral tests indicate that bpV(pis) promotes functional recovery in ICH animals.
Conclusion: This study provides first and direct evidence for a potential role of bpV(pis) in ICH therapy.
Keywords: bpV(pis), intracerebral hemorrhage, PTEN, AKT, ERK1/2, neuroprotection
Introduction
Intracerebral hemorrhage (ICH) is mainly caused by a rupture of the basilar artery in the brain, leading to high rates of disability and death in adults.1 It is critical to treat the relatively controllable secondary injury in ICH. The main factors responsible for secondary injury include the inflammatory response, the toxicity from secondary metabolites, the destruction of the blood–brain barrier, and the formation of secondary edema.2
PTEN (phosphatase and tensin homolog deleted on chromosome 10), a tumor suppressor, has both lipid and protein phosphatase activity. In our previous study we have revealed that inhibiting PTEN phosphatase activity confers neuroprotection through various signaling pathways.3 PTEN also directly regulates the activation of AKT. AKT, phosphorylated by PI3K, promotes cell growth and survival via E2F1, NF-kB, and mTOR signaling.3 Increasing phosphorylation of AKT at S473 by suppressing PTEN contributes to survival. Among various pathways, PI3K/AKT regulated by PTEN lipid phosphatase confers neuroprotection in neuronal injury such as ischemia/reperfusion.4,5
ERK1/2 is the extracellular signal-regulated kinase. The oncogene Ras activates ERK1/2 cascade and promotes cell cycling and proliferation.6,7 Increasing ERK1/2 phosphorylation level improves survival rate after ischemia stroke.8 ERK has a co-function with AKT, indicating the internal relationship of both.9,10
Peroxovanadium (pv) compound is an oxygen vanadium complex with a core of four or five valence vanadium after excessive oxidation by hydrogen peroxide, which can combine with specific ligands and shape into a coordination compound. bpV makes PTP (protein tyrosine phosphatases) catalytic activity allosteric and inactive in the domain of cysteine residues.11 PTEN, one of the subtypes of PTP, has a more lenient structure, which could be inhibited by bpV in a low concentration.12,13 Bisperoxovandium (pyridine-2-carboxyl)[bpV(pic)], a commercially available PTEN inhibitor, confers a protective effect in brain injury and cognitive deficits of isoflurane exposures.14,15 Squaramide is a functional group existing in many bioactive compounds that have various biological activities.16–19 Because of its functional activity, stability, low cost of starting materials, and straightforward synthesis, squaramide is an appropriate molecule for the development of affordable agents. We, therefore, designed and synthesized the compound, the bpV(pis), in the presence of pyridine-2-squaramide and V2O5.20
It has been reported that different vanadium compounds inhibit PTEN and also activate AKT and ERK1/2.21 The neuroprotective effects of bisperoxovandium on cerebral ischemia through ERK1/2 and AKT activations were shown by our group, and also others.22,23 To develop a new compound that may have more effective neuroprotection, we have designed and synthesized a new compound, bpV(pis), which inhibits the activity of PTEN and enhances ERK1/2 activation.20 Importantly, bpV(pis) plays a neuroprotective role in cerebral ischemia/reperfusion injury.20 As ICH-induced brain injury results in severe clinical outcome, we tested whether bpV(pis) is neuroprotective in ICH. Our study provides the first evidence that bpV(pis) protects against ICH-induced brain injury via PTEN inhibition and ERK1/2 activation.
Materials and methods
Animals and ICH model
Adult male SD rats (n=186, weighing 280–300 g) were housed in a light and temperature controlled (23–25°C) environment with unlimited access to food and water. Sixteen adult pregnant female rats were used for cortical neuronal cultures. All animal experiments were carried out in compliance with the National Institutes of Health guidelines and the Animal Care and Ethics Committee of Wuhan University School of Medicine. Randomization was used to assign samples to the experimental groups, and to collect and process data.
Hemorrhagic stroke in rats was induced as previously reported.4 Briefly, rats were anesthetized with 4% isoflurane in 70% N2O and 30% O2 using a mask. A craniotomy was performed and a 23-gauge needle was inserted into the caudate nucleus (stereotactic coordinates from bregma: 0.2 mm anterior, 3.5 mm lateral, and 5.5 mm in depth). The autologous whole blood (80 μL) was injected at a rate of 10 μL/min. The needle was left in place for an additional 10 minutes after the completed infusion. Finally, the burr hole was sealed with bone wax after withdrawing the needle. Sham animals received the same surgical procedures, except for the blood injection.
Intracerebroventricular administration
As was previously reported,24 the rat was anesthetized in a sealed box, with a mixture of 4% isoflurane in 70% N2O and 30% O2. When the rat was deeply anesthetized, the head was secured in position in a stereotaxic frame using ear bars and the upper incisor bar, then the rat was anesthetized continuously with 4% isoflurane. A small mid-sagittal incision was made and the bregma was located as the anatomical reference point. Drug infusion to the cerebral ventricle (from the bregma: posterior, 0.8 mm; lateral, 1.5 mm; depth, 3.5 mm) was performed using a 23-gauge needle attached via polyethylene tubing to a Hamilton microsyringe at a rate of 1.0 μL/min. When the administration was finished, the needle was left in place for an additional 5 minutes, then removed slowly.
Western blotting analysis
Total protein was extracted and processed as previously described. Equal amounts of protein were separated by 10–12% SDS polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were electronically transferred to polyvinylidene difluoride membrane (Millipore, USA) and incubated with a blocking buffer (5% non-fat milk) for 1 hour at room temperature. The polyvinylidene difluoride membrane was incubated with primary antibody against ERK 1/2 (Rabbit, Cell Signaling Technology, Beverly, MA, USA, cat. no. 9102, 1:1,000), phospho-ERK1/2 (Thr202/Tyr204) (Rabbit, Cell Signaling Technology, cat. no. 9101, 1:1,000), AKT (Mouse, Cell Signaling Technology, cat. no. 2920, 1:1,000), and phospho-AKT (Rabbit, Cell Signaling Technology, cat. no. 4060, 1:1,000). Primary antibodies were labeled with horseradish peroxidase-conjugated secondary antibody, and protein bands were imaged using SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL, USA). The EC3 Imaging System (UVP, LLC, Upland, CA, USA) was used to obtain blot images directly from the PVDF membrane. The quantification of Western blot data was performed using Image J software (Image J, USA).
Fluoro-Jade C staining
Experimental procedures for preparing frozen sections were the same as for immunofluorescence staining. FJC staining was performed using a standard protocol. Briefly, brain tissue sections were immersed in 1% sodium hydroxide in 80% ethanol for 5 minutes, then rinsed for 2 minutes in 70% ethanol, for 2 minutes in distilled water, followed by incubating in 0.06% potassium permanganate solution for 10 minutes. After a 1–2 minute water rinse, the slides were transferred to a 0.0001% solution of Fluoro-Jade C (Sigma Aldrich, USA) dissolved in 0.1% acetic acid vehicle for 10 minutes. The slides were then rinsed through three changes of distilled water for 1 minute per change, then air dried on a slide warmer at 50°C for at least 5 minutes. Last, sections were immersed in xylene for 1 minute and mounted with DPX.
Brain water content and evans blue (EB) extravasation
Brain tissues were isolated at 24 hours after surgery and weighed as wet weight. Then they were dried at 100°C for 24 hours and weighed as dry weight. The percentage of water content was calculated as (wet weight–dry weight)/wet weight×100%.
Evans blue (EB) extravasation was carried out as reported at 24 hours after the operation.25 The Evans blue dye (2%, 5 mL/kg; Aladdin, Shanghai, China) was injected and administered >2 minutes into the femoral vein. One hour later, the tissue of brain was collected, homogenized in saline, and centrifuged at 15,000 g for 30 minutes. Next, an equal volume of trichloroacetic acid was added to the resultant supernatant. The samples were incubated at 4°C overnight and centrifuged at 15,000 g for 30 minutes. The resultant supernatant was then spectrophotometrically quantified for the extravasated Evans blue dye at 620 nm.
Hematoma volume analysis
Rats were sacrificed, infused with 0.9% saline. Tissues of hematoma were cut into contiguous coronal slices. The hematoma volume was measured on images and calculated by using Image J software package (Image J).
Cells culture and treatment
The cortical neuronal cultures were prepared from Sprague–Dawley rats at gestation day 17, as described in our previous report.25 The pregnant rats were anesthetized with 4% isoflurane in 70% N2O and 30% O2 and sacrificed by cervical dislocation. The brain of the embryo was quickly removed and the cortices were placed in ice-cold plating medium (Neurobasal medium, 2% B-27 supplement, 0.5% FBS, 0.5 mM L-glutamax, and 25 mM glutamic acid). The cortical neurons were suspended in plating medium and plated on Petri dishes coated with poly-D-lysine. Half of the plating medium was removed and replaced with maintenance medium (Neurobasal medium, 2% B-27 supplement, and 0.5 mM L-glutamine) the next day. The maintenance medium was refreshed every 3 days. After 12 days, the cultured neurons were used. On the treatment day, cells were treated with 100 μM of hemin (Sigma Aldrich) or vehicle (0.1% dimethyl sulfoxide) for 24 hours. On the following day, the cells were exposed to bpV(pis) for 1 hour and then harvested for the next experiments.
Cell viability and LDH release assays
The viability of the cells was assessed by their ability to uptake thiazolyl blue tetrazolium bromide (MTT). Cells were incubated with MTT then the lysates were read on a plate reader (PowerWave X, Bio-Tek) at the absorbance wavelength of 540 nm. The LDH release was measured using a CytoTox 96 Cytotoxicity kit based on the manufacturer’s instructions (Promega, Madison, WI, USA).
Neurobehavioral tests
Neurological severity scores
The rats were subjected to a modified neurological severity score (mNSS) test, as reported previously.26 These tests are a battery of motor, sensory, reflex, and balance tests, which are similar to the contralateral neglect tests in humans. Neurological function was graded on a scale of 0–18 (normal score, 0; maximal deficit score, 18).
Beam walk test
The beam walk test measures the animals’ neuromotor function.27 The animal was timed as it walked a (100x2 cm) beam. A box for the animal to feel safe was placed at one end of the beam. A loud noise was created to stimulate the animal to walk toward and into the box. Scoring was based upon the time it took the rat to go into the box. The higher the score, the more severe was the neurological deficit.
Adhesive-removal test
A modified sticky-tape (MST) test was performed to evaluate forelimb function.28 A sleeve was created using a 3.0×1.0 cm piece of yellow paper tape, and was subsequently wrapped around the forepaw so that the tape attached to itself and allowed the digits to protrude slightly from the sleeve. The typical response is for the rat to vigorously attempt to remove the sleeve by either pulling at the tape with its mouth or brushing the tape with its contralateral paw. The rat was placed in its cage and observed for 30 seconds. Two timers were started: the first ran without interruption and the second was turned on only while the animal attempted to remove the tape sleeve. The ratio of the right/left forelimb performance was recorded. The contralateral and ipsilateral limbs were tested separately. The test was repeated three times per test day, and the best two scores of the day were averaged. The lower the ratio, the more severe is the neurological deficit.
Materials
bpV(pis) was synthesized in the Faculty of Pharmacy, Wuhan University School of Medicine.
Statistics
All results are presented as mean±SE. ANOVA test was used to examine the statistical significance of the differences between groups of data. Bonferroni tests were used for post-hoc comparisons when appropriate. P<0.05 was considered statistically significant.
Results
bpV(pis) reduces brain damage in ICH rats
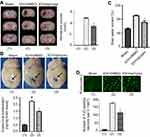
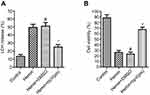
The experiment protocol for this study is shown in Figure 1. As is reported in our previous study, we designed and synthetized bpV(pis) to be neuroprotective in cerebral ischemia injury.20 In this study, we tested whether bpV(pis) is neuroprotective in the ICH model.20 bpV(pis) was administrated to the contralateral cerebral ventricle 1 hour after ICH. We showed that bpV(pis) decreased hematoma volume at 72 hours after ICH (Figure 2A). bpV(pis) treatment also reduced the extravasation of Evans blue in the injured cortex at 72 hours after ICH (Figure 2B). In addition, we found that ICH-induced brain edema was relieved by bpV(pis) treatment at 72 hours after ICH (Figure 2C). Furthermore, Fluor Jade C (FJC) assay labeling degenerating neurons showed that the number of FJC positive neurons was reduced by bpV(pis) treatment at 24 hours after ICH (Figure 2D). To verify the neuroprotective effect of bpV(pis) in vitro, we established the hemin injury model in the cortical neuronal cultures.29 LDH release assay and MTT assay showed that bpV(pis) decreases hemin-induced cortical neuronal death at 24 hours after insult (Figures 3A and B). Together, these results indicate that bpV(pis) is neuroprotective in ICH rats.
bpV(pis) prevents the decrease of AKT phosphorylation in ICH
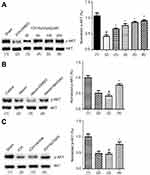
Our study has verified that bpV(pis) inhibits PTEN but enhances Erk1/2 activation independent of PTEN inhibition in cerebral ischemia injury.20 It has been well established that AKT activation is negatively regulated by PTEN. To test whether bpV(pis) inhibits PTEN in our experimental conditions, we set up to use altered AKT activation to index the change of PTEN in ICH by measuring p-AKT (the phosphorylation level of AKT).30 To determine the activation of AKT, the phosphorylation of AKT at S473 was measured.30 In the in vivo ICH models, we found that p-AKT level was decreased after ICH (Figure 4A). To test whether bpV(pis) rescues the decreased AKT activation, bpV(pis) was administrated to lateral ventricle after ICH injury. We showed that p-AKT in the ICH + bpV(pis) group was increased compared to the ICH+DMSO group (Figure 4A). Decreased p-AKT was alleviated by bpV(pis) in a dose-dependent manner (Figure 4A). In the in vitro experiments, similar results showed that suppressed p-AKT in cortical neurons infracted by hemin was upregulated by bpV(pis) (Figure 4B). To support this finding, we show that bpV(pis) prevents ICH-induced reduction of p-AKT (Figure 4C). These results suggest that PTEN suppression by bpV(pis) leads to the enhancement of AKT activation in ICH.
Inhibiting AKT reduces the neuroprotective effect of bpV(pis) in hemin-induced neuronal injury
To determine whether bpV(pis) reduces neuronal death through PTEN/AKT signaling pathway after ICH, we used AKT inhibitor IV to suppress AKT. We carried out LDH and cell viability assay to test death or viability in cultured cortical neurons. We demonstrated that pre-treatment of AKT inhibitor IV blocked bpV(pis)-induced neuronal survival after the hemin insult (Figure 5). These results imply that bpV(pis) protects against hemin insult-induced neuronal injury via PTEN inhibition.
bpV(pis) prevents the reduction of ERK1/2 phosphorylation after ICH
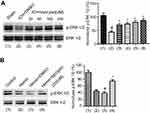
ERK1/2 is activated when it is phosphorylated on Thr202/Tyr204.31 Our previous experiments have revealed that enhancing ERK1/2 activation is neuroprotective in ischemia stroke.20 To explore whether ERK1/2 activation is mediated by bpV(pis) in ICH, we tested the phosphorylation of ERK1/2 (p-ERK1/2) on Thr202/Tyr204 by Western blotting. In the in vivo ICH model, we found that p-ERK1/2 was decreased significantly, but increased in bpV(pis) treatment group after ICH insult (Figure 6A). bpV(pis) rescued ICH-induced decrease of p-ERK1/2 in a dose-dependent manner (Figure 6A). In the in vitro experiments, cultured cortical neurons were insulted by hemin. The bpV(pis) treatment group showed a higher level of p-ERK1/2 than hemin and Hemin+DMSO group (Figure 6B). Consistent with those in vivo data, bpV(pis) alleviated the reduction of p-ERK1/2 caused by hemin. These results indicate bpV(pis) preserves ERK1/2 activation after ICH.
ERK1/2 inhibitor reduces the neuroprotective effect of bpV(pis) in ICH
In order to testify whether preserved ERK1/2 activation by bpV(pis) has a neuroprotective effect, we treated the cultured cortical neurons with ERK1/2 inhibitor (U0126) at 30 minutes before bpV(pis) treatment. In LDH and MTT assays, the Hemin+bpV(pis) group showed a higher neuronal survival rate and lower neuronal death rate compared to the hemin treatment group (Figure 7). These data suggest a neuroprotective role of bpV(pis) in hemin-induced injury. Compared to Hemin+bpV(pis) group, Hemin+U0126+bpV(pis) or Hemin+U0126+AKT inhibitor+bpV(pis) groups show increased LDH release and reduced cell viability (Figure 7), indicating that inactivating ERK1/2 and AKT blocks bpV(pis)-induced neuroprotection. Thus, bpV(pis) protects against hemin-induced neuronal injury through ERK1/2 activation and PTEN inhibition.
bpV(pis) treatment improves functional recovery of ICH animals
To further verify the functional outcome of bpV(pis)-induced neuroprotection, we conducted a series of behavioral tests on rats 1 day before ICH, and 1, 3, 7, and 14 days after ICH. mNSS scores of the rats treated with bpV(pis) were reduced on the 7th and 14th day, and beam-walking test scores of rats treated with bpV(pis) were reduced on the 3rd, 7th, and 14th day (Figures 8A and B). However, the MST ratio was improved on the 7th and 14th day after bpV(pis) treatment compared with the ICH+DMSO group (Figure 8C). Taken together, these results provided behavioral evidence for the neuroprotective role of bpV(pis) in ICH injury.
Discussion
We have previously reported for the first time that suppressing PTEN confers neuroprotection in ischemic neuronal injury.3,30 We further investigated the underlying mechanisms mediating the neuroprotective effect of PTEN downregulation in ischemic neuronal injury.3,30,32,33 However, the role of PTEN downregulation in ICH-induced neuronal injury remains unclear. Here we provide evidence that suppressing PTEN protects against ICH-induced neuronal injury, suggesting a general neuroprotective role of PTEN downregulation in neuronal injury.
It has been established that the bisperoxovandium compound at nanomole concentration reduces the lipid phosphatase activity of PTEN.13 However, the specific effect of this compound remains unclear. We recently designed and synthesized the new compound bpV(pis) by pyridine-2-squaramide and V2O5. Interestingly, we discovered that bpV(pis) not only suppresses PTEN activity, but also enhances ERK1/2 activation, and the ERK1/2 activation does not require PTEN inhibition by bpV(pis).20 Furthermore, we demonstrate that bpV(pis) protects against cerebral ischemia injury through PTEN inhibition and ERK1/2 activation. We also show that the commercially available PTEN inhibitor bpV(pic) protects against ischemic neuronal injury through ERK1/2 activation.23 These data suggest that the bisperoxovandium compound is also an ERK1/2 activator and is neuroprotective in cerebral ischemia injury. In this study, we show that bpV(pis) also reduces ICH-induced neuronal injury. Therefore, the neuroprotective effect of bpV(pis) in ICH is mediated by PTEN inhibition and ERK1/2 activation. The dual neuroprotective effect of bpV(pis) or bpV(pic) through two different signal targets, PTEN and ERK1/2, leads us to conclude that bpV(pis) is a potential drug candidate for stroke therapy. Based on the function of bpV(pis), it is predicable that bpV(pis) may act as an adjuvant compound in cell therapy in diseases like diabetes or aging. Further investigation is warranted.
We show that bpV(pis) alleviates the hematoma and brain edema, and preserves the integrity of the brain–blood barrier after ICH. The underlying mechanisms are not yet clear. It is likely that multiple cellular and molecular mechanisms are involved. A simple hypothesis is that bpV(pis) may act through both PTEN and ERK1/2 to alleviate the hematoma and brain edema, and preserves the integrity of the brain–blood barrier. We will aim to explore other possible mechanisms in our future studies.
AKT is known to be negatively regulated by PTEN.3 In the present study we used AKT activation as an index of PTEN suppression because our early study has carefully characterized the inhibition of PTEN by bpV(pis) and the subsequent AKT activation. ERK1/2 belongs to the MAPK family that can be activated by the upstream signal MAPKKs(MEK1/MEK2). When activated, ERK1/2 regulates the phosphorylation of transcription factors that are closely related to cell differentiation, proliferation and apoptosis.34 Previous studies have shown that ERK1/2 mediates axonal regeneration and promotes the survival of cortical neurons in cerebral hemorrhage injury.35,36 Enhancing ERK1/2 phosphorylation also confers neuroprotection after cerebral ischemia injury.8 The observed effect of bisperoxovandium compound as an ERK1/2 activator in both cerebral ischemia and ICH further support the neuroprotective role of ERK1/2 in CNS injury. It is possible that the neuroprotective effect of bpV(pis) may be mediated through other cellular and molecular mechanisms. For example, according to the functional roles of bone morphogenetic protein (BMP), sonic hedgehog signaling, and Notch signaling in the CNS.37–39 This signaling may be regulated by bpV(pis). Further study will be performed to test these possibilities. Synaptic dysfunction is the earliest sign of neurodegeneration. As a neuroprotective compound, bpV(pis) may act on a single synaptic site to exert its therapeutic effect. Future investigations can be done by performing a Mass Synaptometry assay.40
Compliance with ethical standards
All animal use and experimental protocols were approved and carried out in compliance with the IACUC guidelines and the Animal Care and Ethics Committee of Wuhan University School of Medicine.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi:10.1016/S0140-6736(09)60371-8
2. Xiong XY, Wang J, Qian ZM, Yang QW. Iron and intracerebral hemorrhage: from mechanism to translation. Transl Stroke Res. 2014;5:429–441. doi:10.1007/s12975-013-0317-7
3. Chang N, El-Hayek YH, Gomez E, Wan Q. Phosphatase PTEN in neuronal injury and brain disorders. Trends Neurosci. 2007;30:581–586. doi:10.1016/j.tins.2007.08.006
4. Jiang B, Li L, Chen Q, et al. Role of glibenclamide in brain injury after intracerebral hemorrhage. Transl Stroke Res. 2017;8:183–193. doi:10.1007/s12975-016-0506-2
5. Li Y, Wang H, Muffat J, et al. Global transcriptional and translational repression in human-embryonic-stem-cell-derived rett syndrome neurons. Cell Stem Cell. 2013;13:446–458. doi:10.1016/j.stem.2013.09.001
6. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi:10.1126/science.1072682
7. Sanclemente M, Francoz S, Esteban-Burgos L, et al. c-RAF ablation induces regression of advanced Kras/Trp53 mutant lung adenocarcinomas by a mechanism independent of MAPK Signaling. Cancer Cell. 2018;33:217–228.e214. doi:10.1016/j.ccell.2017.12.014
8. Han J, Feng Z, Xie Y, et al. Oncostatin M-induced upregulation of SDF-1 improves bone marrow stromal cell migration in a rat middle cerebral artery occlusion stroke model. Exp Neurol. 2019;313:49–59. doi:10.1016/j.expneurol.2018.09.005
9. Baumgartner C, Toifl S, Farlik M, et al. An ERK-dependent feedback mechanism prevents hematopoietic stem cell exhaustion. Cell Stem Cell. 2018;22:879–892.e876. doi:10.1016/j.stem.2018.05.003
10. Gwinn DM, Lee AG, Briones-Martin-Del-Campo M, et al. Oncogenic KRAS regulates amino acid homeostasis and asparagine biosynthesis via ATF4 and alters sensitivity to L-Asparaginase. Cancer Cell. 2018;33:91–107.e106. doi:10.1016/j.ccell.2017.12.003
11. Huyer G, Liu S, Kelly J, et al. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem. 1997;272:843–851.
12. Rosivatz E, Matthews JG, McDonald NQ, et al. A small molecule inhibitor for phosphatase and tensin homologue deleted on chromosome 10 (PTEN). ACS Chem Biol. 2006;1:780–790. doi:10.1021/cb600352f
13. Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38. doi:10.1016/j.febslet.2004.03.102
14. Sury MD, Vorlet-Fawer L, Agarinis C, et al. Restoration of Akt activity by the bisperoxovanadium compound bpV(pic) attenuates hippocampal apoptosis in experimental neonatal pneumococcal meningitis. Neurobiol Dis. 2011;41:201–208. doi:10.1016/j.nbd.2010.09.007
15. Tan L, Chen X, Wang W, et al. Pharmacological inhibition of PTEN attenuates cognitive deficits caused by neonatal repeated exposures to isoflurane via inhibition of NR2B-mediated tau phosphorylation in rats. Neuropharmacology. 2017;114:135–145. doi:10.1016/j.neuropharm.2016.11.008
16. Marin C, Ximenis M, Ramirez-Macias I, et al. Effective anti-leishmanial activity of minimalist squaramide-based compounds. Exp Parasitol. 2016;170:36–49. doi:10.1016/j.exppara.2016.07.013
17. Olmo F, Rotger C, Ramirez-Macias I, et al. Synthesis and biological evaluation of N,N‘-squaramides with high in vivo efficacy and low toxicity: toward a low-cost drug against chagas disease. J Med Chem. 2014;57:987–999. doi:10.1021/jm4017015
18. Chan PC, Roon RJ, Koerner JF, Taylor NJ, Honek JF. A 3-amino-4-hydroxy-3-cyclobutene-1,2-dione-containing glutamate analogue exhibiting high affinity to excitatory amino acid receptors. J Med Chem. 1995;38:4433–4438.
19. Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300–304. doi:10.1016/j.clindermatol.2014.12.001
20. Zhang ZF, Chen J, Han X, et al. Bisperoxovandium (pyridin-2-squaramide) targets both PTEN and ERK1/2 to confer neuroprotection. Br J Pharmacol. 2017;174:641–656. doi:10.1111/bph.13727
21. Mehdi MZ, Pandey SK, Theberge JF, Srivastava AK. Insulin signal mimicry as a mechanism for the insulin-like effects of vanadium. Cell Biochem Biophys. 2006;44:73–81. doi:10.1385/Cbb:44:1:073
22. Mao LL, Hao DL, Mao XW, et al. Neuroprotective effects of bisperoxovanadium on cerebral ischemia by inflammation inhibition. Neurosci Lett. 2015;602:120–125. doi:10.1016/j.neulet.2015.06.040
23. Liu R, Tang JC, Pan MX, et al. ERK 1/2 activation mediates the neuroprotective effect of bpV(pic) in focal cerebral ischemia-reperfusion injury. Neurochem Res. 2018;43:1424–1438. doi:10.1007/s11064-018-2558-z
24. Zhang QG, Wu DN, Han D, Zhang GY. Critical role of PTEN in the coupling between PI3K/Akt and JNK1/2 signaling in ischemic brain injury. FEBS Lett. 2007;581:495–505. doi:10.1016/j.febslet.2006.12.055
25. Chen Y, Zhang Y, Tang J, et al. Norrin protected blood-brain barrier via frizzled-4/beta-catenin pathway after subarachnoid hemorrhage in rats. Stroke. 2015;46:529–536. doi:10.1161/STROKEAHA.114.007265
26. Moisan A, Favre I, Rome C, et al. Intravenous injection of clinical grade human MSCs after experimental stroke: functional benefit and microvascular effect. Cell Transplant. 2016;25:2157–2171. doi:10.3727/096368916X691132
27. Sharma S, Taliyan R. High fat diet feeding induced insulin resistance exacerbates 6-OHDA mediated neurotoxicity and behavioral abnormalities in rats. Behav Brain Res. 2018;351:17–23. doi:10.1016/j.bbr.2018.05.025
28. Yang C, DeMars KM, Alexander JC, Febo M, Candelario-Jalil E. Sustained neurological recovery after stroke in aged rats treated with a novel prostacyclin analog. Stroke. 2017;48:1948–1956. doi:10.1161/STROKEAHA.117.016474
29. Dhar D, Antonucci L, Nakagawa H, et al. Liver cancer initiation requires p53 inhibition by CD44-enhanced growth factor signaling. Cancer Cell. 2018;33:1061–1077.e1066. doi:10.1016/j.ccell.2018.05.003
30. Ning K, Pei L, Liao M, et al. Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J Neurosci. 2004;24:4052–4060. doi:10.1523/JNEUROSCI.5449-03.2004
31. Gigliozzi A, Alpini G, Baroni GS, et al. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology. 2004;127:1198–1209.
32. Zheng M, Liao M, Cui T, et al. Regulation of nuclear TDP-43 by NR2A-containing NMDA receptors and PTEN. J Cell Sci. 2012;125:1556–1567. doi:10.1242/jcs.095729
33. Liu B, Li L, Zhang Q, et al. Preservation of GABAA receptor function by PTEN inhibition protects against neuronal death in ischemic stroke. Stroke. 2010;41:1018–1026. doi:10.1161/STROKEAHA.110.579011
34. Roskoski R
35. Kwon KJ, Kim JN, Kim MK, et al. Neuroprotective effects of valproic acid against hemin toxicity: possible involvement of the down-regulation of heme oxygenase-1 by regulating ubiquitin-proteasomal pathway. Neurochem Int. 2013;62:240–250. doi:10.1016/j.neuint.2012.12.019
36. Lou N, Takano T, Pei Y, et al. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci U S A. 2016;113:1074–1079. doi:10.1073/pnas.1520398113
37. Gajera CR, Emich H, Lioubinski O, et al. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci. 2010;123:1922–1930. doi:10.1242/jcs.065912
38. Ihrie RA, Shah JK, Harwell CC, et al. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71:250–262. doi:10.1016/j.neuron.2011.05.018
39. Imayoshi I, Kageyama R. The role of Notch signaling in adult neurogenesis. Mol Neurobiol. 2011;44:7–12. doi:10.1007/s12035-011-8186-0
40. Gajera CR, Fernandez R, Postupna N, et al. Mass synaptometry: high-dimensional multi parametric assay for single synapses. J Neurosci Methods. 2019;312:73–83. doi:10.1016/j.jneumeth.2018.11.008
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.